Abstract
The severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection is linked with inflammatory disorders and the development of oxidative stress in extreme cases. Therefore, anti‐inflammatory and antioxidant drugs may alleviate these complications. Ginkgo biloba L. folium extract (EGb) is a herbal medicine containing various active constituents. This review aims to provide a critical discussion on the potential role of EGb in the management of coronavirus disease 2019 (COVID‐19). The antiviral effect of EGb is mediated by different mechanisms, including blocking SARS‐CoV‐2 3‐chymotrypsin‐like protease that provides trans‐variant effectiveness. Moreover, EGb impedes the development of pulmonary inflammatory disorders through the diminution of neutrophil elastase activity, the release of proinflammatory cytokines, platelet aggregation, and thrombosis. Thus, EGb can attenuate the acute lung injury and acute respiratory distress syndrome in COVID‐19. In conclusion, EGb offers the potential of being used as adjuvant antiviral and symptomatic therapy. Nanosystems enabling targeted delivery, personalization, and booster of effects provide the opportunity for the use of EGb in modern phytotherapy.
Keywords: complementary medicine, Ginkgo biloba, nanomedicines, SARS‐CoV‐2, viral proteases
This review aims to provide a critical discussion on the potential role of Ginkgo biloba L. folium extract (EGb) in the management of COVID‐19. The antiviral effect of EGb is mediated by different mechanisms, for example, by blockage of the SARS‐CoV‐2 3‐chymotrypsin‐like protease. EGb impedes the development of pulmonary inflammatory disorders by the reduction of neutrophil elastase activity, proinflammatory cytokine release, platelet aggregation, and thrombosis.

Abbreviations
- 3CLpro
3‐chymotrypsin like protease
- ACE2
angiotensin‐converting enzyme 2
- ALI
acute lung injury
- Ang II
angiotensin II
- ARDS
acute respiratory distress syndrome
- EGb
Ginkgo biloba extract
- ET‐1
endothelin 1
- GA
ginkgolic acid
- GB
Ginkgo biloba
- HA
hemagglutinin
- HBV
hepatitis B virus
- HNE
human neutrophil elastase
- HO‐1
heme oxygenase‐1
- HSV
herpes simplex virus
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen‐activated protein kinase
- NA
neuraminidase
- nAChR
nicotinic acetylcholine receptor
- NLRP3
node‐like receptor pyrin 3
- NO
nitric oxide
- PAF
platelet‐activating factor
- PEDV
porcine epidemic diarrhea virus
- PLpro
papain‐like protease
- RAS
renin–angiotensin system
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TLR4
toll‐like receptor 4
1. INTRODUCTION
By the end of April 2022, over 510 million individuals had been infected with coronavirus‐2 (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) worldwide, with over 6 million reported deaths globally.[ 1 ] The majority of patients with coronavirus disease 2019 (COVID‐19) present with mild respiratory symptoms. However, acute respiratory distress syndrome (ARDS) is developed in about 5% of cases.[ 2 ] Although most people with COVID‐19 recover, in many cases, they remain in critical condition with various associated serious complications.[ 3 ] The significant risk factors for COVID‐19 severity are old age, male gender, and comorbidities such as hypertension and diabetes mellitus that increase the risk of post‐COVID‐19 complications, which may prove fatal.[ 4 , 5 ] Post‐COVID‐19 sequelae range from mild such as fatigue, to severe such as pulmonary fibrosis, which requires prolonged oxygen supplementation or lung transplantation.[ 6 ] Inflammatory response and oxidative stress are the leading players of the ARDS development during coronavirus infection. Therefore, anti‐inflammatory and antioxidant agents may play a significant role in alleviating the complication of COVID‐19 patients.[ 7 ] In this context, plant‐derived compounds, such as secondary metabolites, offer great potential in the biodiscovery of therapeutics.[ 8 ] Moreover, many published studies on the antiviral activity of plant products and their phytoconstituents indicate their capability to affect various targets at different stages of the virus life cycle.[ 9 ] As recently reviewed, searching multiple databases and computational and a few in vitro studies prove that some of them possess potent activity against SARS‐CoV‐2 or can play an essential role in COVID‐19 management[ 9 , 10 , 11 ] (Figure 1).
Figure 1.
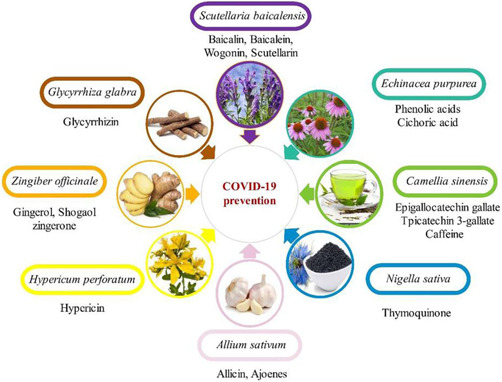
Natural products in coronavirus disease 2019 (COVID‐19) management.[ 12 ]
Ginkgo biloba L. (Gb) is a medicinal herb, and its extract collected from dried green leaves is one of the most widely used phytotherapeutic products globally. The extracts are prepared with different extraction yields depending on the applied method. Active constituents from Gb are extracted and purified with the use of deep eutectic solvents or ionic liquids, graphene oxide‐, ultrasound‐ or mechanically‐assisted enzymatic extractions, silver‐containing adsorbents, or macroporous resin. Validated high‐performance liquid chromatography with various detection methods is used to quantify isolated constituents.[ 13 ] The available herbal medicines based on Ginkgo biloba extract (EGb) are mostly standardized on ginkgo flavon glycosides and terpene lactones—EGb 761® (Tebonin®, Tanakan®).[ 14 ] Recently, strategies for the synthesis, biosynthesis, and biotechnological production of the active Gbʹ phytoconstituents such as terpenoids, flavonoids, and alkyl phenolic acids are also developed.[ 15 ] The EGb‐based products are widely used to treat peripheral vascular diseases, dementia in adults, and age‐related macular degeneration. EGb has beneficial properties for the promotion and management of health because of its active constituents, including flavonoids (such as quercetin, kaempferol, and isorhamnetin), biflavones (such as sciadopitysin and ginkgetin), terpene trilactones (ginkgolides and bilobalide), and alkylphenols (ginkgolic acids [GAs]) with anti‐inflammatory, antioxidant, and antiviral activity[ 16 , 17 ] (Table 1). EGb also contains other active constituents such as adipostatin and bilobol, causing otherwise severe allergic reactions.[ 18 ] The ability to modulate the body's immune functions, besides the anti‐inflammatory, antioxidant, and antiviral activity, supports the therapeutic role of EGb in managing COVID‐19 treatment. While there is enough level of evidence to merit the potential use of this herbal medicine to alleviate symptoms and potentially improve the general wellbeing of patients, recent studies have revealed they act as a coronavirus inhibitor and thus offer the potential for their use as besides symptomatic adjuvant therapy.[ 19 ]
Table 1.
Main chemical constituents of EGb
| Class1 | Compound | Main structure | Percentage | Biological activity |
|---|---|---|---|---|
| Terpenes | Diterpenes: Ginkgolides A, B, C, J |

|
2.8%–3.4% |
Activity: Antioxidant, anti‐inflammatory, antiapoptotic Effects: Neuroprotective, antianxiety, antiplatelet |
| Sesquiterpene: Bilobalide |

|
2.6%–3.2% | ||
| Flavonoids |
Flavanols: Catechins Flavonols: Isorhamnetin, kaempferol, quercetin, myricetin Flavones: Biflavones (BilobetinaGinkgetina) |

|
2% 22%–27% |
Activity: Antioxidant, antibacterial, antiviral, anti‐inflammatory, antithrombin Effects: Neuroprotective, anticancer, antiadipogenesis, antiobesity, hepatoprotective, nephroprotective |
| Organic acids | Alkyl phenolic acids: Ginkgolic acid |

|
≤0.0005% |
Activity: Antioxidant Effects: Antitumor |
| Other | Polysaccharides |
Activity: Anti‐inflammatory, antiproliferative, proapoptotic Effects: Protective in diabetic retinopathy and nonalcoholic fatty liver disease |
Abbreviation: EGb, Ginkgo biloba extract.
EGb and other medicinal plants are gaining popularity because of the various advantages, such as fewer side effects, low cost, and good patient compliance.[ 20 ] A long history of using herbal medicines, including EGb, made the public believe that they are safe for humans as they are natural.[ 18 , 20 ] However, many studies have provided evidence that phytochemicals, even in low doses/concentrations, have the potential for adverse effects.[ 21 , 22 ] Although the safety is promising, EGb, similarly to other herbal medicines, may cause adverse effects and induce herbal–drug interactions.[ 18 , 20 ] It can interact with antiplatelets and anticoagulants, leading to an increased risk of bleeding, particularly in elderly patients.[ 23 ] However, Ryu et al.[ 24 ] have revealed that although EGb enhances the antithrombotic and antiplatelet effects of cilostazol, there was no risk of bleeding. EGb, due to the inhibitory impacts on P‐glycoprotein and cytochrome 450, can also cause interactions with nutritional agents, like grapefruits.[ 25 ] The available toxicological/safety data from clinical trials for EGb 761 prove the low toxicity of G. biloba extract. Although, as depicted in Figure 2, mild adverse effects of GB products, including dizziness, headache, vomiting, nausea, diarrhea, chills, pruritus, rash, and erythema as well as bleeding of several organs, have been demonstrated, these adverse events profiles of EGb products and placebo were similar with no statistically significant differences in the rate of serious adverse events.[ 14 ] Nevertheless, it is necessary to follow the combined use of drugs and nutrition guidelines when using GB products, especially in critically ill patients.[ 26 ]
Figure 2.
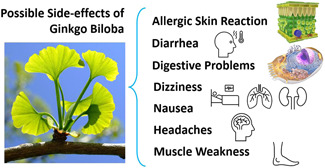
Mild adverse effects of Ginkgo biloba products.[ 14 ]
Paying attention to the significance of herbal products to manage COVID‐19 and the anti‐inflammatory, antiviral, and antioxidant effects of EGb, this review aims to provide a critical overview of the potential use of this phytomedicine in adjuvant antiviral and symptomatic therapy.
2. ANTIVIRAL ACTIVITY OF G. biloba
EGb rich in various phytochemicals with broad‐spectrum antiviral activity can affect the viral life cycle stages, including viral binding, fusion, viral entry, protein expression, and viral protein assembly and release.[ 16 , 19 ] Significantly, the antiviral effects of EGb were observed below the cytotoxic threshold.[ 27 ] Its inhibitory effect has been demonstrated against influenza A (H1N1 and H3N2) and influenza B viruses through the direct disruption of the hemagglutinin (HA) function in adsorption to host cells.[ 27 ] A key factor determining influenza A virus transmissibility is the interaction of the surface glycoproteins HA and neuraminidase (NA) with sialic acid from the cell surface receptor. HA is generally responsible for binding to the sialic acid to allow virus internalization, while the NA is a sialidase responsible for cleaving sialic acid in virus spread and release.[ 28 ] Ginkgetin, a natural biflavone isolated from leaves of GB, has been reported to block the activity of sialidase of influenza virus, thus preventing adsorption of the virus on the host cell surface. The IC50 values of ginkgetin against H1N1 and H3N2 sialidases were 55.00 and 9.78 µg/ml, respectively. Moreover, Ginkgetin–sialic acid conjugates showed a distinct survival effect in the H1N1‐infected mice.[ 29 ] Wang et al.[ 30 ] have demonstrated that polyphenols from EGb exerted antiviral activity against the H3N2 influenza virus and hepatitis B virus (HBV). In in vitro experimental study, the authors established that the polyphenols from GB leaves had 70% viricidal activity at a concentration of 100 µg/ml with a 74.9% protection rate against the H3N2 influenza virus and with a 67.32% inhibition effect on HBV surface antigen.[ 30 ] It is suggested that GB polyphenols exert direct antiviral activity, resulting from disrupting viral fusion and potentially protective effect on the host cell membrane by reducing permeability and improving its stability.[ 31 ] Results from in vivo and in vitro studies indicate that isorhamnetin, also present in EGb, inhibits NA and HA of the influenza H1N1 virus at the gene expression level. Moreover, this flavonoid suppressed virus‐induced autophagy, reactive oxygen species production, and mitogen‐activated protein kinase (MAPK) phosphorylation.[ 32 ]
Polysaccharide from Gb exocarp has been demonstrated to limit binding and entry of porcine epidemic diarrhea virus (PEDV) with 50% inhibitory concentration at 100 µg/ml. This polysaccharide inhibited PEDV infection in Vero cells in a dose‐ and time‐dependent manner.[ 33 ] EGb significantly reduced also the infectivity of herpesviruses (HHV‐1 and HHV‐2) through the interruption of viral adsorption to the cell surface. Most likely, flavonoids, especially isorhamnetin, contribute to the antiviral activity of GB. EGb has been established to be not toxic for normal cells up to 150 µg/ml, though minor toxic effects were observed at concentrations in the range of 200–250 µg/ml. Indeed, nontoxic concentrations of EGb have potential of antiherpesviral agents. Moreover, the potential therapeutic effect of EGb in managing multidrug resistant herpes simplex virus (HSV) infections both of oral and genital types, was suggested.[ 34 ] GA of Gb possesses broad‐spectrum antiviral effects by reducing viral replication in a dose‐dependent manner. It can inhibit infection of arboviruses, including the ZIKA virus, responsible for frequent epidemic outbreaks.[ 35 ] The potential antiviral mechanisms of GA include fusion inhibition of the enveloped viruses, such as human immunodeficiency virus, Ebola virus, influenza A virus, the impedance of nonenveloped viruses' entry, and downregulation of protein synthesis of DNA viruses.[ 16 ] Experimental studies have revealed that GA inhibited viral DNA and protein syntheses and impeded viral fusion by generating positive impulsive curvature, thus inhibiting hemifusion.[ 19 ] Interestingly, GA was also reported to inhibit the formation of HSV‐1 zosteriform vesicles on the skin flanks of BALB/cJ mice.[ 36 ] Likewise, virus inactivation mechanisms exerted by disinfectants target binding viruses to the host cell surface, limiting, therefore, cell–virus interactions.[ 37 ] Moreover, EGb components, specifically GA, can inactivate viral particles by modifying viral genomes and proteins.[ 16 ] The net antiviral effect of GB and its constituents is mediated by different mechanisms, including inhibition of viral binding, entry, replication, assembly, and viral release.
3. ANTI‐INFLAMMATORY EFFECTS OF G. biloba
EGb and its constituents exert wide‐ranging anti‐inflammatory effects mainly via inhibiting proinflammatory cytokines and activating anti‐inflammatory cytokines.[ 38 ] A study by Sochocka et al.[ 39 ] has shown that EGb 761—a standardized extract with 24% flavones and 6% terpenes including bilobalide, enhanced gamma interferon production while inhibiting the release of proinflammatory cytokines in peripheral blood leukocytes stimulated by vesicular stomatitis virus. These findings indicate an improvement in the innate immune response during viral infections upon EGb treatment. It has been established that biflavones of EGb, mainly ginkgetin, strikingly suppressed the abnormal expression of p38 and Akt pathways in the human neutrophil elastase (HNE)‐stimulated adenocarcinomic human alveolar basal epithelial A549 cells. Biflavones also inhibited the messenger RNA expression of respiratory tract mucin (MUC5AC), causing mucociliary clearance failure, both in HNE‐activated A549 cells and in ovalbumin‐sensitized and challenged BALB/c (male/female) mice. In the allergic mice, EGb treatment attenuated allergen‐induced airway inflammation; therefore, it has been suggested as a potential agent for treating airway inflammation.[ 40 ] Tang et al.[ 41 ] have demonstrated that EGb reduced airway inflammation in asthmatic patients by reducing the infiltration of inflammatory cells, mainly lymphocytes, and eosinophils. In addition, it attenuated the activation of protein kinase alpha (PKC‐α) of lymphocytes, thereby decreasing the local release of interleukin 5 (IL‐5) in the respiratory epithelial cells. Therefore, EGb could be considered for managing asthma due to its anti‐inflammatory effects.[ 19 , 42 ] Ginkgolide B, diterpene trilactone of GB, has also been demonstrated to reduce the inflammatory reactions in the airway of asthmatic patients by inhibiting the release of platelet‐activating factor (PAF). This phytoconstituent suppressed the release and action of T‐helper 2 cytokines like IL‐5 and IL‐13 in bronchoalveolar lavage fluid with a significant reduction of eosinophils accumulation.[ 43 ] Ginkgolide A downregulated in vitro the MAPK and nuclear factor kappa‐B (NF‐κB) while promoting the AMP‐activated protein kinase (AMPK pathway) in lipopolysaccharide (LPS)‐stimulated mouse peritoneal macrophages as well as in RAW264.7 cells and differentiated human monocytes, thus inhibiting proinflammatory mediators production. Consistently, in mice, ginkgolide A inhibited the LPS‐stimulated systemic release of tumor necrosis factor‐alpha (TNF‐α) and IL‐6.[ 44 ] As AMPK activation exerts potent anti‐inflammatory effects, modulation of this pathway is suggested as a pharmacological target in treating acute lung injury (ALI) and other neutrophil‐driven acute inflammatory conditions.[ 45 ] Gb‐derived polysaccharides by affecting the expression of p‐p65 p‐IκBα, TNF‐α, and IL‐1β proteins can also modulate the inflammation signaling pathway.[ 46 ] Kotakadi et al.[ 47 ] have shown that EGb 761 inhibited macrophage activations. In colitis mice, treatment with this extract downregulated inflammatory stress marker p53 and proinflammatory cytokines, like IL‐6 and TNF‐α, with suppression of CD4 effector.
EGb is regarded to exert anti‐inflammatory and antioxidant effects by suppressing the inflammatory response and improving human endogenous antioxidant capacity.[ 48 ] It enhances immune function by controlling organ index and T lymphocyte subsets. Besides this, EGb suppresses the release of TNF‐α and IL‐1β and upregulates anti‐inflammatory cytokines IL‐4 and IL‐10 with the regulation of the Th17/Treg axis.[ 49 , 50 ] EGb reduced expression of retinoic acid‐related orphan receptor γt and upregulated expression of forkhead winged‐helix transcription factor 3 in peripheral blood, thus attenuating the imbalance of Th17/Treg cells and the propagation of inflammatory reactions.[ 50 , 51 ] On the other hand, ginkgolide B inhibited acute spinal cord injury in rats by suppressing the signal transducer and activator transcription 3 signaling pathway.[ 52 ]
What is more, EGb (Ginaton) attenuated angiotensin II (Ang II)‐induced inflammation, vascular injury, endothelial dysfunction, and hypoxia in human vascular endothelial cells by regulating mitochondrial integrity and inhibiting endothelin‐mediated endothelial damage.[ 53 ]
Based on the above, as shown in Figure 3, EGb may modulate the immune responses in various inflammatory conditions by regulating inflammatory signaling pathways.
Figure 3.
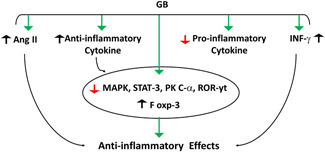
Anti‐inflammatory effects of Ginkgo biloba (GB): GB stimulates the release of interferon‐gamma (INF‐γ) and release of anti‐inflammatory cytokines, inhibits angiotensin II (Ang II) and release of proinflammatory cytokines. GB suppresses inflammatory mediators, including; mitogen‐activated protein kinase (MAPK), signal transducer and activator transcription 3 (STAT‐3), protein kinase C alpha (PKC‐α), and retinoic acid‐related orphan nuclear receptor (ROR‐γt) with upregulation of forkhead winged‐helix transcription factor 3 (Fox‐3).
4. G. biloba AND COVID‐19
The replication of SARS‐CoV‐2 is mainly processed through the 3‐chymotrypsin‐like protease (3CLpro) enzyme, which is regarded as a plausible target for direct‐acting antiviral agents attributable to its indispensable role in virus replication.[ 54 ] Jukič et al.[ 55 ] have noted that this protease with no mutations in the reported pandemically relevant variants may be a valuable therapeutic target providing trans‐variant effectiveness. EGb, particularly present therein GA and bioflavone—sciadopitysin, can block SARS‐CoV‐2 3CLpro as demonstrated in inhibition kinetic studies and docking simulation.[ 56 ] In in vitro experiments, EGb effectively inhibited SARS‐CoV‐2 3CLpro activity at a concentration of 6.68 µg/ml,[ 56 ] while GA at a concentration of 16.30 μM.[ 57 ] A molecular docking study by Cherrak et al.[ 58 ] revealed that glycosylated flavonoids present in EGb, like the quercetin and rutin derivatives, exerted inhibitory effects on the replication of SARS‐CoV‐2 by constraining the 3CLpro function. Among the tested flavonoid derivatives, quercetin‐3‐O‐rhamnoside represented the highest binding affinity (‐9,7 kcal/mol). The binding affinity to the active site of SARS‐CoV‐2 protease is mainly structure‐dependent. Based on the dynamic behavior and stability of the protein in complex, rutin demonstrated the highest potential to inhibit the function of 3CLpro of coronavirus. Although these compounds showed superior inhibitory effects on the SARS‐CoV‐2 protease, the authors stressed the need to verify this activity experimentally.[ 58 ] A glycosylated derivative of kaempferol—also present in EGb, has been selected alongside other four phytochemicals with the highest binding energies for SARS‐CoV‐2 main protease from screened 6570 herbal compounds. Moreover, the docking results support the ability of kaempferol to inhibit SARS‐CoV‐2 protease with modulating effects on intracellular and extracellular signaling pathways.[ 59 ] Besides 3CLpro, the papain‐like protease (PLpro) is the second viral cysteine protease critical for the translation of the viral RNA and therefore attractive antiviral drug target. Chen et al.,[ 57 ] in an enzymatic inhibition assay, found that GA acts as an irreversible inhibitor also against PLpro at a nontoxic concentration of 1.79 μM. Similarly, quercetin, the main component of the EGb flavonoid fraction, has been demonstrated to interfere with the replication of SARS‐CoV‐2 through inhibition of 3CLpro and PLpro with corresponding docking energy of 6.25–4.62 kcal/mol.[ 60 ] The mitigation of associated coagulopathy and hyperinflammatory reactions is indicated. Indeed, this flavonoid may inhibit SARS‐CoV‐2 replication and protect against associated inflammatory and prothrombotic conditions.[ 60 ] Moreover, kaempferol and quercetin with robust channel blocking activity inhibit the SARS‐CoV envelope protein E, suppressing virus activity and proliferation.[ 61 ] Broad‐spectrum antiviral activity and its potential role in COVID‐19 management of GA should be emphasized as well. Bhutta et al.[ 62 ] have demonstrated that this phytochemical significantly protected against human coronavirus strain 229E (HCoV‐229E) infection in human epithelial lung cells (MRC‐5) by restricting progeny virus production, viral protein expression, and cytopathic effects. Significantly, the anti‐inflammatory activity of quercetin through stabilization of mast cells, modulation of dendritic cells activity, and inhibition of proinflammatory cytokines release from activated macrophages in the lung has also been reported.[ 63 , 64 ] Quercetin and quercetin‐rich extract suppress oxidative stress and inflammatory reactions by inhibiting the NF‐κB signaling pathway as demonstrated in various cell lines.[ 65 ]
Due to the wide range of quercetin antiviral activity, its coadministration with vitamin C has been suggested to be an effective combination in preventing and treating COVID‐19 as an adjuvant with anti‐SARS‐CoV‐2 medications such as a convalescent plasma or remdesivir.[ 66 ] Vitamin C exerts direct antiviral activity, scavenges radical species, inhibits vascular inflammation and endothelial dysfunction with the restoration of mitochondrial function.[ 67 , 68 ] Quercetin‐rich extracts trigger various biological effects, including anti‐inflammatory, antioxidant, and antiplatelet effects, and inhibit proinflammatory mediators such as phospholipase A2 and lipooxygenase (LOX).[ 69 ] Quercetin anti‐inflammatory activity is mediated mainly by regulating prostaglandin/leukotriene pathways and threonine/serine kinases such as inducible nitric oxide synthase (iNOS) and phosphatidylinositol kinase.[ 70 ] As reviewed, there is evidence that coadministration with vitamin C and quercetin can provide synergistic therapy for the prevention and treatment of COVID‐19. This combination leads to synergistic antiviral effects against SARS‐CoV‐2. Overlapping of immunomodulatory effects on the development of cytokine storms by inhibiting the release of IL‐6 and other proinflammatory cytokines‐induced ALI is also suggested. Notably, the capacity of ascorbate to recycle quercetin is relevant for increasing its efficacy.[ 66 ]
Furthermore, in SARS‐CoV‐2 infections, deregulation of inflammatory responses is characterized by activation of node‐like receptor pyrin 3 (NLRP3) inflammasome and TH17 that increase proinflammatory cytokines and neutrophil release recruitments, respectively.[ 71 ] Quercetin and other phytochemicals found in EGb block activation of NLRP3 inflammasome and TH17, thus mitigating hyperinflammation and hypercytokinemia state with suppression development of cytokine storm.[ 72 ] The adjuvant treatment with this flavonoid shortened the time to virus clearance as well as reduced severe symptoms and negative predictors of COVID‐19 patients.[ 73 ] Quercetin and kaempferol blocked phosphorylation of protein kinase and the expression of a cation‐selective channel (3A channel) in the infected cells by SARS‐CoV‐2. They also suppress the release of proinflammatory cytokines, expression of iNOS, and production of reactive free radicals.[ 74 ] Thus, quercetin and kaempferol might apply beneficial effects in the treatment of COVID‐19 because of their antioxidant, immunomodulatory, anti‐inflammatory, and antiviral effects.
SARS‐CoV‐2 infection is linked with the development of various immunological and inflammatory hyperactivations that per se may cause tissue injury, including ALI and ARDS.[ 75 ] Both LOX and cyclooxygenase (COX) enzymes are activated in SARS‐CoV‐2 infection leading to bronchial inflammatory reactions and progression of ALI in patients with severe COVID‐19.[ 4 , 76 ] EGb, mainly its constituent ginkgetin, inhibited prostaglandin and leukotriene pathways due to the suppression of phospholipase A2 enzyme activity in the mast cells.[ 77 ] EGb modulated inflammatory reactions in an experimental model with chronic asthma, and its role as an alternative and complementary therapy in asthma management has been suggested.[ 78 ] Tao et al.[ 40 ] have demonstrated that EGb and ginkgetin could inhibit the development of pulmonary inflammatory disorders through the diminution of HNE activity. Taken together, these findings support the EGb use to attenuate ALI/ARDS.
Recently, several studies have revealed the therapeutic potential of bilobalide in ALI. This GB's terpenoid prevented sepsis‐induced ALI by reducing proinflammatory activation and suppression of NF‐κB and COX‐2 activity.[ 79 ] Mechanistic studies demonstrated that pretreatment with bilobalide reduced the expression of COX‐2, heme oxygenase‐1 (HO‐1), iNOS, and phosphorylation of p65 with IkB activation in the lung in mice with ALI. Bilobalide treatment attenuated oxidative stress‐induced ALI via upregulation of antioxidant enzymes and HO‐1.[ 79 ] Therefore, EGb, mainly bilobalide, might be beneficial in preventing ALI by mitigating inflammation and oxidative stress. Moreover, this EGb's constituent regulated the expression of toll‐like receptor 4 (TLR4), NF‐κB, and myeloid differentiation factor 88, preventing the development of ALI.[ 80 ] Significantly, these proinflammatory mediators, mainly TLR4 and NF‐κB, contribute to an inflammatory response with subsequent development of ALI/ARDS in severe cases of SARS‐CoV‐2 infection.[ 81 , 82 ] Therefore, it is believed that GB products may prevent the development of ALI and cytokine storm in COVID‐19 by downregulation of lung proinflammatory signaling pathways, mainly NF‐κB pathway and expression TLR4.[ 83 ]
The receptors for binding and entry of SARS‐CoV‐2 are angiotensin‐converting enzyme 2 (ACE2), cluster differentiation 147, and dipeptidyl peptidase 4. ACE2 is the most dominant and highly expressed receptor in pulmonary alveolar type 2 cells.[ 84 ] The interaction between SARS‐CoV‐2 and ACE2 leads to its notable downregulation. ACE is involved in controlling and regulating the renin–angiotensin system (RAS) through conversion of the renin–angiotensin vasoconstrictor Ang II into vasodilator anti‐inflammatory Ang 1–7 and Ang 1–9. Accordingly, downregulation of ACE2 and progression of high circulating Ang II during SARS‐CoV‐2 infection might be the conceivable mechanism overdue initiation of inflammatory instabilities and progression of ALI.[ 85 , 86 ] It has been reported that extract EGb 50 blocked activation of local RAS through mitigation of over‐stimulated NF‐κB/TLR4 pathway, thus reducing the increased expression levels of angiotensinogen and AT1a receptors in cardiomyocytes.[ 87 ] EGb 50 is a concentrated extract of G. biloba leaves containing 6.4% ginkgolides, 26.4% flavonoid glycosides, and 44.1% total flavonoids.[ 88 ] EGb 761, comprising 24% flavonoid glycosides and 6% terpenoids, inhibited platelet activation, removed free radicals, and mitigated p53‐mediated and mitochondrion‐dependent proapoptotic effects doxorubicin‐induced cardiac toxicity.[ 89 ] EGb 50 also exerted beneficial effects against myocardial ischemia–reperfusion (IR) by reducing the oxidative stress in the reperfused myocardium and increasing immunity and antioxidant activities in IR rats.[ 90 ] Moreover, EGb 50, by inhibiting the p38MAPK and NF‐κB pathways, attenuated inflammatory response also in microglial cells.[ 91 ] Liu et al.[ 92 ] demonstrated that EGb 50 is a potential anti‐inflammatory herbal agent suppressing NLRP3 inflammasome‐induced microglial activation. Thus, EGb could play an important role in reducing SARS‐CoV‐2‐induced inflammatory and oxidative stress disorders with attenuation of RAS deregulation.
Some peptides isolated from EGb also exhibit a potent inhibitory effect on the ACE activity, signifying the hypotensive effect of the extract through reduction of vasoconstrictor effect of Ang II levels. This finding supports the protective impact of EGb against Ang II‐mediated ALI in COVID‐19.[ 93 ] A meta‐analysis study illustrated that combination of EGb with antihypertensive drugs might improve blood pressure and renal function in patients with hypertensive nephropathy.[ 94 ] Significantly, EGb protects endothelial cells by inhibiting thromboxane A2 activity and promoting the production and release of prostacyclin I2 and nitric oxide (NO). Likewise, EGb inhibited endothelin 1 (ET‐1) release, thereby controlling tissue blood flow and vascular tension in patients with coronary artery disease.[ 95 ] Both NO and ET‐1 are highly disturbed in patients with severe COVID‐19. NO, which decreased level is associated with vascular dysfunction and immune inflammation in patients with COVID‐19, is considered a possible therapeutic strategy for managing COVID‐19‐induced ARDS.[ 96 , 97 ] Importantly, Farhangrazi et al.[ 98 ] showed that high circulating ET‐1 level is linked with COVID‐19 severity due to activation of NF‐κB and development of ARDS. Indeed, ET‐1 antagonist bosentan reduced COVID‐19 severity by inhibiting the necroptosis pathway and altering microvascular endothelial cells. Thus, EGb may reduce COVID‐19 severity by modulation of endothelial NO and inhibition of ET‐1.
Moreover, high circulating Ang II levels, together with SARS‐CoV‐2 infection, lead to endothelial dysfunction through activation of p38MAPK.[ 99 ] Li et al.[ 100 ] found that EGb impeded the progression of inflammatory reactions in myocardial injury by suppressing the p38MAPK pathway. EGb rich in biflavones with potent human thrombin inhibitor activity can prevent p38MAPK‐induced thrombosis and platelet activations.[ 101 , 102 ] Moreover, GB's ginkgolides inhibit PAF‐mediated aggregation of human platelets,[ 103 ] which is crucial for mitigating cardiovascular complications. Likewise, EGb attenuates endothelial dysfunction, which is triggered by inflammatory mediators and oxidative stress via the regulation of endothelial cells NO.[ 104 ] Therefore, EGb may reduce the progression of SARS‐CoV‐2‐induced ALI through modulation of the Ang II/p38MAPK axis with attenuation of endothelial dysfunction and pulmonary microvascular thrombosis.
On the other hand, escalation of COVID‐19 severity is related to the sympathetic storm and vagal suppression that forms fatal COVID‐19 complications like cytokine storm. Therefore, vagal stimulation could be appreciated in COVID‐19 patients by suppressing the sympathetic storm and releasing proinflammatory cytokines.[ 105 ] Indeed, cholinergic agonists inhibit inflammation by clamping inflammatory signals such as high mobility group protein 1 (HMGB1). Also, a molecular docking study has shown that the nicotinic acetylcholine receptor (nAChR) might be a possible requisite receptor for SARS‐CoV‐2. Blocking nAChR by SARS‐CoV‐2 leads to the progression of cytokine storm through inhibition of vagal anti‐inflammatory effect.[ 106 ] The SARS‐CoV‐2 also invades the brain, causing downregulation of ACE2 in the inhibitory GABAergic neurons, leading to the augmentation of sympathetic flow with the development of the sympathetic storm.[ 107 ] Therefore, sympathetic storm in SARS‐CoV‐2 is due to a central inhibition of GABAergic neurons and peripheral inhibition of the parasympathetic‐nACh axis. It has been reported that EGb, mainly flavonoids, and ginkgolides, exert inhibitory effects on the acetylcholinesterase activity that increases acetylcholine activity, which may counterbalance the sympathetic over‐activity and inflammatory reactions through nAChRs.[ 108 , 109 ] On the other hand, EGb may antagonize the anti‐inflammatory and central inhibitory effect of GABAergic neurons, increasing cortical neuronal activity with the risk of convulsion.[ 110 ] However, a meta‐analysis study has confirmed that the anxiolytic effect of EGb that occurs through modulation of GABAergic neurons is without risk of convulsion.[ 111 ] These findings highlight the potential role of EGb in the modulation of the autonomic nervous system and augmentation of the anti‐inflammatory role of parasympathetic division in COVID‐19 patients.
Besides this, anxiety disorders are developed in COVID‐19 patients that might be due to sympathetic overactivity or high proinflammatory cytokines that affect brain neurotransmitters leading to a stressful state with a risk of cardiopulmonary complications.[ 107 ] EGb has been reported to reduce anxiety and stress disorders, particularly in elderly patients with a cognitive decline, by improving brain neurotransmitters.[ 112 ]
In the COVID‐19 era, with substantial development of anti‐SARS‐CoV‐2 vaccines, antibody titers postvaccination, however, may not be persistent in immune‐comprised patients. Thus, it is suggested that some patients whose antibody titers diminish more rapidly than in healthy individuals will need a booster (comparable with hepatitis B vaccination).[ 113 ] Though, EGb, through its immunomodulating effects, may enhance the host immune response to the vaccines.[ 114 ] In line with this, Al‐Kuraishy et al.[ 115 ] have reported that levamisole enhanced the humoral immune response to the hepatitis B vaccine. As it has been shown that EGb improves human humoral immune response,[ 116 ] it appears to be a promising immune response booster for anti‐SARS‐CoV‐2 vaccines.
Given the above evidence, GB preparations might be considered potential candidates for adjuvant therapy in managing COVID‐19 through different mechanisms (Figure 4). However, experimental and clinical trial studies are recommended to verify the beneficial effect of EGb in the management of COVID‐19.
Figure 4.
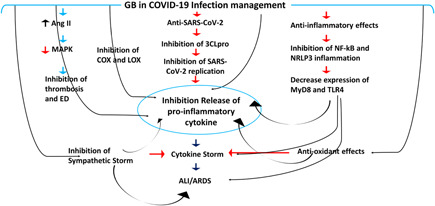
Role of Ginkgo biloba (GB) in COVID‐19: GB inhibits replication of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) through inhibition of 3‐chymotrypsin like protease (3CLpro). GB inhibits the release of proinflammatory cytokines via suppression of nuclear factor kappa B (NF‐κB) and node‐like receptor pyrin 3 (NLRP3) inflammasome. GB inhibits thrombosis and endothelial dysfunction (ED) via inhibition of angiotensin II (Ang II) and mitogen‐activated protein kinase (MAPK). Also, GB reduces the activity of cyclooxygenase (COX) and lipoxygenase (LOX) involved in releasing proinflammatory cytokines. GB antioxidant effects inhibit the expression of toll‐like receptor 4 (TLR4) and myeloid differentiation primary response (MyD88), thereby attenuating proinflammatory cytokines. GB antioxidant effects also inhibit cytokine storm and the development of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). GB blocks the development of the sympathetic storm, which increases the risk of ALI, ARDS, cytokine storm, and release of proinflammatory cytokines.
5. PERSPECTIVES AND CHALLENGES
Taken together, the herbal medicine, as it is EGb, offers its use as an adjuvant both in the symptomatic and antiviral therapy to COVID‐19. However, there is a need to validate its therapeutic potential and safety profile to scientifically support recommendations on the benefits and risks of its use in managing COVID‐19 treatment.[ 117 ] Regarding effectiveness, poor membrane permeability, solubility, and oral bioavailability, exhibited by GB phytochemicals, require considerable attention. To overcome this obstacle, effective delivery nanosystems crossing biological barriers and enabling sustained release to achieve therapeutic concentrations in target tissues are developed.[ 56 , 118 ] Several nanotechnology‐based systems, such as polymeric, solid lipid, magnetic, metal, and inorganic nanoparticles, polymeric micelles, liposomes, nanospheres, nanocapsules, quantum dots, nanoemulsions, and dendrimers have been developed for the successful delivery of natural products. Moreover, nanocarriers with stimuli‐responsive characteristics sensitive to temperature, pH, electric or magnetic field, light, ultrasound, and salt content enable controlled medication administration.[ 119 ] Based on patient medical history and using the power of artificial intelligence, nanomedicine can even be developed towards therapy optimized at the individual level by achieving maximum efficacy and the least amount of adverse effects.[ 120 ] Moreover, given the ability of nanoparticles to inhibit viral entry and infected cell protein fusion during initial attachment and membrane fusion, their direct antiviral activity could potentiate EGb effects in the management at various stages of COVID‐19.[ 118 , 121 ] As the SARS‐CoV‐2 virus exhibits strain variation, managing its treatment is challenging. High‐performance nanotechnology with manipulative features can address the consequences of newer SARS‐CoV‐2 variants efficiently and promptly.[ 122 ] The outcomes of such manipulative therapy, consisting of an anti‐SARS‐CoV‐2 virus agent and nanocarriers with the variant independent activity, appear to be a promising approach.[ 123 , 124 ]
6. CONCLUSION
EGb has potent anti‐inflammatory, antioxidant, and antiviral properties, including anti‐SARS‐CoV‐2 effects through modulation of proinflammatory cytokines release, endothelial dysfunction‐mediated thrombotic complications, and progression of ALI/ARDS in patients with COVID‐19. Moreover, structural studies alongside biological evaluation experiments on the inhibiting activity of GB against SARS‐CoV‐2 3CLpro support its trans‐variant effectiveness. Therefore, EGb could be a potential therapeutic modality in managing COVID‐19. However, there is a need to assess the benefits/risks balance of this herbal medicine to scientifically substantiate future recommendations on their use in the management of COVID‐19. Clinical trials with experimental studies are necessary to authorize the use of EGb in the reviewed indication. Moreover, as nanotechnology offers improved delivery to the target tissue and action enhancement, the opportunity to benefit from GB‐based nanomedicine in modern phytotherapy is worth considering.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors acknowledge Mustansiriyia University.
Al‐kuraishy H. M., Al‐Gareeb A. I., Kaushik A., Kujawska M., Batiha G. E.‐S., Arch. Pharm. 2022;355:e2200188. 10.1002/ardp.202200188
Contributor Information
Małgorzata Kujawska, Email: kujawska@ump.edu.pl.
Gaber El‐Saber Batiha, Email: gaberbatiha@gmail.com.
REFERENCES
- 1. @JohnsHopkins .COVID‐19 Map—Johns Hopkins Coronavirus Resource Center. 2022. Accessed May 4, 2022. https://coronavirus.jhu.edu/map.html
- 2. Al‐Kuraishy H. M., Al‐Gareeb A. I., Qusty N., Cruz‐Martins N., El‐Saber Batiha G., Pulm. Pharmacol. Ther. 2021, 67, 102008. 10.1016/j.pupt.2021.102008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Kuraishy H. M., Al‐Gareeb A. I., Alblihed M., Cruz‐Martins N., Batiha G. E., Front. Med. 2021, 8, 644295. 10.3389/fmed.2021.644295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Kuraishy H. M., Al‐Gareeb A. I., Almulaiky Y. Q., Cruz‐Martins N., El‐Saber Batiha G., Eur. J. Pharmacol. 2021, 904, 174196. 10.1016/j.ejphar.2021.174196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamamoto V., Bolanos J. F., Fiallos J., Strand S. E., Morris K., Shahrokhinia S., Cushing T. R., Hopp L., Tiwari A., Hariri R., Sokolov R., Wheeler C., Kaushik A., Elsayegh A., Eliashiv D., Hedrick R., Jafari B., Johnson J. P., Khorsandi M., Gonzalez N., Balakhani G., Lahiri S., Ghavidel K., Amaya M., Kloor H., Hussain N., Huang E., Cormier J., Wesson Ashford J., Wang J. C., Yaghobian S., Khorrami P., Shamloo B., Moon C., Shadi P., Kateb B., J. Alzheimer's Dis. 2020, 77, 459. 10.3233/JAD-200831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lugnier C., Al‐Kuraishy H. M., Rousseau E., Biochem. Pharmacol. 2021, 185, 114431. 10.1016/j.bcp.2021.114431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al‐Kuraishy H. M., Al‐Gareeb A. I., Alqarni M., Cruz‐Martins N., El‐Saber Batiha G., Front. Pharmacol. 2021, 12, 642822. 10.3389/fphar.2021.642822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeshi K., Crayn D., Ritmejerytė E., Wangchuk P., Molecules 2022, 27, 313. 10.3390/molecules27010313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Owen L., Laird K., Shivkumar M., Lett. Appl. Microbiol. 2021. 10.1111/lam.13637 [DOI] [PMC free article] [PubMed]
- 10. Mani J. S., Johnson J. B., Steel J. C., Broszczak D. A., Neilsen P. M., Walsh K. B., Naiker M., Virus Res. 2020, 284, 197989. 10.1016/j.virusres.2020.197989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demeke C. A., Woldeyohanins A. E., Kifle Z. D., Metabol. Open 2021, 12, 100141. 10.1016/j.metop.2021.100141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boozari M., Hosseinzadeh H., Phytother. Res. 2021, 35, 864. 10.1002/ptr.6873 [DOI] [PubMed] [Google Scholar]
- 13. Liu L., Wang Y., Zhang J., Wang S., J. Pharm. Biomed. Anal. 2021, 193, 113704. 10.1016/j.jpba.2020.113704 [DOI] [PubMed] [Google Scholar]
- 14. EMA/HMPC/321095/2012 . Committee on Herbal Medicinal Products [HMPC] Assessment report on Ginkgo biloba L., folium. 2014. https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-ginkgo-biloba-l-folium_en.pdf (accessed: January 2014).
- 15. Liu X. G., Lu X., Gao W., Li P., Yang H., Nat. Prod. Rep. 2022, 39, 474. 10.1039/d1np00026h [DOI] [PubMed] [Google Scholar]
- 16. Borenstein R., Hanson B. A., Markosyan R. M., Gallo E. S., Narasipura S. D., Bhutta M., Shechter O., Lurain N. S., Cohen F. S., Al‐Harthi L., Nicholson D. A., Sci. Rep. 2020, 10, 4746. 10.1038/s41598-020-61700-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Beek T. A., J. Chromatogr. A 2002, 967, 21. 10.1016/s0021-9673(02)00172-3 [DOI] [PubMed] [Google Scholar]
- 18. Mei N., Guo X., Ren Z., Kobayashi D., Wada K., Guo L., J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev. 2017, 35, 1. 10.1080/10590501.2016.1278298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ibrahim M. A., Ramadan H. H., Mohammed R. N., J. Basic Clin. Physiol. Pharmacol. 2021, 32, 131. 10.1515/jbcpp-2020-0310 [DOI] [PubMed] [Google Scholar]
- 20. Abad M. J., Bedoya L. M., Bermejo P., Curr. Drug Metab. 2010, 11, 171. 10.2174/138920010791110818 [DOI] [PubMed] [Google Scholar]
- 21. Jodynis‐Liebert J., Kujawska M., J. Clin. Med. 2020, 9. 10.3390/jcm9030718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kujawska M., Ewertowska M., Ignatowicz E., Adamska T., Szaefer H., Gramza‐Michałowska A., Korczak J., Jodynis‐Liebert J., J. Med. Food 2016, 19, 330. 10.1089/jmf.2015.0114 [DOI] [PubMed] [Google Scholar]
- 23. Chan A. L., Leung H. W., Wu J. W., Chien T. W., J. Altern. Complement. Med. 2011, 17, 513. 10.1089/acm.2010.0295 [DOI] [PubMed] [Google Scholar]
- 24. Ryu K. H., Han H. Y., Lee S. Y., Jeon S. D., Im G. J., Lee B. Y., Kim K., Lim K. M., Chung J. H., Thromb. Res. 2009, 124, 328. 10.1016/j.thromres.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 25. Etheridge A. S., Black S. R., Patel P. R., So J., Mathews J. M., Planta Med. 2007, 73, 731. 10.1055/s-2007-981550 [DOI] [PubMed] [Google Scholar]
- 26. Nguyen T., Talal A., Ginkgo biloba, StatPearls Publishing LLC,Treasure Island (FL), US 2021. [Google Scholar]
- 27. Haruyama T., Nagata K., J. Nat. Med. 2013, 67, 636. 10.1007/s11418-012-0725-0 [DOI] [PubMed] [Google Scholar]
- 28. Benton D. J., Wharton S. A., Martin S. R., McCauley J. W., J. Virol. 2017, 91. 10.1128/JVI.02293-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miki K., Nagai T., Suzuki K., Tsujimura R., Koyama K., Kinoshita K., Furuhata K., Yamada H., Takahashi K., Bioorg. Med. Chem. Lett. 2007, 17, 772. 10.1016/j.bmcl.2006.10.075 [DOI] [PubMed] [Google Scholar]
- 30. Wang C. Z., Li W. J., Tao R., Ye J. Z., Zhang H. Y., Molecules 2015, 20, 5137. 10.3390/molecules20035137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferreira J. V., Capello T. M., Siqueira L. J., Lago J. H., Caseli L., Langmuir 2016, 32, 3234. 10.1021/acs.langmuir.6b00600 [DOI] [PubMed] [Google Scholar]
- 32. Abdal Dayem A., Choi H. Y., Kim Y. B., Cho S. G., PLoS One 2015, 10, e0121610. 10.1371/journal.pone.0121610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee J. H., Park J. S., Lee S. W., Hwang S. Y., Young B. E., Choi H. J., Virus Res. 2015, 195, 148. 10.1016/j.virusres.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 34. Sochocka M., Sobczyński M., Ochnik M., Zwolińska K., Leszek J., Front. Microbiol. 2019, 10, 2367. 10.3389/fmicb.2019.02367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Campos D., Navarro S., Llamas‐González Y. Y., Sugasti M., González‐Santamaría J., Viruses 2020, 12, 449. 10.3390/v12040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhutta M. S., Shechter O., Gallo E. S., Martin S. D., Jones E., Doncel G. F., Borenstein R., Viruses 2021, 13, 86. 10.3390/v13010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wigginton K. R., Pecson B. M., Sigstam T., Bosshard F., Kohn T., Environ. Sci. Technol. 2012, 46, 12069. 10.1021/es3029473 [DOI] [PubMed] [Google Scholar]
- 38. Achete De Souza G., De Marqui S. V., Matias J. N., Guiguer E. L., Barbalho S. M., Planta Med. 2020, 86, 376. 10.1055/a-1109-3405 [DOI] [PubMed] [Google Scholar]
- 39. Sochocka M., Taboł A., Sobczyński M., Zaczyńska E., Czarny A., Leszek J., Open Life Sci. 2014, 9, 359. 10.2478/s11535-013-0278-6 [DOI] [Google Scholar]
- 40. Tao Z., Jin W., Ao M., Zhai S., Xu H., Yu L., Food Funct. 2019, 10, 2209. 10.1039/c8fo02506a [DOI] [PubMed] [Google Scholar]
- 41. Tang Y., Xu Y., Xiong S., Ni W., Chen S., Gao B., Ye T., Cao Y., Du C., J. Huazhong Univ. Sci. Technol., Med. Sci. 2007, 27, 375. 10.1007/s11596-007-0407-4 [DOI] [PubMed] [Google Scholar]
- 42. Gaber A., Refat M. S., Belal A., El‐Deen I. M., Hassan N., Zakaria R., Alhomrani M., Alamri A. S., Alsanie W. F., M Saied E., Molecules 2021, 26. 10.3390/molecules26082288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chu X., Ci X., He J., Wei M., Yang X., Cao Q., Li H., Guan S., Deng Y., Pang D., Deng X., Molecules 2011, 16, 7634. 10.3390/molecules16097634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Y., Wu Y., Yao X., Hao F., Yu C., Bao Y., Wu Y., Song Z., Sun Y., Zheng L., Wang G., Huang Y., Sun L., Li Y., Int. J. Mol. Sci. 2017, 18, 794. 10.3390/ijms18040794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao X., Zmijewski J. W., Lorne E., Liu G., Park Y. J., Tsuruta Y., Abraham E., Am. J. Physiol.: Lung Cell. Mol. Physiol. 2008, 295, L497. 10.1152/ajplung.90210.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Y., Sheng Y., Liu J., Xu G., Yu W., Cui Q., Lu X., Du P., An L., Carbohydr. Polym. 2022, 278, 118811. 10.1016/j.carbpol.2021.118811 [DOI] [PubMed] [Google Scholar]
- 47. Kotakadi V. S., Jin Y., Hofseth A. B., Ying L., Cui X., Volate S., Chumanevich A., Wood P. A., Price R. L., McNeal A., Singh U. P., Singh N. P., Nagarkatti M., Nagarkatti P. S., Matesic L. E., Auclair K., Wargovich M. J., Hofseth L. J., Carcinogenesis 2008, 29, 1799. 10.1093/carcin/bgn143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tulsulkar J., Shah Z. A., Neurochem. Int. 2013, 62, 189. 10.1016/j.neuint.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ye J., Ye C., Huang Y., Zhang N., Zhang X., Xiao M., J. Sci. Food Agric. 2019, 99, 2329. 10.1002/jsfa.9431 [DOI] [PubMed] [Google Scholar]
- 50. Xia S., Sun Q., Zou Z., Liu Y., Fang X., Sun B., Wei S., Wang D., Zhang A., Liu Q., Int. J. Biol. Sci. 2020, 16, 483. 10.7150/ijbs.39351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen S., Zhang J., Yu W. B., Zhuang J. C., Xiao W., Wu Z. Y., Xiao B. G., Int. J. Biol. Sci. 2021, 17, 50. 10.7150/ijbs.50041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song Y., Zeng Z., Jin C., Zhang J., Ding B., Zhang F., Neurochem. Res. 2013, 38, 610. 10.1007/s11064-012-0959-y [DOI] [PubMed] [Google Scholar]
- 53. Han L., Li M., Vasc. Endovascular Surg. 2013, 47, 546. 10.1177/1538574413497106 [DOI] [PubMed] [Google Scholar]
- 54. Theerawatanasirikul S., Kuo C. J., Phetcharat N., Lekcharoensuk P., Antiviral Res. 2020, 174, 104697. 10.1016/j.antiviral.2019.104697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jukič M., Škrlj B., Tomšič G., Pleško S., Podlipnik Č., Bren U., Molecules 2021, 26, 3003. 10.3390/molecules26103003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiong Y., Zhu G. H., Wang H. N., Hu Q., Chen L. L., Guan X. Q., Li H. L., Chen H. Z., Tang H., Ge G. B., Fitoterapia 2021, 152, 104909. 10.1016/j.fitote.2021.104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen Z., Cui Q., Cooper L., Zhang P., Lee H., Chen Z., Wang Y., Liu X., Rong L., Du R., Cell Biosci. 2021, 11, 45. 10.1186/s13578-021-00564-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cherrak S. A., Merzouk H., Mokhtari‐Soulimane N., PLoS One 2020, 15, e0240653. 10.1371/journal.pone.0240653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dowlati Beirami A., Hatamabadi D., Iranpanah S., Rezaei M., Ziai S. A., Sch. Med. Stud. J. 2020, 2, 2. 10.22037/smsj.v2i3.31997 [DOI]
- 60. Derosa G., Maffioli P., D'Angelo A., Di Pierro F., Phytother. Res. 2021, 35, 1230. 10.1002/ptr.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Breitinger U., Ali N. K. M., Sticht H., Breitinger H. G., Front. Microbiol. 2021, 12, 692423. 10.3389/fmicb.2021.692423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bhutta M. S., Sausen D. G., Gallo E. S., Dahari H., Doncel G. F., Borenstein R., Pharmaceuticals 2021, 14, 980. 10.3390/ph14100980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kleemann R., Verschuren L., Morrison M., Zadelaar S., van Erk M. J., Wielinga P. Y., Kooistra T., Atherosclerosis 2011, 218, 44. 10.1016/j.atherosclerosis.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 64. Li Y., Yao J., Han C., Yang J., Chaudhry M. T., Wang S., Liu H., Yin Y., Nutrients 2016, 8, 167. 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fuentes J., de Camargo A. C., Atala E., Gotteland M., Olea‐Azar C., Speisky H., J. Agric. Food Chem. 2021, 69, 2157. 10.1021/acs.jafc.0c07085 [DOI] [PubMed] [Google Scholar]
- 66. Colunga Biancatelli R. M. L., Berrill M., Catravas J. D., Marik P. E., Front. Immunol. 2020, 11, 1451. 10.3389/fimmu.2020.01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Furuya A., Uozaki M., Yamasaki H., Arakawa T., Arita M., Koyama A. H., Int. J. Mol. Med. 2008, 22, 541. [PubMed] [Google Scholar]
- 68. May J. M., Harrison F. E., Antioxid. Redox Signaling 2013, 19, 2068. 10.1089/ars.2013.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thibane V. S., Ndhlala A. R., Finnie J. F., Van Staden J., S. Afr. J. Bot. 2019, 120, 198. 10.1016/j.sajb.2018.06.001 [DOI] [Google Scholar]
- 70. Malekmohammad K., Sewell R. D. E., Rafieian‐Kopaei M., Curr. Pharm. Des. 2020, 26, 2591. 10.2174/1381612826666200318152049 [DOI] [PubMed] [Google Scholar]
- 71. Freeman T. L., Swartz T. H., Front. Immunol. 2020, 11, 1518. 10.3389/fimmu.2020.01518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Saeedi‐Boroujeni A., Mahmoudian‐Sani M.‐R., J. Inflamm. 2021, 18, 3. 10.1186/s12950-021-00268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Di Pierro F., Iqtadar S., Khan A., Ullah Mumtaz S., Masud Chaudhry M., Bertuccioli A., Derosa G., Maffioli P., Togni S., Riva A., Allegrini P., Khan S., Int. J. Gen. Med. 2021, 14, 2807. 10.2147/IJGM.S318949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Khazdair M. K., Anaeigoudari A., Agbor G., Asian Pac. J. Trop. Biomed. 2021, 11, 327. 10.4103/2221-1691.319567 [DOI] [Google Scholar]
- 75. Weatherhead J. E., Clark E., Vogel T. P., Atmar R. L., Kulkarni P. A., J. Clin. Invest. 2020, 130, 6194. 10.1172/JCI145301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen J. S., Alfajaro M. M., Wei J., Chow R. D., Filler R. B., Eisenbarth S. C., Wilen C. B., bioRxiv 2020. 10.1101/2020.09.24.312769 [DOI]
- 77. Son J. K., Son M. J., Lee E., Moon T. C., Son K. H., Kim C. H., Kim H. P., Kang S. S., Chang H. W., Biol. Pharm. Bull. 2005, 28, 2181. 10.1248/bpb.28.2181 [DOI] [PubMed] [Google Scholar]
- 78. Babayigit A., Olmez D., Karaman O., Ozogul C., Yilmaz O., Kivcak B., Erbil G., Uzuner N., Allergy Asthma Proc. 2009, 30, 186. 10.2500/aap.2009.30.3187 [DOI] [PubMed] [Google Scholar]
- 79. Wang F., Huang J., Li J., Chen K., Zhang X., Zhang Y., Zhu Y., Pharmacogn. Mag. 2021, 17, 163. 10.4103/pm.pm_448_20 [DOI] [Google Scholar]
- 80. Liu T., Jiang S., Jia S., Fan F., Curr. Top. Nutraceutical Res. 2021, 19, 227 [Google Scholar]
- 81. Kaushik D., Bhandari R., Kuhad A., Expert Opin. Ther. Targets 2021, 25, 491. 10.1080/14728222.2021.1918103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gholami M., Nozarnezhad R., Motaghinejad M., Iran. J. Med. Sci. 2021, 46, 228. 10.30476/ijms.2021.88513.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gour A., Manhas D., Bag S., Gorain B., Nandi U., Phytother. Res. 2021, 35, 4258. 10.1002/ptr.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Al‐Amri J. S., Hagras M. M., Mohamed I. M., Indian J. Exp. Biol. 2013, 51, 357. [PubMed] [Google Scholar]
- 85. Al‐Kuraishy H. M., Al‐Gareeb A. I., Alblihed M., Guerreiro S. G., Cruz‐Martins N., Batiha G. E., Front. Cardiovasc. Med. 2021, 8, 644095. 10.3389/fcvm.2021.644095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. South A. M., Brady T. M., Flynn J. T., Hypertension 2020, 76, 16. 10.1161/HYPERTENSIONAHA.120.15291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jiang H., Qu P., Exp. Ther. Med. 2017, 14, 5857. 10.3892/etm.2017.5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li L., Yang L., Yang F., Zhao X. L., Xue S., Gong F. H., J. Inflamm. Res. 2021, 14, 1959. 10.2147/JIR.S302934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu T. J., Yeh Y. C., Ting C. T., Lee W. L., Wang L. C., Lee H. W., Wang K. Y., Lai H. C., Lai H. C., Cardiovasc. Res. 2008, 80, 227. 10.1093/cvr/cvn192 [DOI] [PubMed] [Google Scholar]
- 90. Lu S., Guo X., Zhao P., Molecules 2011, 16, 9194. 10.3390/molecules16119194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. He G. Y., Yuan C. G., Hao L., Xu Y., Zhang Z. X., Evidence‐Based Complementary Altern. Med. 2014, 2014, 368598. 10.1155/2014/368598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu F. Q., Gao Q., Wang D. D., Zhang Z. X., Zhongguo Zhongyao Zazhi 2018, 43, 3346. 10.19540/j.cnki.cjcmm.20180504.001 [DOI] [PubMed] [Google Scholar]
- 93. Liskova A., Samec M., Koklesova L., Samuel S. M., Zhai K., Al‐Ishaq R. K., Abotaleb M., Nosal V., Kajo K., Ashrafizadeh M., Zarrabi A., Brockmueller A., Shakibaei M., Sabaka P., Mozos I., Ullrich D., Prosecky R., La Rocca G., Caprnda M., Büsselberg D., Rodrigo L., Kruzliak P., Kubatka P., Biomed. Pharmacother. 2021, 138, 111430. 10.1016/j.biopha.2021.111430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jialiken D., Qian L., Ren S., Wu L., Xu J., Zou C., Medicine 2021, 100, e25852. 10.1097/MD.0000000000025852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu Y. Z., Li S. Q., Zu X. G., Du J., Wang F. F., Phytother. Res. 2008, 22, 734. 10.1002/ptr.2335 [DOI] [PubMed] [Google Scholar]
- 96. Guan S. P., Seet R. C. S., Kennedy B. K., Ageing Res. Rev. 2020, 64, 101201. 10.1016/j.arr.2020.101201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., Ghiladi R. A., Wang J., Free Radic. Biol. Med. 2021, 163, 153. 10.1016/j.freeradbiomed.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Farhangrazi Z. S., Moghimi S. M., Precis. Nanomed. 2020, 3, 622. 10.33218/001c.13525 [DOI] [Google Scholar]
- 99. Grimes J. M., Grimes K. V., J. Mol. Cell. Cardiol. 2020, 144, 63. 10.1016/j.yjmcc.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Y., Zhang Y., Wen M., Zhang J., Zhao X., Zhao Y., Deng J., Mol. Med. Rep. 2017, 16, 3657. 10.3892/mmr.2017.6999 [DOI] [PubMed] [Google Scholar]
- 101. Chen T. R., Wei L. H., Guan X. Q., Huang C., Liu Z. Y., Wang F. J., Hou J., Jin Q., Liu Y. F., Wen P. H., Zhang S. J., Ge G. B., Guo W. Z., Bioorg. Chem. 2019, 92, 103199. 10.1016/j.bioorg.2019.103199 [DOI] [PubMed] [Google Scholar]
- 102. Giri H., Cai X., Panicker S. R., Biswas I., Rezaie A. R., Int. J. Mol. Sci. 2019, 20. 10.3390/ijms20081851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Koch E., Phytomedicine 2005, 12, 10. 10.1016/j.phymed.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 104. Koltermann A., Hartkorn A., Koch E., Fürst R., Vollmar A. M., Zahler S., Cell. Mol. Life Sci. 2007, 64, 1715. 10.1007/s00018-007-7085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Al‐Kuraishy H. M., Al‐Gareeb A. I., Qusti S., Alshammari E. M., Gyebi G. A., Batiha G. E., ASN Neuro 2021, 13, 17590914211057635. 10.1177/17590914211057635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Andersson U., Mol. Med. 2020, 26, 64. 10.1186/s10020-020-00184-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Porzionato A., Emmi A., Barbon S., Boscolo‐Berto R., Stecco C., Stocco E., Macchi V., De Caro R., FEBS J. 2020, 287, 3681. 10.1111/febs.15481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee B. H., Choi S. H., Shin T. J., Pyo M. K., Hwang S. H., Lee S. M., Paik H. D., Kim H. C., Nah S. Y., Eur. J. Pharmacol. 2011, 650, 79. 10.1016/j.ejphar.2010.09.079 [DOI] [PubMed] [Google Scholar]
- 109. Nedumpully‐Govindan P. et al., Phys. Chem. Chem. Phys. 2016, 18, 94. 10.1039/c5cp05924k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ivic L., Sands T. T. J., Fishkin N., Nakanishi K., Kriegstein A. R., Strømgaard K., J. Biol. Chem. 2003, 278, 49279. 10.1074/jbc.M304034200 [DOI] [PubMed] [Google Scholar]
- 111. Spiegel R., Kalla R., Mantokoudis G., Maire R., Mueller H., Hoerr R., Ihl R., Clin. Interv. Aging. 2018, 13, 1121. 10.2147/CIA.S157877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Alsmadi A. M., Tawalbeh L. I., Gammoh O. S., Shawagfeh M. Q., Zalloum W., Ashour A., Attarian H., Andersson U., Mol Med. 2020, 26, 64. 10.1186/s10020-020-00184-0 [DOI] [Google Scholar]
- 113. Geisen U. M., Berner D. K., Tran F., Sümbül M., Vullriede L., Ciripoi M., Reid H. M., Schaffarzyk A., Longardt A. C., Franzenburg J., Hoff P., Schirmer J. H., Zeuner R., Friedrichs A., Steinbach A., Knies C., Markewitz R. D., Morrison P. J., Gerdes S., Schreiber S., Hoyer B. F., Ann. Rheum. Dis. 2021, 80, 1306. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Khanna K., Kohli S. K., Kaur R., Bhardwaj A., Bhardwaj V., Ohri P., Sharma A., Ahmad A., Bhardwaj R., Ahmad P., Phytomedicine 2021, 85, 153361. 10.1016/j.phymed.2020.153361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Al‐Kuraishy H. M., Al‐Gareeb A. I., Alkazmi L., Alexiou A., Batiha G. E., Viral Immunol. 2021, 34, 722. 10.1089/vim.2021.0042 [DOI] [PubMed] [Google Scholar]
- 116. Huang Q. C., He Y. Q., Li Y., Yang X. Y., Chin. Pharmacol. Bull. 2010, 26, 278. [Google Scholar]
- 117. Yu L., Feng L., Xiong L., Li S., Xu Q., Pan X., Xiao Y., Nanoscale 2021, 13, 11188. 10.1039/d1nr02036f [DOI] [PubMed] [Google Scholar]
- 118. Varahachalam S. P., Lahooti B., Chamaneh M., Bagchi S., Chhibber T., Morris K., Bolanos J. F., Kim N. Y., Kaushik A., Int. J. Nanomedicine. 2021, 16, 539. 10.2147/IJN.S283686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dubey A. K., Chaudhry S. K., Singh H. B., Gupta V. K., Kaushik A., Biotechnol. Rep. 2022, 33, e00712. 10.1016/j.btre.2022.e00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Paliwal P., Sargolzaei S., Bhardwaj S. K., Bhardwaj V., Dixit C., Kaushik A., Front. Nanotechnol. 2020, 2. 10.3389/fnano.2020.571284 [DOI] [Google Scholar]
- 121. Sadique M. A., Yadav S., Ranjan P., Verma S., Salammal S. T., Khan M. A., Kaushik A., Khan R., J. Mater. Chem. B 2021, 9, 4620. 10.1039/d1tb00472g [DOI] [PubMed] [Google Scholar]
- 122. Gage A., Brunson K., Morris K., Wallen S. L., Dhau J., Gohel H., Kaushik A., Front. Nanotechnol. 2021, 3. 10.3389/fnano.2021.700888 [DOI] [Google Scholar]
- 123. Tiwari S., Juneja S., Ghosal A., Bandara N., Khan R., Wallen S. L., Ramakrishna S., Kaushik A., Curr. Opin. Biomed. Eng. 2021, 21, 100363. 10.1016/j.cobme.2021.100363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kaushik A., Expert. Opin. Drug. Deliv. 2021, 18, 531. 10.1080/17425247.2021.1860938 [DOI] [PubMed] [Google Scholar]


