Abstract
It is unclear if there is an association between COVID‐19 and cryptococcosis. Therefore, this study aimed to describe the clinical features, risk factors, and outcomes associated with cryptococcosis in hospitalised patients with COVID‐19. The objectives of this study were to determine the incidence of and examine factors associated with cryptococcosis after a diagnosis of COVID‐19. We used TriNetX to identify and sort patients 18 years and older hospitalised with COVID‐19 into two cohorts based on the presence or absence of a diagnosis of cryptococcosis following diagnosis of COVID‐19. Outcomes of interest included the incidence of cryptococcosis following the diagnosis of COVID‐19 as well as the proportion of patients in each group who had underlying comorbidities, received immunomodulatory therapy, required ICU admission or mechanical ventilation (MV), or died. Propensity score matching was used to adjust for confounding. Among 212,479 hospitalised patients with COVID‐19, 65 developed cryptococcosis. The incidence of cryptococcosis following COVID‐19 was 0.022%. Patients with cryptococcosis were more likely to be male and have underlying comorbidities. Among cases, 32% were people with HIV. Patients with cryptococcosis were more likely to have received tocilizumab (p < .0001) or baricitinib (p < .0001), but not dexamethasone (p = .0840). ICU admission (38% vs 29%), MV (23% vs 11%), and mortality (36% vs 14%) were significantly higher among patients with cryptococcosis. Mortality remained elevated after adjusted propensity score matching. Cryptococcosis occurred most often in hospitalised patients with COVID‐19 who had traditional risk factors, comparable to findings in patients without COVID‐19. Cryptococcosis was associated with increased ICU admission, MV, and mortality.
Keywords: COVID‐19, cryptococcus, cytokine release syndrome, immunotherapy, SARS‐CoV‐2
1. INTRODUCTION
The dysregulated immune response in patients with COVID‐19 is multifactorial due to comorbidities, cytokine dysregulation, impaired cell‐mediated immunity, and receipt of immunomodulatory therapies. 1 , 2 , 3 As a result, there is an increased risk for reactivation of previously latent diseases or development of secondary opportunistic infections. Invasive fungal infections (IFIs) have been reported in patients with COVID‐19, especially those with neutropenia, lymphopenia, comorbidities, as well as treatment with antibacterial or immunomodulatory therapies, such as corticosteroids, interleukin (IL)‐1 and IL‐6 inhibitors, or Janus kinase (JAK) inhibitors. 4 , 5 , 6 , 7 , 8
Invasive pulmonary aspergillosis has been extensively reported in patients with COVID‐19. The incidence of coronavirus disease‐associated pulmonary aspergillosis (CAPA) ranges from 2% in one post‐mortem study 9 to 30% in patients on mechanical ventilation (MV) or in the intensive care unit (ICU). 10 , 11 A recent meta‐analysis found a pooled prevalence of 10% in ICU patients. 12 Risk factors for CAPA include advanced age, chronic pulmonary disease, ICU admission, MV, and treatment with antibacterial therapy, corticosteroids, or IL‐6 inhibitors. 5 , 6 , 13 However, differences in study designs and challenges in diagnosing and defining CAPA, especially in early case reports and series, limit these findings.
Cases of COVID‐19 associated mucormycosis (CAM) have also been reported, 4 but to a lesser extent than CAPA. Though the highest number of cases have emerged in literature from India, 14 , 15 CAM has been reported in patients across the world. 4 , 15 , 16 , 17 Candidiasis 18 , 19 and pneumocystosis 20 in patients with COVID‐19 have also been documented, and are often identified in patients with other established risk factors.
Despite the ubiquitous presence of Cryptococcus spp. coupled with the immunomodulatory therapies used for COVID‐19 and impaired immunologic response (lymphopenia and a paucity of peripheral T cells) associated with SARS‐CoV‐2, 2 , 3 few cases of cryptococcosis in patients with COVID‐19 have been reported compared to the above IFIs. 8 , 21 However, it is unclear if cryptococcosis represents a superinfection in these cases and if there is an association between COVID‐19 and cryptococcosis. The epidemiology, natural history, and characteristics of patients who develop cryptococcosis following COVID‐19 remain unknown and likely underrecognised. Therefore, the purpose of this study was to describe the clinical features, risk factors, and outcomes associated with cryptococcosis in patients hospitalised with COVID‐19.
2. METHODS
The objectives were to determine the incidence of and examine factors associated with cryptococcosis after a diagnosis of COVID‐19.
2.1. Study design and population
We used TriNetX a global federated research network that captures anonymous data from electronic medical records (EMRs) of 57 healthcare organisations (Appendix S1). Available data include demographic characteristics, diagnoses, procedures, medications, and measurements (e.g., laboratory test results).
Patients 18 years and older hospitalised with COVID‐19 were identified from TriNetX between February 19, 2020, and April 17, 2022. Diagnosis of COVID‐19 was defined by logical observation identifiers names and codes (LOINCs) for SARS‐CoV‐2 or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) diagnosis codes of “COVID‐19” or “pneumonia due to COVID‐19” (Appendix S1), whereas Current Procedural Terminology (CPT) codes were used to determine hospitalisation (Table S1).
The patient population was divided into two cohorts based on the presence or absence of a diagnosis of cryptococcosis following diagnosis of COVID‐19. Patients diagnosed with cryptococcosis within 3 months of the most recent COVID‐19 diagnosis (cases) were identified by ICD‐10‐CM diagnosis code for cryptococcosis, pulmonary cryptococcosis, cerebral cryptococcosis, cutaneous cryptococcosis, osseous cryptococcosis, disseminated cryptococcosis, other forms of cryptococcosis, or unspecified cryptococcosis (Table S2). 22 , 23 The earliest encounter for cryptococcosis was identified as the index encounter in patients with multiple encounters. Cases were also stratified by HIV status. Controls were defined as patients diagnosed and hospitalised with COVID‐19 but without a diagnosis of cryptococcosis (Appendix S1).
Demographics, underlying comorbidities, medications, laboratory data, and outcomes were examined. Demographic information included age at index event (years), sex, race, and ethnicity, as entered into TriNetX from EMRs. ICD‐10‐CM diagnosis codes were used to identify underlying comorbidities during the 30 days prior to COVID‐19 diagnosis and included HIV infection, immunodeficiency with predominantly antibody defects, combined immunodeficiencies, common variable immunodeficiency, other immunodeficiencies, malnutrition, type 2 diabetes mellitus (DM2), heart failure, hepatic fibrosis and cirrhosis, sarcoidosis, systemic connective tissue disorders, rheumatoid arthritis, non‐infective enteritis and colitis, chronic kidney disease (CKD), neoplasms, and transplanted organs or tissues (Table S3). Laboratory data, defined as the most recent labs between the index event and 30 days before, included leukocytes, lymphocytes, CD4 cell counts, haemoglobin A1c, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, albumin, serum creatinine, ferritin, C‐reactive protein (CRP), and lactate dehydrogenase (LDH) (Table S4). Types of cryptococcosis were based on ICD‐10‐CM diagnosis codes and included pulmonary cryptococcosis, cutaneous cryptococcosis, cerebral cryptococcosis, osseous cryptococcosis, disseminated cryptococcosis, other forms of cryptococcosis, and unspecified cryptococcosis (Table S1). Medication use was characterised by receipt of immunomodulatory therapies as part of the management of COVID‐19, including dexamethasone, tocilizumab, and baricitinib (Table S5). Outcomes determined by CPT codes included ICU admission, receipt of MV, and death (Table S1).
2.2. Outcome measures
The primary outcome was the incidence of cryptococcosis following the diagnosis of COVID‐19 among hospitalised patients. The secondary outcomes included the proportion of patients in each group who had underlying comorbidities, received immunomodulatory therapy, required ICU admission or MV, or died.
2.3. Statistical analysis
Statistical analyses were completed on the TriNetX platform. Descriptive statistics were presented as means and standard deviations for continuous variables, and as frequency and proportions for categorical variables. Continuous data were compared using independent t‐tests, whereas categorical data were compared using χ2 or Fisher's exact test, as appropriate. Outcome analysis was reported before and after propensity score matching. Propensity score matching was performed to control for differences between groups based on age, male sex, Hispanic or Latino ethnicity, HIV, transplant, or neoplasm using a 1:1 Greedy nearest‐neighbour algorithm. These variables were selected because they are established risk factors for cryptococcosis and associated with increased Cryptococcus spp.‐related mortality. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) for receipt of immunomodulatory therapies, ICU admission, MV, and mortality, with p < .05 as the cut off for statistical significance.
The incidence and prevalence of cryptococcosis among all hospitalised patients with COVID‐19 and those requiring ICU admission was analysed for the timeframe between February 19, 2020, and April 17, 2022.
2.4. Ethics statement
Research utilising TriNetX does not require ethical approval because patient‐identifiable information is not accessible to users. The authors have adhered to that the ethical policies of the journal, as noted on the journal's author guidelines page.
3. RESULTS
3.1. Clinical features of hospitalised patients with COVID‐19 complicated with cryptococcosis
A total of 212,479 hospitalised patients with COVID‐19 were included of which 65 patients were diagnosed with cryptococcosis. Of those with cryptococcosis, 88% (n = 57), 40% (n = 26), 28% (n = 18), and 23% (n = 15) had ICD‐10‐CM diagnosis codes for cryptococcosis, cerebral cryptococcosis, pulmonary cryptococcosis, and disseminated cryptococcosis, respectively. Fifteen percent (n = 10) had cutaneous cryptococcosis, whereas 42% (n = 27) and 15% (n = 10) had unspecified and other forms cryptococcosis, respectively, based on ICD‐10‐CM diagnosis codes.
Demographic characteristics were similar between groups, but patients with cryptococcosis were more likely to be male than those without cryptococcosis (Table 1). More patients with cryptococcosis had underlying comorbidities than those without. Immunodeficiencies, such as HIV and transplanted organs or tissues, were more frequent in patients with cryptococcosis (Figure 1). Additionally, a higher proportion of patients with cryptococcosis had autoimmune diseases, DM2, heart failure, hepatic fibrosis and cirrhosis, CKD, and malnutrition.
TABLE 1.
Comparison of baseline characteristics between hospitalised patients diagnosed with cryptococcosis within 3 months of the most recent COVID‐19 diagnosis and hospitalised patients without a diagnosis of cryptococcosis within 3 months of COVID‐19 diagnosis
| Variable | Cryptococcosis following COVID‐19 (n = 65) | COVID‐19 without cryptococcosis (n = 212,414) | p value |
|---|---|---|---|
| Age at index event (years), mean (SD) | 56.8 (14.5) | 56 (22.4) | .7692 |
| Male sex | 52 (80) | 108,802 (51) | <.0001 |
| BMI (kg/m2), mean (SD) | 26.8 (6.93) | 29.3 (7.69) | 1036 |
| Race | |||
| White | 41 (63) | 144,234 (68) | .4047 |
| Black or African American | 17 (26) | 41,868 (20) | .1917 |
| Asian | 0 (0) | 410,916 (2) | .2571 |
| Unknown race | 10 (15) | 20,982 (10) | .1368 |
| Ethnicity | |||
| Hispanic or Latino | 14 (22) | 29,260 (14) | .0694 |
| Non‐Hispanic | 48 (74) | 161,555 (76) | .6763 |
| Underlying comorbidities | |||
| HIV | 21 (32) | 3461 (2) | <.0001 |
| Transplanted organs or tissues | 18 (28) | 5659 (3) | <.0001 |
| Neoplasm | 17 (26) | 46,629 (22) | .4132 |
| Immunodeficiency with predominantly antibody defects | 10 (15) | 589 (<1) | <.0001 |
| Combined immunodeficiencies | 0 (0) | 67 (<1) | .8861 |
| Common variable immunodeficiency | 0 (0) | 137 (<1) | .8377 |
| Other immunodeficiencies | 21 (32) | 5592 (3) | <.0001 |
| Sarcoidosis | 10 (15) | 981 (<1) | <.0001 |
| Systemic connective tissue disorders | 10 (15) | 8378 (4) | <.0001 |
| Rheumatoid arthritis | 10 (15) | 596 (<1) | <.0001 |
| Non‐infective enteritis and colitis | 10 (15) | 10,007 (5) | <.0001 |
| Hepatic fibrosis and cirrhosis | 10 (15) | 5351 (3) | <.0001 |
| Type 2 diabetes mellitus | 27 (42) | 64,126 (30) | .0463 |
| Heart failure | 18 (28) | 36,674 (17) | .0262 |
| Malnutrition | 17 (26) | 11,436 (5) | <.0001 |
| Chronic kidney disease | 26 (40) | 51,074 (24) | .0026 |
| Laboratory values | |||
| Leukocytes (K/μl), mean (SD) | 9.18 (6.9) | 9.11 (21.8) | .9818 |
| Lymphocytes (K/μl), mean (SD) | 1.97 (4.31) | 3.68 (10.5) | .3472 |
| CD4 cells (cells/μl), mean (SD) | 73 (68.9) | 299 (316) | .0242 |
| AST (units/L), mean (SD) | 80.3 (235) | 58.2 (256) | .5582 |
| ALT (units/L), mean (SD) | 85.1 (393) | 45.4 (157) | .0769 |
| Alkaline phosphatase (units/L), mean (SD) | 139 (141) | 101 (101) | .0097 |
| Serum creatinine (mg/dl) | 1.61 (1.37) | 1.36 (1.79) | .2923 |
| Albumin (mg/dl), mean (SD) | 3.02 (0.767) | 3.46 (0.665) | <.0001 |
| Haemoglobin A1C (%), mean (SD) | 7.34 (2.46) | 7.29 (2.36) | 0.9404 |
| Ferritin (ng/ml), mean (SD) | 5253 (17950) | 941 (2344) | <.0001 |
| C‐reactive protein (mg/dl), mean (SD) | 81.7 (84.3) | 79.4 (82.6) | .8972 |
| Lactate dehydrogenase (units/L) | 1006 (1798) | 433 (501) | <.0001 |
Note: Data are presented as n (%) unless otherwise noted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; COVID‐19, coronavirus disease 2019; SD, standard deviation.
FIGURE 1.
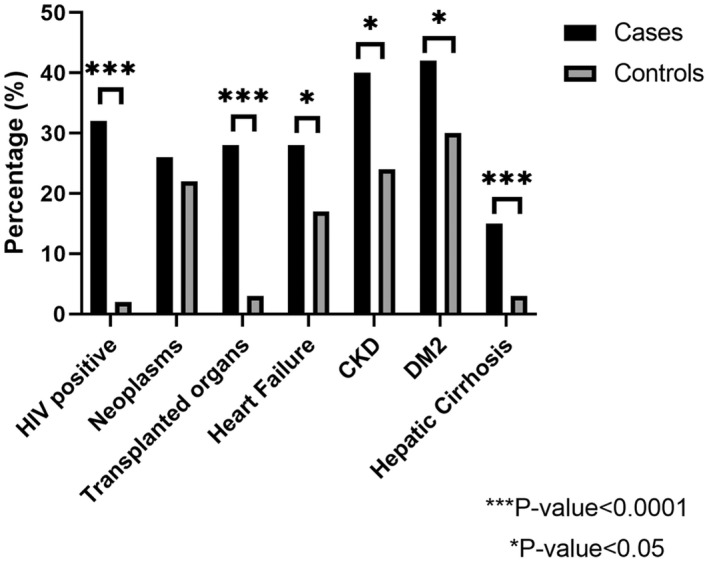
Distribution of underlying comorbidities among hospitalised patients with COVID‐19 stratified based on a diagnosis of cryptococcosis. The figure compares the percentage of HIV infection, neoplasms, transplanted organs or tissues, heart failure, chronic kidney disease (CKD), type 2 diabetes mellitus (DM2), and hepatic fibrosis and cirrhosis among hospitalised patients based on the presence (cases) or absence (control) of a diagnosis of cryptococcosis following diagnosis of COVID‐19. The asterisks denotes a statistically significant difference between groups
Mean leukocyte and lymphocyte counts were similar between groups. However, patients with cryptococcosis had significantly lower CD4 cell counts. Patients with cryptococcosis also had higher ALT, alkaline phosphatase, ferritin, and LDH, but lower albumin.
A similar proportion of patients from each group received dexamethasone (p = .0848), whereas tocilizumab (p < .0001) and baricitinib (p < .0001) were administered to more patients with cryptococcosis (Figure 2). Compared to patients without cryptococcosis, those with cryptococcosis were significantly more likely to have received tocilizumab (OR 18.6, 95% CI 9.5–36.3, p < .0001) or baricitinib (OR 12.4, 95% CI 6.4–24.1, p < .0001), but not dexamethasone (OR 0.7, 95% CI 0.4–1.2, p = .222).
FIGURE 2.
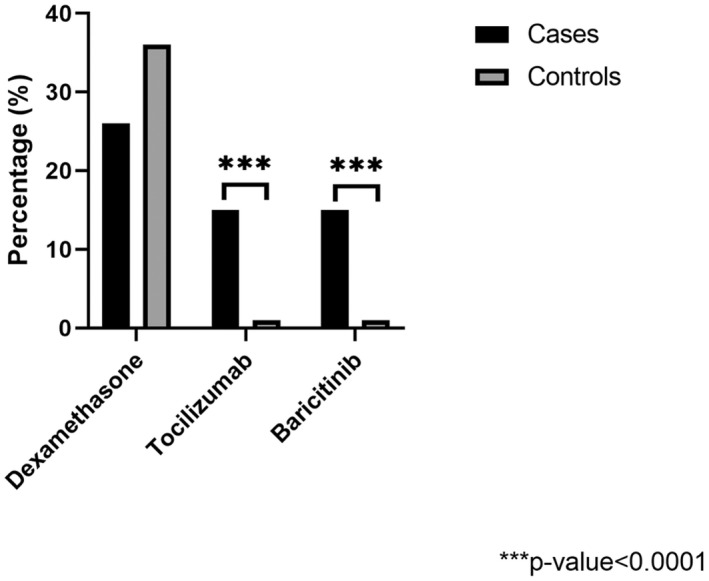
Percentage of hospitalised patients with COVID‐19 who received immunomodulatory therapies stratified based on a diagnosis of cryptococcosis. The figure compares the percentage of hospitalised patients with COVID‐19 with cryptococcosis (cases) to those without cryptococcosis (controls) who received dexamethasone, tocilizumab, or baricitinib. The asterisks denotes a statistically significant difference between groups
3.2. Clinical features of people with HIV hospitalised with COVID‐19 complicated with cryptococcosis
Hospitalised people with HIV (PWH) who developed cryptococcosis were more likely to be male (88% vs 48%, p < .0001) and Black or African American (42% vs 21%, p = .0154) compared to hospitalised PWH without cryptococcosis. Additionally, cases with HIV were less likely to be White (42% vs 71%, p = .0154) and non‐Hispanic (71% vs 91%, p = .0008). Systemic connective tissue disorders (42% vs 14%, p = .0001) and hepatic fibrosis and cirrhosis (42% vs 7%, p < .0001) were more common in cases with HIV, while neoplasms (42% vs 64%, p = .0235) were less common. Leukocyte and lymphocyte counts were similar (7.02 ± 4.24 cells/μl vs 9.46 ± 21.8 cells/μl, p = .6255 and 3.09 ± 5.99 cells/μl vs 5.97 ± 12.6 cells/μl, p = .3591, respectively), though CD4 cell counts (73 ± 68.9 cells/μl vs 295 ± 296 cells/μl, p = .0188) were significantly lower among those who developed cryptococcosis. Cerebral cryptococcosis was the most common reported form (63%) among cases. Significantly more hospitalised PWH with COVID‐19 who developed cryptococcosis received dexamethasone (42% vs 18%, p = .0027), while use of tocilizumab and baricitinib were infrequent among both groups (0% vs 1%, p = .5605 and 0% vs <1%, p = .8233, respectively). Hospitalised PWH with COVID‐19 who developed cryptococcosis were significantly more likely to be admitted to the ICU (50% vs 19%, p < .0001), require MV (42% vs 6%, p < .0001), and die (42% vs 18%, p = .0030) compared to those with HIV who did not develop cryptococcosis.
3.3. Outcome analysis
Unmatched analysis showed a significantly higher proportion of patients who developed cryptococcosis following COVID‐19 required MV than patients without cryptococcosis (Table 2). In addition, mortality was significantly higher in patients with cryptococcosis (Figure 3). In the propensity score matching analysis adjusted by age, male sex, Hispanic or Latino ethnicity, HIV status, transplant, and neoplasm; patients with cryptococcosis remained at significantly higher odds of death compared to patients without cryptococcosis.
TABLE 2.
Differences in outcomes between hospitalised patients diagnosed with cryptococcosis within 3 months of the most recent COVID‐19 diagnosis and hospitalised patients without a diagnosis of cryptococcosis within 3 months of COVID‐19 diagnosis
| Before matching | After matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Cryptococcosis following COVID‐19 (n = 64) a | COVID‐19 without cryptococcosis (n = 212,414) | OR (95% CI) | p value | Cryptococcosis following COVID‐19 (n = 63) | COVID‐19 without cryptococcosis (n = 63) | OR (95% CI) | p value |
| ICU admission | 24 (38) | 60,645 (29) | 1.5 (0.9–2.5) | .1129 | 24 (38) | 24 (38) | 1 (0.5–2.1) | 1 |
| Mechanical ventilation | 15 (23) | 23,888 (11) | 2.4 (1.4–4.3) | .0020 | 15 (24) | 12 (19) | 1.3 (0.6–3.1) | .5148 |
| Deceased | 23 (36) | 29,747 (14) | 3.4 (2.1–5.7) | <.0001 | 23 (37) | 10 (16) | 3.0 (1.3–7.1) | .0084 |
Note: Data are presented as n (%) unless otherwise noted.
Abbreviations: COVID‐19, coronavirus disease 2019; CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Outcome data of interest were unavailable for one patient.
FIGURE 3.
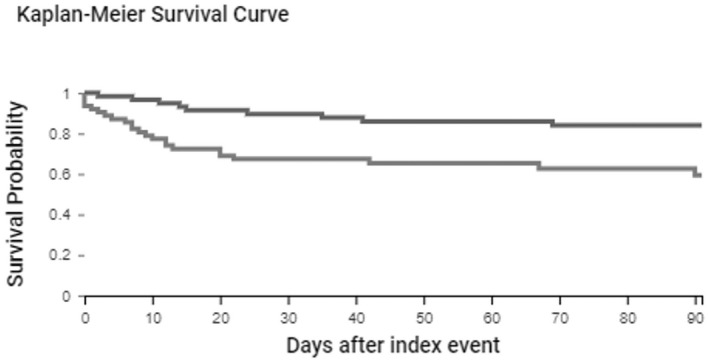
Kaplan–Meier survival analysis of hospitalised patients with COVID‐19 stratified based on a diagnosis of cryptococcosisThe figure displays the survival probability of hospitalised patients diagnosed with cryptococcosis within 3 months of the most recent COVID‐19 diagnosis (light grey line) and hospitalised patients without a diagnosis of cryptococcosis within 3 months of COVID‐19 diagnosis (dark grey line)
3.4. Frequency analysis
Of 212,479 hospitalised patients with COVID‐19 available in the TriNetX system, the incidence of cryptococcosis was 0.022%, while the prevalence was 0.059%. Most episodes of cryptococcosis occurred within 10 days after COVID‐19 diagnosis (Figure 4). The incidence and prevalence were higher among males (0.034% and 0.087%, respectively). Hispanic or Latino patients had a higher incidence (0.029%) and prevalence (0.063%) compared to non‐Hispanic patients (0.022% and 0.061%, respectively). Risk of cryptococcosis remained higher among Hispanic or Latino patients than non‐Hispanic patients after adjusting for age, gender, and HIV status.
FIGURE 4.
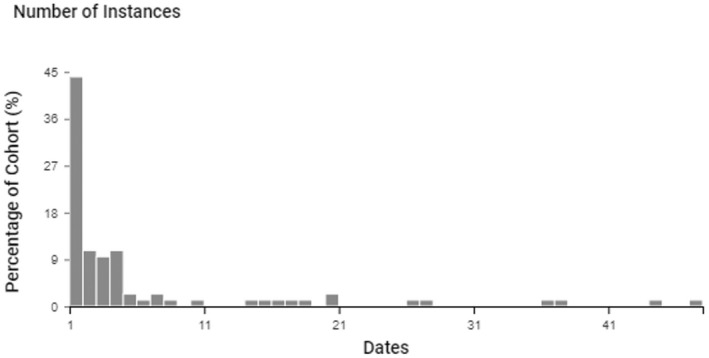
Instances of cryptococcosis following COVID‐19 among hospitalised patients. The figure displays the number of instances of cryptococcosis among hospitalised patients with COVID‐19 represented as a percentage of the cohort stratified by the number of days (dates) from COVID‐19 diagnosis to diagnosis of cryptococcosis
Similarly, the prevalence was 0.043% among patients 60–64 years and 0.035% among those 70–74 years. The risk of cryptococcosis was 0.06% among 64,607 patients with COVID‐19 requiring ICU admission, with a prevalence of 0.123%. In addition, the risk of cryptococcosis increased to 0.618% among COVID‐19 patients requiring ICU admission who were 30–34 years and to 0.16% among Hispanic or Latino patients with COVID‐19 requiring ICU admission.
4. DISCUSSION
We evaluated the epidemiology and characteristics of hospitalised patients diagnosed with cryptococcosis within 3 months of the most recent COVID‐19 diagnosis from a multicentre research network. The overall incidence of cryptococcosis was 0.022% among 212,479 hospitalised patients with COVID‐19. Notably, the distribution of cryptococcosis cases in our study was similar to that reported in patients without COVID‐19 with a higher prevalence of immunodeficiencies in patients diagnosed with cryptococcosis. Multiple IFIs, including CAPA, CAM, and candidiasis, are becoming increasingly recognised as complications in patients with COVID‐19. 4 , 9 , 10 , 11 , 14 , 15 , 16 , 17 , 18 , 19 While an association between COVID‐19 and cryptococcosis is unclear, the low incidence of cryptococcosis in patients with COVID‐19 may reflect lack of recognition and underdiagnosis in this population.
In our study, cryptococcosis occurred significantly more often in males with underlying comorbidities or immunodeficiencies, of which 32% had HIV, and 28% were transplant recipients. These findings support broader literature suggesting non‐HIV non transplant patients are an emerging group at risk for cryptococcosis and increasing recognition of DM2, CKD, neoplasms, autoimmune diseases, and other immunodeficiencies as risk factors for cryptococcosis. 23 , 24 Among previous cases of cryptococcosis in patients with COVID‐19 summarised in a recent case report and literature review, 21 chronic comorbidities, such as hypertension and DM2, were common. One patient was receiving prednisone for autoimmune haemolytic anaemia, 25 one patient with a renal transplant was taking tacrolimus with prednisone, 26 while one patient was newly diagnosed with HIV. 27 However, traditional risk factors associated with cryptococcosis were not detected in 56% of patients in those previous reports. We found higher rates of cryptococcosis in patients with COVID‐19 who had heart failure, DM2, and CKD. CD4 cell counts were significantly lower among patients with COVID‐19 who developed cryptococcosis, suggesting impaired cell‐mediated immunity may contribute to the pathogenesis of this opportunistic IFI.
Receipt of immunomodulatory therapies, many of which are used routinely in the medical management of COVID‐19, 1 could also increase susceptibility to cryptococcosis. Following initial infection, Cryptococcus spp. may persist as a pulmonary granuloma without causing overt clinical symptoms. 28 , 29 Administration of corticosteroids impairs the binding and phagocytic function of alveolar macrophages against Cryptococcus spp., 30 in addition to increasing fungal burden and promoting extrapulmonary dissemination. 28 , 29 Increased Cryptococcus spp. cell proliferation has also been observed following exposure to either dexamethasone or methylprednisolone. 31 Surprisingly, dexamethasone administration in our study was similar among patients with COVID‐19 who developed cryptococcosis and those without cryptococcosis. These findings differ from an analysis of nine previous reports whereby eight patients with COVID‐19 received corticosteroids prior to diagnosis of cryptococcosis. 21
IL‐6 inhibitors have been administered to patients with COVID‐19 to modulate IL‐6 concentrations associated with the dysregulated host immune response 1 despite the potential increased risk of bacterial, fungal, and non‐SARS‐CoV‐2 viral infections. Previous data suggest IL‐6‐deficient mice are more susceptible to Cryptococcus spp. infection, suggesting a potential role for IL‐6 in the host defence against cryptococcosis. 32 Higher IL‐6 concentrations restrict the growth of Cryptococcus spp., while suppression of IL‐6 with IL‐6 inhibitors may allow Cryptococcus spp.to subvert host immune response. 1 , 33 In our study, patients with cryptococcosis were almost 19 times more likely to have received tocilizumab versus those without cryptococcosis, which is consistent with previous reports. 21
Recently, JAK inhibitors have emerged as a potential treatment option in patients with COVID‐19 to inhibit cytokine signalling, thus limiting immune activation and inflammation. 1 However, interferon (INF)‐γ may function in a protective role against cryptococcosis, by way of lymphocyte infiltration and macrophage activation. 34 Post‐influenza cryptococcosis, while uncommon, is associated with lower concentrations of INF‐γ. 35 Cases of pulmonary cryptococcosis have occurred in patients treated with baricitinib, 36 but none had been reported in patients with COVID‐19, until now. In our study, patients with COVID‐19 who developed cryptococcosis were 12 times more likely to have received baricitinib compared to those without cryptococcosis.
Dexamethasone, tocilizumab, and baricitinib have been recommended in select patients with COVID‐19, 1 but widespread, indiscriminate use may lead to impaired host immunity and increased risk of opportunistic infections. Though the mechanism of cryptococcosis in patients infected with SARS‐CoV‐2 remains to be studied further, it has been proposed to be the result of reactivation of latent Cryptococcus spp.infection 28 , 29 , 37 due to (1) SARS‐CoV‐2 associated lymphopenia, 2 , 3 (2) vomocytosis of Cryptococcus spp. from macrophages following SARS‐CoV‐2 infection, 38 or (3) receipt of immunomodulatory therapy. 28 Cryptococcosis following COVID‐19 is likely multifactorial. The presence of epidemiologic risk factors increasing Cryptococcus spp. latency and T‐cell immunity defects—from COVID‐19 severity associated immunodeficiency to use of immunomodulatory agents—likely play a central role.
However, most cases of cryptococcosis occurred within 10 days after the index hospitalisation with COVID‐19 (Figure 4), which may represent unrecognised cryptococcosis before hospitalisation or receipt of immunomodulatory therapy for COVID‐19. While early‐onset reactivation of cryptococcosis is rare, a previous report of a cluster of C. neoformans pulmonary and bloodstream infections found an association between receipt of short‐term corticosteroids in the ICU and increased risk of cryptococcosis. 39 It is unknown whether patients in our study who developed cryptococcosis represent reactivation of latent Cryptococcus spp. infection following receipt of immunomodulatory therapy or acute infection.
Data describing outcomes among patients with COVID‐19 who developed cryptococcosis are limited. Higher rates of ICU admission, MV, and death were observed in patients with cryptococcosis in our study. The significantly increased rate of death among persons with cryptococcosis persisted after propensity score matching variables, which are established risk factors for cryptococcosis and Cryptococcus spp.‐related mortality. Upon review of previous reports, three of nine patients with COVID‐19 died prior to the identification of cryptococcosis. 21 Early suspicion leading to prompt identification and treatment of cryptococcosis is critical. Serum cryptococcal antigen (CrAg) screening could improve detection of asymptomatic infections or early disease in patients with COVID‐19, especially those with risk factors for cryptococcosis or those who clinically deteriorate after receipt of immunomodulatory therapy. However, the sensitivity of serum CrAg is unknown in this population.
Although we described the epidemiology and characteristics of a large number of hospitalised patients with COVID‐19 and cryptococcosis, our study was retrospective and utilised a multicentre research network. In some cases, COVID‐19 may have represented an incidental diagnosis where other well‐established risk factors for cryptococcosis were present. Though all patients in our study were hospitalised, the reason for hospitalisation is unknown (manifestations of COVID‐19 or cryptococcosis). Our data are based on EMR data aggregation, which may be limited by data entry or coding errors. Collection of ICD‐10‐CM diagnosis codes, CPT codes, and LOINCs may be subjected to data inaccuracies due to unrecorded, under coding and/or misclassification of diseases. Additionally, granular details, such as medication dosage, duration, and timeframe prior to diagnosis of cryptococcosis, as well as microbiologic data, could not be assessed. Lastly, laboratory tests were not obtained for all patients due to differences in institutional practices, reflected by the fact that CD4 cell counts were obtained in only 15% of patients who developed cryptococcosis and less than 1% of those without cryptococcosis.
This study is the first to report factors associated with hospitalised patients who developed cryptococcosis following COVID‐19. Cryptococcosis occurred most often in patients with COVID‐19 who also had traditional risk factors, comparable to findings observed in patients without COVID‐19. Clinicians must be aware of traditional and lesser known or recognised risk factors for cryptococcosis among patients with COVID‐19 and have a low threshold to screen for cryptococcosis.
AUTHOR CONTRIBUTIONS
D.B.C. involved in conceptualization, data curation, formal analysis, methodology, visualisation, writing—original draft, writing—review and editing. V.M.K. involved in conceptualization, formal analysis, methodology, visualisation, writing—original draft, writing—review and editing. S.G., B.T.J., L.V.B. and G.R.T. involved in writing—review and editing. C.F.P. involved in conceptualization, writing—review and editing. A.F.H.M. involved in conceptualization, data curation, formal analysis, methodology, visualisation, writing—original draft, writing—review and editing.
CONFLICT OF INTEREST
None.
Supporting information
Appendix S1
Chastain DB, Kung VM, Golpayegany S, et al.. Cryptococcosis among hospitalised patients with COVID‐19: A multicentre research network study. Mycoses. 2022;65:815‐823. doi: 10.1111/myc.13476
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from TriNetX. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from TriNetX through a third‐party agreement option.
REFERENCES
- 1. Chastain DB, Stitt TM, Ly PT, Henao‐Martinez AF, Franco‐Paredes C, Osae SP. Countermeasures to Coronavirus Disease 2019: are immunomodulators rational treatment options‐a critical review of the evidence. Open Forum Infect Dis. 2020;7(7):ofaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992‐1000.e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg D, Muthu V, Sehgal IS, et al. Coronavirus Disease (Covid‐19) Associated Mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID‐19‐associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149‐e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marr KA, Platt A, Tornheim JA, et al. Aspergillosis complicating severe coronavirus disease. Emerg Infect Dis. 2021;27(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez‐Morales AJ, Sah R, Millan‐Oñate J, et al. COVID‐19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs. Ther Adv Infect Dis. 2021;8:20499361211027065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baddley JW, Thompson GR, 3rd , Chen SC, et al. Coronavirus disease 2019‐associated invasive fungal infection Open Forum Infect Dis 2021;8(12):ofab510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kula BE, Clancy CJ, Hong Nguyen M, Schwartz IS. Invasive mould disease in fatal COVID‐19: a systematic review of autopsies. Lancet Microbe 2021;2(8):e405‐e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID‐19: a prospective study. Clin Infect Dis. 2021;73(11):e3606‐e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Grootveld R, van Paassen J, de Boer MGJ, Claas ECJ, Kuijper EJ, van der Beek MT. Systematic screening for COVID‐19 associated invasive aspergillosis in ICU patients by culture and PCR on tracheal aspirate. Mycoses. 2021;64(6):641‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kariyawasam RM, Dingle TC, Kula BE, Vandermeer B, Sligl WI, Schwartz IS. Defining COVID‐19 associated pulmonary aspergillosis: systematic review and meta‐analysis. Clin Microbiol Infect. 2022. doi: 10.1016/j.cmi.2022.01.027. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salmanton‐García J, Sprute R, Stemler J, et al. COVID‐19‐associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis. 2021;27(4):1077‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sen M, Honavar SG, Bansal R, et al. Epidemiology, clinical profile, management, and outcome of COVID‐19‐associated rhino‐orbital‐cerebral mucormycosis in 2826 patients in India–Collaborative OPAI‐IJO Study on Mucormycosis in COVID‐19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69(7):1670‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID‐19 converge: the perfect storm for mucormycosis. J fungi. 2021;7(4):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UKpatients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe. 2020;1(6):e245‐e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med. 2021;42:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nucci M, Barreiros G, Guimarães LF, Deriquehem VAS, Castiñeiras AC, Nouér SA. Increased incidence of candidemia in a tertiary care hospital with the COVID‐19 pandemic. Mycoses. 2021;64(2):152‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mastrangelo A, Germinario BN, Ferrante M, et al. Candidemia in Coronavirus Disease 2019 (COVID‐19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non–COVID‐19 Controls. Clin Infect Dis. 2020;73(9):e2838‐e2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menon AA, Berg DD, Brea EJ, et al. A Case of COVID‐19 and Pneumocystis jirovecii Coinfection. Am J Respir Crit Care Med. 2020;202(1):136‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chastain DB, Henao‐Martínez AF, Dykes AC, et al. Missed opportunities to identify cryptococcosis in COVID‐19 patients: a case report and literature review. Ther Adv Infect Dis. 2022;9:20499361211066363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. George IA, Spec A, Powderly WG, Santos CAQ. Comparative epidemiology and outcomes of human immunodeficiency virus (HIV), non‐HIV non‐transplant, and solid organ transplant associated cryptococcosis: a population‐based study. Clin Infect Dis. 2018;66(4):608‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kashef Hamadani BH, Franco‐Paredes C, McCollister B, Shapiro L, Beckham JD, Henao‐Martínez AF. Cryptococcosis and cryptococcal meningitis: New predictors and clinical outcomes at a United States academic medical centre. Mycoses. 2018;61(5):314‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woldie IL, Brown IG, Nwadiaro NF, et al. Autoimmune hemolytic anemia in a 24‐year‐old patient with COVID‐19 complicated by secondary cryptococcemia and acute necrotizing encephalitis: a case report and review of literature. J Med Cases. 2020;11(11):362‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thota DR, Ray B, Hasan M, Sharma K. Cryptococcal meningoencephalitis during convalescence from severe COVID‐19 pneumonia. Neurohospitalist. 2022;12(1):96‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heller HM, Gonzalez RG, Edlow BL, Ard KL, Gogakos T. Case 40‐2020: A 24‐year‐old man with headache and covid‐19. N Engl J Med. 2020;383(26):2572‐2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldman DL, Lee SC, Mednick AJ, Montella L, Casadevall A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun. 2000;68(2):832‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ristow LC, Davis JM. The granuloma in cryptococcal disease. PLoS Pathog. 2021;17(3):e1009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gross NT, Chinchilla M, Camner P, Jarstrand C. Anticryptococcal activity by alveolar macrophages from rats treated with cortisone acetate during different periods of time. Mycopathologia. 1996;136(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 31. Araújo GRS, Alves V, Martins‐de‐Souza PH, et al. Dexamethasone and methylprednisolone promote cell proliferation, capsule enlargement, and in vivo dissemination of C. neoformans . Frontiers in Fungal Biol. 2021;3:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beenhouwer DO, Shapiro S, Feldmesser M, Casadevall A, Scharff MD. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect Immun. 2001;69(10):6445‐6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siddiqui AA, Shattock RJ, Harrison TS. Role of capsule and interleukin‐6 in long‐term immune control of Cryptococcus neoformans infection by specifically activated human peripheral blood mononuclear cells. Infect Immun. 2006;74(9):5302‐5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL Jr. Protective immunity against pulmonary cryptococcosis is associated with STAT1‐mediated classical macrophage activation. J Immunol. 2012;189(8):4060‐4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliveira LVN, Costa MC, Magalhães TFF, et al. Influenza A virus as a predisposing factor for cryptococcosis. Front Cell Infect Microbiol. 2017;7:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Yu Y, Xie J, Jiang Y, Lu L. Cryptococcal pneumonia in a patient with rheumatoid arthritis treated with baricitinib. Rheumatology. 2021;61(1):e6‐e7. [DOI] [PubMed] [Google Scholar]
- 37. Goldman DL, Khine H, Abadi J, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107(5):E66. [DOI] [PubMed] [Google Scholar]
- 38. Seoane PI, Taylor‐Smith LM, Stirling D, et al. Viral infection triggers interferon‐induced expulsion of live Cryptococcus neoformans by macrophages. PLoS Pathog. 2020;16(2):e1008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vallabhaneni S, Haselow D, Lloyd S, et al. Cluster of Cryptococcus neoformans Infections in Intensive Care Unit, Arkansas, USA, 2013. Emerg Infect Dis. 2015;21(10):1719‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from TriNetX. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from TriNetX through a third‐party agreement option.


