Abstract
Background
Guidelines recommend that patients with haemophilia should preferably receive vaccination subcutaneously. COVID‐19 and other vaccines, however, are only licenced for intramuscular application.
Aims
To assess the safety of intramuscular COVID‐19 vaccination in patients living with haemophilia.
Methods
Part A of this prospective observational study enrolled consecutive patients with haemophilia A (HA) and B (HB) of all ages and severities and assessed injection site bleeding and other complications within 30 days of vaccination. Part B enrolled patients providing informed consent for detailed data collection including medication and prophylaxis around the time of vaccination. Logistic regression was performed to assess potential risk factors for bleeding.
Results
Four hundred and sixty‐one patients were enrolled into part A. The primary endpoint injection site bleeding occurred in seven patients (1.5%, 95% confidence interval .7–3.1%). Comprehensive analysis of 214 patients (404 vaccinations, part B) revealed that 97% of patients with severe haemophilia had prophylaxis before vaccination, either as part of their routine prophylaxis or using additional doses. 56% and 30% of patients with moderate and mild haemophilia, respectively, received prophylaxis before vaccination. Among the seven bleeds recorded, three occurred when intramuscular vaccination was done without prophylaxis (odds ratio 12).
Conclusions
This is the first prospective study reporting on the safety of intramuscular vaccination in haemophilia. The rate of injection site bleeding was low in mild haemophilia, and in moderate and severe haemophilia if patients received factor prophylaxis.
Keywords: blood coagulation disorders, COVID‐19 vaccines, emicizumab, Factor IX, Factor VIII, humans, observational study
1. INTRODUCTION
Intramuscular injections have traditionally been avoided in persons with haemophilia because of the need of factor concentrate prophylaxis and the risk of serious haemorrhage. 1 , 2 , 3 Muscle bleeds in haemophilia are difficult to manage and may require several days or weeks of clotting factor treatment. 4 , 5 , 6 Feared sequelae include fibrosis and contracture. 6 , 7 The World Federation of Haemophilia (WFH) guideline recommended that children and adults with haemophilia should preferably receive vaccines subcutaneously rather than intramuscularly, as it is safe and effective as the latter and does not require clotting factor infusion. 8
The haemophilia treatment landscape is rapidly evolving, 9 and the COVID‐19 pandemic poses new challenges and risks to the haemophilia community. 10 , 11 Vaccination has unanimously been regarded as the preferred way of coping with it. 12 Vaccines against the severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV2) have been studied and licenced for intramuscular injection only, and their efficacy and safety when given through other routes is unclear. Therefore, an international board of experts suggested that COVID‐19 vaccines should be administered in people with haemophilia through intramuscular injection after prophylaxis with clotting factor concentrate. 12
The German, Austrian, and Swiss Society on Thrombosis and Haemostasis (GTH) working group on haemophilia set out to study the safety of COVID‐19 vaccines, including their intramuscular injection, in people with haemophilia. The group issued detailed consensus recommendations for the use of these vaccines in Germany, Austria, and Switzerland, 13 and initiated a sufficiently powered, prospective observational study in accordance with the German Infection Protection Act. Here we report the main results of the study, demonstrating the safety of intramuscular vaccination under adequate protection with clotting factor concentrate.
2. METHODS
2.1. Study design
This prospective observational study was performed in six tertiary care centres and consisted of two parts (Figure 1). Part A enrolled consecutive, anonymised patients with HA or HB of any severity and age, who received COVID‐19 vaccination between January 1, 2021 and December 31, 2021. Patients were approached during routine site visits or telephone calls that often replaced site visits during the pandemic. Part B enrolled patients for detailed data collection if they provided informed consent. Only three of the six institutions participated in part B.
FIGURE 1.
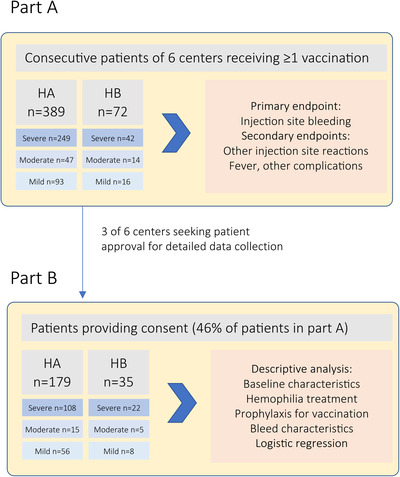
Study design and patient disposition
Management decisions were at the discretion of the treating physicians and not part of the study protocol. Guidance was available from recent GTH recommendations, 14 and the German Robert Koch Institute. 15 The study was approved by local ethics committees of all institutions, by the regulatory body of the city of Hannover, Lower Saxony, Germany. Advice on study conduct was also obtained from the national competent authority, the Paul Ehrlich Institute.
2.2. Data collection
For part A, centres documented patients receiving vaccinations and obtained information as part of routine management within the study period on complications until 30 days after the second vaccination. The following anonymised data were entered into the electronic case report form (eCRF): haemophilia type and severity, decade of age, date of vaccinations, complications and adverse events including injection site hematoma, injection site granuloma, other injection site reactions, fever ≥38.5°C, and other complications. In the case of other complications, free text descriptions were requested.
For part B, patients received data capture forms to collect information on regular prophylaxis, the last factor VIII (FVIII) or factor IX (FIX) injection before vaccination (as part of routine prophylaxis or on‐demand), type of concentrate (plasma‐derived or recombinant standard half‐life [SHL] or recombinant extended half‐life [EHL]), type of vaccine, injection needle size, anatomic region and depth of injection, adverse events, and treatment of bleeding events. Forms were filled in by patients themselves or physicians in vaccination centres as appropriate. Additional information was retrieved from medical records, including information on haemophilia, body weight (BW), concomitant disorders, and medication. Pseudonymised data were entered into the eCRF (Ninox Software GmbH, Berlin, Germany, www.ninox.com).
2.3. Endpoints
The primary endpoint, analysed in part A of the study, was the cumulative incidence of injection site bleeds within the observational period starting on the day of the first vaccination and ending 30 days after the second vaccination. Secondary endpoints included the cumulative incidence of other injection site reactions, fever, and other adverse events. Analysis was per patient (i.e., events were only counted once per patient). In part B, information was collected separately for the first and second vaccinations and additional data on severity of bleeding, haematoma size, pain, and treatment was also evaluated.
2.4. Statistical analysis
Descriptive statics were used to summarize data, with absolute numbers and proportions (in percentage) for categorical data, mean and standard deviation (SD) reported for normally distributed data, and median and interquartile range (IQR) for continuous data not following normal distribution.
The primary and secondary endpoints were reported by proportions (in percentage) with the Wilson 95% confidence interval (CI) for binomial distribution. Statistical power considerations performed at the time of study design estimated that about 500 patients were required assuming a proportion of 2% for the primary endpoint and an acceptable confidence interval width of about 2%.
Logistic regression was performed on data from part B to assess the occurrence of the primary endpoint in association with haemophilia severity, use of antithrombotic drugs, body weight, age, and prophylaxis at the time of vaccination. Regarding the latter, two models were tested: adequate prophylaxis defined as (model 1) FVIII concentrate (SHL or EHL), or FIX concentrate (SHL) given within 24 h or FIX concentrate (EHL) within the last 48 h prior to vaccination, or emicizumab; (model 2) FVIII concentrate (SHL or EHL) or FIX concentrate (SHL) given within 48 h or FIX concentrate (EHL) within the last 96 h prior to vaccination, or emicizumab. Data are only presented for model 2 that performed significantly better. Haemophilia severity was part of all models, whereas other covariates were selected using backward selection. A P‐value of < .05 was considered statistically significant.
3. RESULTS
3.1. Part A
Demographic data of the 461 patients enrolled in part A are shown in Table 1. The mean proportion of patients enrolled out of the total number of haemophilia patients attending the centres was 37% (range 14–69%). The distribution of haemophilia type and severity was similar to previous studies performed in German haemophilia centres. 16 , 17 , 18 The distribution of ages showed an underrepresentation of children due to the licensing status of COVID‐19 vaccines in 2021. The time course of vaccinations is shown in Figure 2.
TABLE 1.
Demographics (part A)
| Characteristic | Patients | |
|---|---|---|
| n (%) | ||
| Patients enroled | 461 | |
| Haemophilia type | ||
| Haemophilia A | 389 (84) | |
| Haemophilia B | 72 (16) | |
| Severity | Severe | 291 (63) |
| Moderate | 61 (13) | |
| Mild | 109 (24) | |
| Age group (years) | <10 | 4 (1) |
| 10‐ < 20 | 51 (11) | |
| 20‐ < 30 | 80 (17) | |
| 30‐ < 40 | 72 (16) | |
| 40‐ < 50 | 63 (14) | |
| 50‐ < 60 | 83 (18) | |
| 60‐ < 70 | 55 (12) | |
| 70‐ < 80 | 32 (7) | |
| 80‐ < 90 | 16 (3) | |
| ≥90 | 2 (0) | |
| Not reported | 3 (1) |
FIGURE 2.
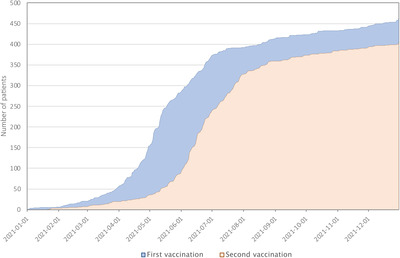
Timeline of first and second COVID‐19 vaccinations (part A)
The primary endpoint, injection site bleeding after the first or second vaccination, occurred in seven of 461 patients (1.5%, 95% CI .7–3.1%, Table 2).
TABLE 2.
Primary and secondary endpoints (part A)
| Endpoint | Patients n / N | Proportion in percentage (95% CI) a |
|---|---|---|
| Injection site bleeding | 7 / 461 | 1.5 (.7‐3.1) |
| Other injection site reactions | 12 / 461 | 2.6 (1.5‐4.5) |
| Fever ≥38.5°C | 26 / 461 | 5.6 (3.9‐8.1) |
| Other | 39 / 461 | 8.5 (6.3‐11.4) |
Wilson 95% confidence interval.
Other injection site reactions occurring in 12 patients (2.6%) included pain (five patients), erythema (five patients), and injection site granuloma (two patients). Fever ≥38.5°C was reported in 5.6%.
Other adverse events were reported in 8.5% of patients, including fatigue (19 patients), headache (10 patients), chills (eight patients), muscle or joint pain (eight patients), subfebrile temperature (four patients), circulatory problems (three patients), lymph node swelling (two patients), and nausea (one patient).
3.2. Part B
Data of the 214 patients in part B are summarised in Table S1. This population was about half of the population in part A and had a similar distribution. 49% and 69% of HA and HB patients, respectively, were on regular prophylaxis including all but two patients with severe haemophilia. Twenty one patients with severe HA were on emicizumab prophylaxis. Antithrombotic drugs were used by 5% of patients.
For all 214 patients, a first vaccination was documented, and for 190 (89%) participants also a second. In 10 patients, the Ad26.COV2‐S vaccine was used that was licenced for a single vaccination at that time. Of note, a potential third "booster" vaccination was not part of the protocol. A summary on factor prophylaxis before vaccination is provided in Table 3. Altogether, 97%, 56%, and 30% of severe, moderate, and mild haemophilia patients had effective prophylaxis at the time of vaccination.
TABLE 3.
Summary on factor prophylaxis for vaccination (part B)
| Patients receiving prophylaxis before vaccination a | 1st vaccination (n = 214) | 2nd vaccination (n = 190) | Both vaccinations (n = 404) |
|---|---|---|---|
| Severe, n/n (%) | 128/130 (98) | 108/113 (96) | 236/243 (97) |
| Moderate, n/n (%) | 11/20 (55) | 11/19 (58) | 22/39 (56) |
| Mild, n/n (%) | 15/64 (23) | 22/58 (38) | 37/122 (30) |
Defined as FVIII SHL or EHL concentrate or FIX SHL concentrate within the last 48 h, or FIX EHL concentrate within the last 96 h, or emicizumab.
Details are provided in Table S2. Patients using on‐demand treatment rather than prophylaxis did not receive factor in 76% and 75% of the first and second vaccinations, respectively. This included most patients with mild but also more than half of the patients with moderate haemophilia. If factor was used, a mean dose of 35 IU/kg BW was given about 2–4 h before vaccination. Patients on regular factor prophylaxis (including almost all severe patients not on emicizumab) did not receive additional doses in 67% and 74% of first and second vaccinations, respectively. They received a mean dose of 40 IU/kg BW as part of their regular regimen, usually on the day of vaccination or the day before. If additional factor doses were given, a mean dose of 36 IU/kg was used. Patients on emicizumab did not receive additional factor in 90% and 94% of first and second vaccinations, respectively.
Most injections were with mRNA COVID‐19 vaccinations and were given intramuscularly (91–95%) into the left (82%) or right shoulder (15%). Injection needle size was often not reported, but usually 25 G (Table S3).
Injection site bleeds occurred seven times in six patients enrolled into part B (Table 4). Five of them were > 50 years old; none was on antithrombotic therapy. Three patients with severe HA had hematomas despite factor injection before vaccination. One patient with severe HA (patient 6) did not bleed after the first vaccination, when he had received an additional dose of factor. He bled after the second vaccination, when he did not receive an additional dose and his last prophylactic dose was about 3 days ago. The two patients with moderate or mild haemophilia had not received factor concentrate for either vaccination. They bled on one occasion each. Injection site bleeding was considered mild in all cases and caused mild to moderate pain. Three of the seven bleeds were treated with an additional dose of factor.
TABLE 4.
Details of patients with injection site bleeding (part B)
| P1 | P2 | P3 | P4 | P5 | P6 | |
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Age, years | 58 | 57 | 34 | 57 | 50 | 63 |
| Haemophilia | HA | HA | HA | HA | HB | HA |
| Severity | Severe | severe | severe | moderate | mild | Severe |
| Inhibitor | Never | never | never | never | never | Never |
| Body weight, kg | 92 | 72 | 68 | 95 | 95 | 83 |
| Regular prophylaxis | y | y | Y | n | n | N |
| Drug | SHL | SHL | SHL | – | – | SHL |
| Last factor dose administered before 1st/2nd vaccination | ||||||
| Dose, IU/kg | 22/22 | 28/28 | 44/44 | 0/0 | 0/0 | 36/36 |
| Time, h | 17/17 | 16/13 | 2/3 | – | – | 2/72 |
| In addition to routine prophylaxis | y/y | y/y | n/n | – | – | y/n |
| Bleeds after 1st/2nd vaccination | ||||||
| Local Hematoma | y/y | y/n | y/n | y/n | n/y | n/y |
| Diameter, cm | 5/5 | 1/‐ | 7/‐ | 3/‐ | ‐/3 | ‐/6 |
| Pain score, VAS | 1/2 | 1/‐ | 2/‐ | 0/‐ | ‐/6 | ‐/0 |
| Doses for treatment, n | 1/1 | 0/‐ | 0/‐ | 0/‐ | ‐/0 | ‐/1 |
Abbreviations: n, no; P, patient; SHL, standard half‐life factor concentrate; VAS, visual analogue scale; y, yes.
Other adverse events were generally mild and of low frequency. The few vaccinations given subcutaneously did not result in increased injection site reactions or other adverse events. The study did not assess the efficacy of intramuscular or subcutaneous vaccination.
Logistic regression analysis identified severity of haemophilia and prophylaxis before vaccination as the only covariates associated with the risk of bleeding (Table S4). The predicted risk from the model closely matched the observed risk shown in Figure 3. The risk appeared low in persons with mild haemophilia but increased to 6% and 14% in moderate and severe haemophilia, respectively, if no prophylaxis was given before vaccination.
FIGURE 3.
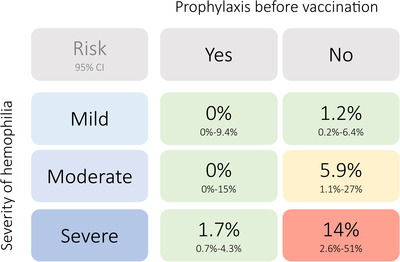
Observed bleeding risk according to haemophilia severity and prophylaxis before vaccination. Data was derived from part B of the study. Compare Table 3 for absolute patient numbers per group. The risk is provided by its point estimate (number of observed bleeds / number vaccinations per group, in percentage) and the Wilson 95% confidence interval. Prophylaxis before vaccination (“yes”) was defined as FVIII SHL or EHL or FIX concentrate within the last 48 h, or FIX EHL concentrate within the last 96 h, or emicizumab
4. DISCUSSION
At the time, when the WFH issued its recommendation to give vaccination in persons with haemophilia preferably via the subcutaneous route, an increasing body of evidence supported the subcutaneous administration is as effective as intramuscular and did not require factor prophylaxis. This had been demonstrated for both hepatitis vaccination and diphtheria and tetanus vaccines in children and adults. 19 , 20 , 21 , 22 The situation changed when SARS‐CoV2 vaccines were first licenced during the unfolding COVID‐19 pandemic. Although it cannot be excluded that these vaccines would also be safe and effective when given subcutaneously, the available evidence and the urgency of effective vaccination did not allow to recommend other than the established intramuscular route of administration. Vaccines based on mRNA or adenovirus technology require transduction of human body cells to produce the SARS‐CoV2 spike protein, and it remains to be demonstrated whether this will also happen effectively in the subcutaneous fatty tissue. Very limited data suggest that subcutaneous administration of the mRNA vaccine BNT162b1 can result in neutralising antibodies. 23 However, other reports reinforced intramuscular administration in the light of poor evidence for equal immunogenicity of subcutaneous administration and because of recurrent injection site reactions. 24 , 25 , 26 Emerging data suggest that COVID‐19 peptide vaccines are safe and effective when given subcutaneously but these have not been fully studied or licenced. 27
In this situation, several haemophilia experts panels published ad hoc recommendations that COVID‐19 vaccines should be given via the intramuscular route in persons with haemophilia. In accordance with the current WFH guideline, that factor prophylaxis should be provided in persons with severe or moderate haemophilia when intramuscular injection must be the route of administration, we and others suggested that patients on routine prophylaxis should preferably receive vaccination on the day of factor injection. If this was not feasible, given the dynamics of vaccination programs in the pandemic, additional doses were recommended to ensure that adequate factor levels could be expected.
Our prospective observational study demonstrates the feasibility and safety of this approach. Bleeding rates were low in persons, who received prophylaxis close to intramuscular vaccination. The same was true in persons using prophylaxis with emicizumab, who were advised to receive vaccination without additional factor. These results are in line with smaller studies or published single centre experience that intramuscular injections are generally safe in persons with haemophilia, 28 , 29 but can sometimes result in injection site bleeds of unpredictable severity. 30
Strengths of our study include the cohort size, the prospective design, and the consecutive enrollment in part A. The 95% confidence interval of the primary endpoint is narrow enough to firmly recommend intramuscular vaccination in persons with haemophilia under the conditions studied here. This includes factor prophylaxis to severe and moderate patients unless these are treated with emicizumab. Given the noninterventional nature of our study, some patients with severe or moderate haemophilia did not receive prophylaxis close before vaccination, and their bleeding risk was remarkably higher. This observation reinforces the WFH recommendation to provide prophylaxis to all patients with severe or moderate haemophilia before intramuscular injection.
Limitations of our study include the smaller size of subgroups in part B. Therefore, the confidence intervals for the bleeding risk in patients receiving vaccination without prophylaxis are quite large, although certainly higher than for patients on prophylaxis. The latter was reinforced by logistic regression that identified lack of prophylaxis as the predominant risk factor for bleeding. Another limitation of our study is the small number of children due to the licensing status of COVID‐19 vaccines in 2021. Risks and benefits of COVID‐19 vaccination need to be carefully discussed with caregivers in the light of the developing pandemic. Another limitation that should not be ignored in a global context is that an approach of providing factor prophylaxis before vaccination may not be feasible in all communities and for all patients depending on their health insurance status. A factor was used in 94 out of 404 (23%) first and second vaccinations, either in on‐demand patients or in addition to routine prophylaxis. This can be summed up, at an average dose of 36 IU/kg and a body weight of 82 kg in our cohort, to a total consumption of 280,000 IU of factor.
5. CONCLUSION
This study provides evidence on the safety of intramuscular vaccination in persons with haemophilia. Until vaccines become available that have proven safety and efficacy when given subcutaneously, physicians should follow the WFH recommendation on intramuscular injection that is supported by our data.
DISCLOSURES
Andreas Tiede reports grants for studies and research from Bayer, Biotest, Chugai/Roche, Novo Nordisk, Octapharma, Pfizer, and Takeda outside the submitted work, and personal fees for lectures or consultancy from Bayer, Biotest, Chugai/Roche, CSL Behring, Novo Nordisk, Octapharma, Pfizer, SOBI, and Takeda outside the submitted work. H.L. has nothing to disclose. Silvia Horneff reports personal fees for lectures from Chugai/Roche, SOBI, Octapharma, Takeda, Alexion Pharma, Novartis Pharma and Pfizer; travel coasts/congress participation from NovoNordisk, Octapharma, Takeda, Bayer and CSL Behring. Johannes Oldenburg reports grants for studies and research from Bayer, Biotest, CSL‐Behring, Octapharma, Pfizer, SOBI, Takeda outside the submitted work, and personal fees for lectures or consultancy from Bayer, Biogen Idec, Biomarin, Biotest, CSL‐Behring, Chugai, Freeline, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sparks, Swedish Orphan Biovitrum, Takeda outside the submitted work. Susan Halimeh reports grants for studies and research from Bayer Healthcare GmbH, Biotest AG, CSL Behring GmbH, Novo Nordisk Pharma GmbH, Octapharma GmbH, Pfizer Pharma GmbH, Swedish Orphan Biovitrum GmbH, and Takeda, and personal fees for lectures or consultancy Bayer Healthcare GmbH, Biotest AG, CSL Behring GmbH, Chugai Pharma GmbH, Novo Nordisk Pharma, GmbH, Octapharma GmbH, Pfizer Pharma, Roche Pharma AG, and Swedish Orphan Biovitrum GmbH. Christine Heller reports personal fees for lectures or advisory board fees from Biotest, Sobi, Chugai/Roche, Bayer, Novo Nordisk, Takeda, and Pfizer. Christoph Königs reports institutional research support (incl. GEPHARD cohort) from Bayer, Biotest, CSL Behring, Intersero, Novo Nordisk, Pfizer, Takeda, Sobi/Sanofi, EU H2020 ITN, and personal fees for lectures or consultancy from Bayer, Biotest, BFSH, CSL Behring, MSD, Novo Nordisk, Roche/Chugai, Sobi/Sanofi, Takeda outside the submitted work. Katharina Holstein reports grants for studies and research from Bayer, Chugai/Roche, CSL Behring, Pfizer and Sobi outside the submitted work, and personal fees for lectures or consultancy from Bayer, Biotest, Chugai/Roche, CSL Behring, Novo Nordisk, Pfizer, Sobi, and Takeda outside the submitted work. Christian Pfrepper reports grants for studies and research from Chugai/Roche, LeoPharma, Zacros, and Takeda outside the submitted work, and personal fees for lectures or consultancy from Bayer, Chugai/Roche, CSL Behring, Novo Nordisk, Pfizer, BMS, SOBI, and Takeda outside the submitted work.
AUTHOR CONTRIBUTIONS
All authors contributed to study design, enroled patients, and analysed data. AT was responsible for study design and oversight, performed statistical analysis, and wrote the paper. All authors critically revised the manuscript and agreed to its final content.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
Data collection was supported by an educational grant from Ninox GmbH. Otherwise, no funding was obtained for this study.
Open Access funding enabled and organized by Projekt DEAL.
Tiede A, Leise H, Horneff S, et al. Safety of intramuscular COVID‐19 vaccination in patients with haemophilia. Haemophilia. 2022;1‐7. 10.1111/hae.14586
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Santagostino E, Riva A, Cesaro S, et al. Consensus statements on vaccination in patients with haemophilia‐Results from the Italian haemophilia and vaccinations (HEVA) project. Haemophilia. 2019;25(4):656‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De la Corte‐Rodriguez H, Rodriguez‐Merchan EC, Alvarez‐Roman MT, Martin‐Salces M, Jimenez‐Yuste V. Do not do' recommendations in hemophilia. Cardiovasc Hematol Disord Drug Targets. 2020;20(3):168‐174. [DOI] [PubMed] [Google Scholar]
- 3. Pfrepper C, Krause M, Sigl‐Kraetzig M, Konigs C, Wendisch J, Olivieri M. Vaccination in patients with haemophilia‐Results from an online survey among haemophilia treatment centres in Germany. Haemophilia. 2019;25(4):e304‐e306. [DOI] [PubMed] [Google Scholar]
- 4. Sorensen B, Benson GM, Bladen M, et al. Management of muscle haematomas in patients with severe haemophilia in an evidence‐poor world. Haemophilia. 2012;18(4):598‐606. [DOI] [PubMed] [Google Scholar]
- 5. Jones P. Intramuscular injection and coagulation defects. Br Med J. 1972;2(5816):770‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atkins RM, Henderson NJ, Duthie RB. Joint contractures in the hemophilias. Clin Orthop Relat Res. 1987(219):97‐106. [PubMed] [Google Scholar]
- 7. Rodriguez‐Merchan EC, De la Corte‐Rodriguez H. Side effects and potential risk factors of botulinum toxin type A intramuscular injections in knee flexion contractures of hemophiliacs. Expert Rev Hematol. 2017;10(7):587‐594. [DOI] [PubMed] [Google Scholar]
- 8. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1‐158. [DOI] [PubMed] [Google Scholar]
- 9. Mancuso ME, Mahlangu JN, Pipe SW. The changing treatment landscape in haemophilia: from standard half‐life clotting factor concentrates to gene editing. Lancet. 2021;397(10274):630‐640. [DOI] [PubMed] [Google Scholar]
- 10. Coppola A, Tagliaferri A, Rivolta GF, Quintavalle G, Franchini M. Confronting COVID‐19: issues in hemophilia and congenital bleeding disorders. Semin Thromb Hemost. 2020;46(7):819‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pipe SW, Kaczmarek R, Srivastava A, et al. Management of COVID‐19‐associated coagulopathy in persons with haemophilia. Haemophilia. 2021;27(1):41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaczmarek R, El Ekiaby M, Hart DP, et al. Vaccination against COVID‐19: rationale, modalities and precautions for patients with haemophilia and other inherited bleeding disorders. Haemophilia. 2021;27(4):515‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfrepper C, Holstein K, Konigs C, et al. Consensus recommendations for intramuscular COVID‐19 vaccination in patients with hemophilia. Hamostaseologie. 2021;41(3):190‐196. [DOI] [PubMed] [Google Scholar]
- 14. Konsensusempfehlungen zur SARS‐CoV‐2 Impfung bei Patienten mit Hämophilie. 2022. Accessed February 15, 2022. https://gth‐online.org/wp‐content/uploads/2021/07/GTH_Konsensempfehlungen_zur_SARS‐CoV‐2_Impfung.pdf
- 15. Impfen bei Blutungsneigung. 2022. Accessed February 15, 2022. https://www.rki.de/DE/Content/Infekt/Impfen/Stichwortliste/G/Injektionsort_Tabelle.pdf?__blob=publicationFile
- 16. Duda H, Hesse J, Haschberger B, Hilger A, Keipert C. The German hemophilia registry: growing with its tasks. J Clin Med. 2020;9(11):3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tiede A, Bonanad S, Santamaria A, et al. Quality of electronic treatment records and adherence to prophylaxis in haemophilia and von Willebrand disease: systematic assessments from an electronic diary. Haemophilia. 2020;26(6):999‐1008. [DOI] [PubMed] [Google Scholar]
- 18. Mahn R, Schilling K, Klamroth R, et al. Development of haemophilia treatment in the eastern part of germany over the last decade in the Kompetenznetz Hamorrhagische Diathese Ost (KHDO). Hamostaseologie. 2020;40(1):119‐127. [DOI] [PubMed] [Google Scholar]
- 19. Ragni MV, Lusher JM, Koerper MA, Manco‐Johnson M, Krause DS. Safety and immunogenicity of subcutaneous hepatitis A vaccine in children with haemophilia. Haemophilia. 2000;6(2):98‐103. [DOI] [PubMed] [Google Scholar]
- 20. Carpenter SL, Soucie JM, Presley RJ, et al. Hepatitis B vaccination is effective by subcutaneous route in children with bleeding disorders: a universal data collection database analysis. Haemophilia. 2015;21(1):e39‐e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaefer BA, Gruppo RA, Mullins ES, Tarango C. Subcutaneous diphtheria and tetanus vaccines in children with haemophilia: a pilot study and review of the literature. Haemophilia. 2017;23(6):904‐909. [DOI] [PubMed] [Google Scholar]
- 22. Nakasone M, Lopes MH, Sartori AMC, et al. Immunogenicity, long term protection and safety of subcutaneous administration of hepatitis A vaccine in patients with hemophilia and other bleeding disorders: a randomized study. Vaccine. 2020;38(26):4162‐4166. [DOI] [PubMed] [Google Scholar]
- 23. Karer M, Stiasny K, Zeitlinger M, Jilma B. Subcutaneous injection of mRNA vaccines against severe acute respiratory syndrome coronavirus 2: an option for severe bleeding disorders or anticoagulated patients?. Blood Coagul Fibrinolysis. 2021;32(6):423‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cook IF. Subcutaneous vaccine administration – an outmoded practice. Hum Vaccin Immunother. 2021;17(5):1329‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng JY. Inadvertent subcutaneous injection of COVID‐19 vaccine. Postgrad Med J. 2021;97(1148):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gyldenlove M, Skov L, Hansen CB, Garred P. Recurrent injection‐site reactions after incorrect subcutaneous administration of a COVID‐19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(9):e545‐e546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heitmann JS, Bilich T, Tandler C, et al. A COVID‐19 peptide vaccine for the induction of SARS‐CoV‐2 T cell immunity. Nature. 2022;601(7894):617‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peck RC, Clark A, Shapiro S. Experience of Covid 19 vaccination in patients with bleeding disorders. Haemophilia. 2022;28(1):e9‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hochart A, Falaise C, Huguenin Y, Meunier S. Intramuscular vaccination of haemophiliacs: is it really a risk for bleeding?. Haemophilia. 2019;25(5):e322‐e323. [DOI] [PubMed] [Google Scholar]
- 30. Lee JS, Chieng CH, Martin M, Toh TH. Spontaneous neonatal scrotal haematoma: an early manifestation of severe haemophilia. BMJ Case Rep. 2021;14(4):e241482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


