Abstract
Background
Indirect calorimetry (IC) is the gold standard for measuring resting energy expenditure. Energy expenditure (EE) estimated by ventilator‐derived carbon dioxide consumption (EEVCO2) has also been proposed. In the absence of IC, predictive weight‐based equations have been recommended to estimate daily energy requirements. This study aims to compare simple predictive weight‐based equations with those estimated by EEVCO2 and IC in mechanically ventilated patients of COVID‐19.
Methods
Retrospective study of a cohort of critically ill adult patients with COVID‐19 requiring mechanical ventilation and artificial nutrition to compare energy estimations by three methods through the calculation of bias and precision agreement, reliability, and accuracy rates.
Results
In 58 mechanically ventilated patients, a total of 117 paired measurements were obtained. The mean estimated energy derived from weight‐based calculations was 2576 ± 469 kcal/24 h, as compared with 1507 ± 499 kcal/24 h when EE was estimated by IC, resulting in a significant bias of 1069 kcal/day (95% CI [−2158 to 18.7 kcal]; P < 0.001). Similarly, estimated mean EEVCO2 was 1388 ± 467 kcal/24 h when compared with estimation of EE from IC. A significant bias of only 118 kcal/day (95% CI [−187 to 422 kcal]; P < 0.001), compared by the Bland‐Altman plot, was noted.
Conclusion
The energy estimated with EEVCO2 correlated better with IC values than energy derived from weight‐based calculations. Our data suggest that the use of simple predictive equations may potentially lead to overfeeding in mechanically ventilated patients with COVID‐19.
Keywords: COVID‐19, EEVCO2 , energy expenditure, indirect calorimetry, predictive equation
CLINICAL RELEVANCY STATEMENT
Both overfeeding and underfeeding of patients in the intensive care unit are associated with worse outcomes. Ideally, the individual energy target is based on the frequent assessment of energy expenditure (EE). Indirect calorimetry (IC) is considered the gold standard but is not always available. EE estimated by ventilator‐derived carbon dioxide consumption (EEVCO2), derived from ventilator and stand‐alone monitors, has also been proposed as an alternative. Guidelines recommend predictive weight‐based dosing when IC is not feasible to estimate daily energy requirements. This study was able to prove that guideline‐recommended weight‐based calculations overestimated the energy requirements, and we were able to arrive at an energy estimation that can be closer to the EE with IC and EEVCO2 among patients with COVID‐19. This study would help in standardizing the more commonly used weight‐based calculations for energy estimation.
INTRODUCTION
The novel SARS‐CoV‐2 virus infection, in its severe form, clinically resembles acute respiratory distress syndrome (ARDS), pneumonia, and septic shock and often necessitates mechanical ventilation. 1 Severe COVID‐19 is complex interplay between the virus and host immune system characterized by a dysregulated immunopathological response, resulting in a hyperinflammatory effect. Many authors have argued that this hyperinflammation and hypermetabolic phase is the primary cause of raised energy, protein, and nutrition requirements. 2 , 3 , 4 Weight loss is noted in this group of patients as a result of the extended inflammatory state and changing energy expenditures (EEs). 5 The optimal initial energy target in critically ill patients in the intensive care unit (ICU) remains controversial 6 , 7 , 8 and confusing among the novel set of critically ill patients with COVID‐19. Nonetheless, accurate EE estimation is critical to avoid overfeeding and underfeeding, both of which are linked to higher mortality and poor outcomes. 9 , 10 , 11 Few research articles have explored EE among patients with COVID‐19. One study by Stapel et al compared the EE estimated by ventilator‐derived carbon dioxide consumption (EEVCO2) with the EE from indirect calorimetry (IC) among critically ill patients without COVID‐19 and found EEVCO2 reasonably accurate and more precise than other predictive equations, 12 but data in mechanically ventilated patients with COVID‐19 are sparse.
Whittle et al, in their longitudinal observational study, described EE in 22 ventilated patients with COVID‐19 measured by IC. 13 Interestingly, measured EE increased progressively from week 1 (1568 kcal/day, range 1175–2215 kcal/day) to week 2 (1830 kcal/day, range 1465–2467 kcal/day) and week 3 (2789 kcal/day, range 1776–3262 kcal/day). 13 , 14 In contrast, in a retrospective case series of seven mechanically ventilated patients, the median EE measured by IC was 4044 kcal/day, which was 235.7 ± 51.7% of that predicted by the Penn State equations. With these variations in EE among patients with COVID‐19 recorded by IC, which is a gold standard, use of predictive weight‐based equations as per guidelines may be inadequate to estimate energy requirements. Additionally, the American Society for Parenteral and Enteral Nutrition (ASPEN) 2016 15 guidelines indicate that >200 predictive equations have been published in research articles, with accuracy ranging from 40% to 75% when compared with IC, and no single equation emerges as being more accurate in an ICU setting. Owing to the large variability of measurements, and in the absence of IC, EEVCO2 may give a better estimation of EE than predictive equations. 16 Most studies conducted in non–COVID‐19 patients (both adults and children) showed significant correlation between EE values obtained by IC and VCO2‐based calorimetry. 12 , 17 , 18 COVID‐19 leads to significant EE/metabolic changes, systemic mitochondrial dysfunction, significant muscle wasting, and loss of function throughout the course of illness and during recovery. We hypothesize metabolic needs will initially decrease in acute illness and subsequently increase as patients transition from the acute phase of the COVID‐19 illness to recovery phases.
This study aimed to retrospectively compare the accuracy of measured EEVCO2 and weight‐based predictive equations in mechanically ventilated, critically ill adult patients with COVID‐19 with EE by IC.
METHODS
The aimed to retrospectively compare whether the VCO2 values and body weight–based calculations used for the EE estimations are comparable to IC (reference standard), which allows accurate measurement of resting EE (REE).
We performed a retrospective observational study in invasively ventilated critically ill patients with COVID‐19 receiving artificial nutrition at a tertiary care hospital. Patients were selected per the criteria, as listed in Figure 1.
Figure 1.
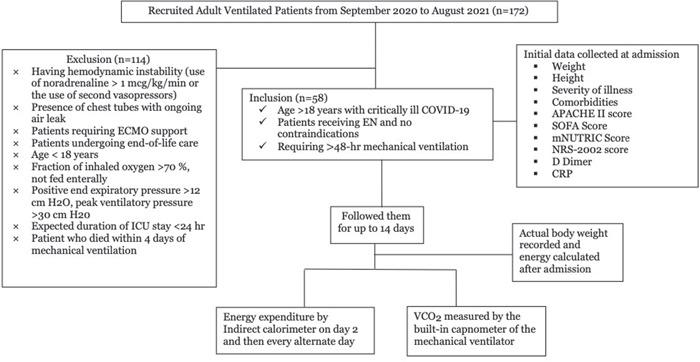
Flow diagram of patients selected for analysis. APACHE, Acute Physiology and Chronic Health Evaluation; CRP, C‐reactive protein; EN, enteral nutrition; ICU, intensive care unit; mNUTRIC, modified Nutrition Risk in Critically Ill; SOFA, Sequential Organ Failure Assessment
Methods of deriving the EE were as follows
No measurements for VCO2 or REE were taken when there were changes made in vasoactive agent dose (>20%, <1 h before or during IC), change in sedative/analgesic dose (>20%, <1 h before and/or during IC), or change in body temperature (>0.5°C, <1 h before and/or during IC).
Assessment of EE derived from ventilator
For each patient, the mean VCO2 was measured by the built‐in capnometer of the mechanical ventilator Carescape R 860, with the help of the metabolic monitor E‐sCOVX (GE Healthcare). The measurement was taken when steady state was achieved, which was defined as the value at which the coefficient of variation for carbon dioxide production and oxygen consumption was <5% for a period of 30 min. These parameters can be generated from a software algorithm in the Carescape R 860 ventilator.
The VCO2 (milliliters per minute) measured by the ventilator was then used to derive the EE using the formula 8.19 × VCO2 ml/min by using a modification of the Weirs formula and an respiratory quotient (RQ) of 0.86. The RQ was assumed as 0.86 because this is usually the RQ of the commonly used nutrition products in our unit. 12 , 19
Assessment of EE derived from weight‐based calculations
EE was calculated as 30 kcal/kg/day per the European Society for Clinical Nutrition and Metabolism (ESPEN) expert statements referred to in the guidelines. 16
Assessment of EE derived from IC
The EE was measured using the E‐sCOVX metabolic monitor (indirect calorimeter) on the CARESCAPE R860 ventilator, which measured VCO2, VO2, the coeffecient of variation of both VCO2 and VO2, and the respiratory quotient.
The steady state was determined, as shown in point 1, and the value of EE was taken when the respiratory quotient was found to be 0.7–0.9 in the same 30‐min window. All patients were started on feeds only after measurement of the last 24‐h EE. This measurement was done once in 2 days. We recorded the first IC measurement on day 2 after ICU admission and then, later, every alternate day while the patients were in the ICU, up to 10–14 days.
Data collection
Initial descriptive data were collected at the time of admission for each patient, including age, predicted body weight per the ARDS net strategy, 20 height, diagnosis, severity of illness, comorbidities, length of ICU stay, Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation III score, modified Nutrition Risk in Critically Ill (mNUTRIC) score, and Nutrition Risk Screening 2002 (NRS‐2002) score. Markers of inflammation, CRP, D‐Dimer, LDH, and ferritin were collected from the patient chart.
Patients were classified as having a high NRS‐2002 score (≥3) and low (<3) risk of malnutrition. Patients were also classified as having either a high (≥5) or low (<5) mNUTRIC score.
Statistical analysis
All data were reported as means ± SD. The EE derived from the two methods were compared by Bland‐Altman plots. Reliability and adequacy (sensitivity, specificity, and positive and negative predictive values) between the methods were tested using receiver operating characteristic curves with к coefficient (reliability coefficient). For coefficient of variation, analysis of variance was used when applicable.
Analysis of descriptive statistics was computed for measured EE by IC, VCO2, and predictive weight‐based equations, and Bland‐Altman analysis was utilized to quantify the mean bias and limits of agreement between devices. Acceptable limits of agreement between the devices was taken as ±20%. VO2 and VCO2 were reported as means (SDs). Sensitivity and specificity tests were also conducted for each of the methods to test the accuracy of the predictive equations relative to the reference standard of IC. 21 Sensitivity and specificity tests are conducted to evaluate the interrater agreement between two methods. Sensitivity rates the level of abnormality between the two methods, and, in contrast, specificity shows the percentage of normality between the two methods.
We performed a primary analysis evaluating the performance of the EEVCO2 compared with EE measured by IC through the determination of accuracy, agreement, reliability, and 10% accuracy rates. Similar tests were conducted with weight‐based energy estimations and EE measured by IC. Each of the energy estimations were also compared with the hospital length of stay to identify any association.
Descriptive data were also computed as median, interquartile range and percentages when appropriate. A P value of <0.05 was considered statistically significant. IBM SPSS Statistics for Windows, version 24.0 (IBM Corporation) was used to perform analyses. MedCalc version 19 (MedCalc bv) was used to create Bland‐Altman plots.
RESULTS
All hospitalized patients with a laboratory‐confirmed diagnosis of COVID‐19 by reverse transcription polymerase chain reaction between September 2020 and August 2021 who were admitted to the ICU were included in the study. Institutional ethical committee approval was obtained for the study (ECR/70/Inst/MH/2013/RR‐19).
During the study period, 450 patients with COVID‐19 were admitted to the ICU. A total of 172 patients were ventilated, of whom 58 were eligible for inclusion and 114 were excluded. Among the excluded patients, 72 had 100% oxygen requirements for >6 days, and in the remaining 42 patients, 12 had pneumothoraxes and 30 died within 4 days of mechanical ventilation (Figure 1).
Baseline characteristics, nutrition, and ventilatory parameters are shown in Table 1. There were 117 measurements that were conducted among the 58 patients with COVID‐19.
Table 1.
Clinical, nutrition, and ventilatory characteristics of patients during measurements in the ICU
| Days of EE recording by IC after admission | ||||
|---|---|---|---|---|
| 0–3 ICU days | 4–7 ICU days | ≥8 ICU days | P value | |
| Total number of patients included from ICU | 58 | |||
| Number of patients, n (%) | 3 (5) | 13 (23) | 42 (72) | – |
| Number of measurements in ICU after admission (n = 117), n (%) | 49 (42) | 58 (50) | 10 (8) | – |
| Age, years, mean + SD | 58.8 ± 15.0 | 61.76 ± 14.34 | 59.85 ± 16.1 | 0.642 |
| BMI, mean + SD | 24.55 ± 3.33 | 24.95 ± 3.6 | 25.36 ± 4.66 | 0.663 |
| Weight, kg, mean + SD | 66.7 ± 10.11 | 67.17 ± 10.6 | 68.42 ± 15.69 | 0.828 |
| Height, cm, mean + SD | 165.08 ± 6.9 | 164.07 ± 6.5 | 163.77 ± 7.9 | 0.684 |
| NRS score (low [<3], high [≥3]), mean + SD | 4.0 ± 0.645 | 4.12 ± 0.67 | 4.08 ± 0.74 | 0.698 |
| mNUTRIC score (low [0–4], high [5−9]), mean + SD | 6.22 ± 1.23 | 6.57 ± 1.33 | 6.69 ± 1.41 | 0.261 |
| Baseline APACHE II, mean + SD | 23.3 ± 4.56 | 24.1 ± 4.52 | 23.96 ± 4.15 | 0.673 |
| SOFA score, mean + SD | 9.0 ± 2.65 | 7.6 ± 3.68 | 6.85 ± 3.6 | 0.470 |
| VO2 (ml/min), mean + SD | 226 ± 93.51 | 221 ± 56.72 | 231 ± 62 | 0.873 |
| VCO2 (ml/min), mean + SD | 169.5 ± 66.9 | 167.4 ± 51.4 | 172.5 ± 47.7 | 0.937 |
| EE by IC (kcal/day), mean + SD | 1489.45 ± 606.6 | 1501.9 ± 406.5 | 1547.6 ± 424.1 | 0.890 |
| EE by VCO2‐based predictive equation (kcal/day), mean + SD | 1389.98 ± 548 | 1372.33 ± 422 | 1414.82 ± 390.9 | 0.937 |
| EE by weight‐based predictive equation (kcal/day), mean + SD | 2549.6 ± 527.7 | 2564.7 ± 440.3 | 2645.5 ± 401.7 | 0.690 |
| CRP, mean + SD | 121.3 ± 96.9 | 121.5 ± 90.8 | 91.9 ± 61.3 | 0.403 |
| D‐Dimer, mean + SD | 4.1 ± 4.9 | 5.6 ± 10.5 | 1.0 ± 0.53 | 0.307 |
| LDH, mean + SD | 613.9 ± 318.6 | 587.3 ± 290 | 413.3 ± 153.5 | 0.060 |
| Ferritin, mean + SD | 1543.8 ± 1763.4 | 1278.2 ± 1199.5 | 1064.1 ± 632.8 | 0.538 |
| LOS, inpatient days, mean + SD | 3.0 ± 0 | 5.3 ± 0.93 | 20.5 ± 10.3 | <0.001 |
| Median (interquartile range), LOS | 3 | 5 (4.5–6) | 18 (11.5–30) | <0.001 |
| MV h, mean + SD | 204.8 ± 199.8 | 253.6 ± 206.7 | 413.5 ± 193.9 | <0.001 |
| Number of comorbidities | ||||
| Nil | 17 | 11 | 6 | |
| ≤2 | 21 | 23 | 15 | |
| ≥3 | 11 | 8 | 5 | |
Note: BMI calculated as weight in kilograms divided by height in meters squared.
Abbreviations: APACHE, acute physiology and chronic health evaluation; BMI, body mass index; CRP, C‐reactive protein; EE, energy expenditure; IC, indirect calorimetry; ICU, intensive care unit; LOS, length of stay; mNUTRIC, modified Nutrition Risk in Critically Ill; MV, mechanical ventilation; NRS, nutritional risk screening; SOFA, sequential organ failure assessment; VCO2, carbon dioxide consumption.
Of the 58 patients, 76% were male (n = 44) and 24% were female (n = 14), with a mean age of 60.09 ± 14.95 years and body mass index of 31.76 ± 5.3 (calculated as weight in kilograms divided by height in meters squared). Among these patients, 18 did not present with any comorbidities, 26 had ≤2 comorbidities, and 14 had ≥3 comorbidities. The mean NRS‐2002 and mNUTRIC scores were 4.06 ± 0.67 and 6.45 ± 1.31, respectively, wherein both indicate that patients were at risk for malnutrition (Table 1).
The estimated mean EE derived from actual body weight–based calculations was 2576 ± 469 kcal/24 h, which was significantly higher when compared with that derived from IC (1507 ± 499 kcal/24 h). This resulted in a significant bias of 1069 kcal/day (95% CI, −2158 to 18.7 kcal; P < 0.001). Bias and precision, as visualized by the limits of agreement between the two methods, are shown in the Bland‐Altman plot in Figure 2, in which the difference is wide but the limits of agreement are within the range. The regression analyses reveal that for every 1‐U change in EE calculated by weight‐based estimation, there is only 0.37‐U change in EE by IC. This correlation is significant but not useful for prediction (r = 0.345; r 2 = 0.119).
Figure 2.
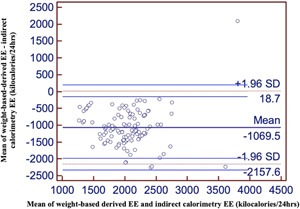
Bland‐Altman plot of EE derived by weight‐based calculations and EE by IC. EE, energy expenditure; EEWB, energy expenditure calculated with weight‐based calculations; IC, indirect calorimetry
The estimated mean EEVCO2 was 1388 ± 467 kcal/24 h compared with an estimation of EE from IC of 1507 ± 499 kcal/24 h. The bias and precision, as visualized by the limits of agreement, are shown in the Bland‐Altman plot in Figure 3, in which there was a significant bias of only 118 kcal/day (95% CI, −187 to 422 kcal; P < 0.001). The regression analyses reveal that for every 1‐U change in EEVCO2 value, there is a 1‐U change in EE by IC. This correlation is significant (r = 0.951; r 2 = 0.904). Similarly, the Bland‐Altman plot tested between estimated mean EEVCO2 and EE derived from weight‐based calculations showed a wide difference, with significant bias of 1187 kcal/day (95% CI −2256 to −118 kcal; P < 0.001).
Figure 3.
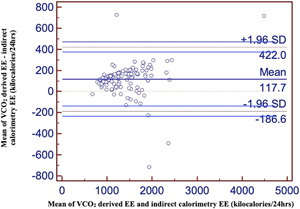
Bland‐Altman plot of EEVCO2 and EE by IC. EE, energy expenditure; EEVCO2, energy expenditure calculated with ventilator‐derived carbon dioxide production; IC, ventilator‐derived indirect calorimetry
The sensitivity test shows an abnormality of 0.629 (62.9%) (95% CI, 0.4969 to 0.7484) between the two methods, and the specificity test shows a normal value of 0.6364 (63.6%) (95% CI, 0.4956 to 0.7619) between the two methods. Sensitivity and specificity were also used to identify a new cutoff value with the standard EE by IC. When we consider the EE as 1500 kcal/day, the area under the curve on sensitivity and specificity is 0.662 (95% CI, 0.565 to 0.761; P = 0.002). The corresponding EE by weight‐based calculations for the given 1500 kcal/day by IC is 2618 kcal/day, with sensitivity of 0.621 and specificity of 0.610. When we consider EE as 1800 kcal/day, the area under the curve did not improve much, which is 0.630 (95% CI, 0.515 to 0.746; P = 0.053). When we consider the EE as 1400 kcal/day, the area under the curve on sensitivity and specificity is 0.978 (95% CI, 0.948 to 1.000; P < 0.001), which is significant. Since 1500 kcal/day, which is the mean EE by IC, is closer to the new cutoff value 1400 kcal/day, we can consider 1400 kcal/day as the mean requirement, which is also the same EE when computed by VCO2 that has significantly correlated.
Therefore, mean EE by IC was further categorized as <1400 and ≥1400 kcal/day and cross‐tabulated with the corresponding mean EE by weight‐based calculations as <2600 and ≥2600 kcal/day (Table 2). The cross‐tabulation showed statistically significant association when all 117 measurements were compared between the groups (P = 0.004). The measurements were not statistically significant when compared for 1–3 and 4–7 days, but in contrast, there was statistically significant association for measurements during ≥8 days of hospital stay (P = 0.003) (Table 2). Similar cross‐tabulation was done between EE by IC and EEVCO2. We had 54 (98%) patient readings in the category of <1400 kcal/day, and 54 (87%) patient readings in which the energy was categorized as ≥1400 kcal/day by both methods, indicating that energy estimations of EE by IC and EEVCO2 significantly correlated (P < 0.001).
Table 2.
Cross‐tabulation of the number of patient measurements with mean EE by IC and mean EE by weight‐based calculations.
| Days of recording the EE | Mean EE by IC kcal/day | Number of patient measurements with corresponding EE by weight‐based calculations, n (%) | P value | |
|---|---|---|---|---|
| <2600 kcal/day | ≥2600 kcal/day | |||
| Total days | <1400 | 35 (64) | 20 (36) | 0.004 |
| ≥1400 | 23 (37) | 39 (63) | ||
| 0–3 ICU days | <1400 | 16 (61) | 10 (39) | 0.336 |
| ≥1400 | 11 (48) | 12 (52) | ||
| 4–7 ICU days | <1400 | 12 (63) | 7 (37) | 0.204 |
| ≥1400 | 10 (43) | 13 (57) | ||
| ≥8 ICU days | <1400 | 7 (70) | 3 (30) | 0.003 |
| ≥1400 | 2 (12) | 14 (88) | ||
Abbreviations: EE, energy expenditure; IC, indirect calorimetry; ICU, intensive care unit.
In the next step of data analysis, we compared the length of hospital stay when the estimated energy was <1400 and ≥1400 kcal/day and when it was <2600 and ≥2600 kcal/day. When the estimated energy was <1400 and ≥1400 kcal/day, the length of hospital stay was 16.6 ± 9.89 and 19.45 ± 12.01 days, respectively. It is observed that there was a statistically significant difference in the length of hospital stay of 14.3 ± 8.93 and 21.8 ± 11.83 days (P < 0.001) when the energy estimation was <2600 and ≥2600 kcal/day, respectively, indicating that the patients were hypermetabolic and probably more catabolic, resulting in longer length of hospital stay.
DISCUSSION
We retrospectively compared the energy estimations with weight‐based equations with the EE measured by IC using 117 measurements among 58 adult critically ill patients with COVID‐19. The estimation of energy by weight‐based equations in this study was poor, shown by a large bias of 1069 kcal when compared with EE by IC. When these data were segregated according to the day of EE recording after admission into 1–3, 4–7, and ≥8 days, they further revealed that 1–3 and 4–7 days showed no agreement and association (P = 0.336, 0.204, respectively). But as the mean of both the measurements during ≥8 days increased, the trend also increased in the Bland‐Altman plots and was with significant agreement and association (P = 003). From this, we can understand that the EE estimations by weight‐based calculations are overestimated for the first 1 week and that they may correlate with the EE by IC after the first week. A similar trend has been shown in the study by Whittle et al. 13 The ASPEN guidelines 22 for critically ill patients with COVID‐19 specify that the initial energy goal can be 15–20 kilocalories per kilogram of actual body weight per day and ESPEN guidelines recommend 27–30 kcal/kg/day. 16 , 23 The estimated mean EE derived from weight‐based calculations was 2576 ± 469 kcal/24 h, which was significantly higher when compared with an estimation of EE from IC of 1507 ± 499 kcal/24 h. This indicates that weight‐based energy calculations may lead to overfeeding among the patients. The mean estimated energy was 1718 ± 313 and 2147 ± 391 kcal/24 h when the weight‐based calculations were recalculated according to 20 kcal and 25 kcal/kg actual body weight. The energy estimation with 20 kcal/kg body weight is closer to the EE by IC value. Taking into consideration the findings of our study, it is our opinion that it may be appropriate to feed at 20 kcal/kg body weight for the first week after admission and then subsequently increase it thereafter.
The study by Pey‐Jen Yu 2 showed that patients with COVID‐19 have EEs >200% of REE. This possibly correlates with the fever and inflammatory state in COVID‐19. Although the study by Pey‐Jen Yu 2 showed that patients with COVID‐19 had EEs >200% of REE, the EE either by VCO2 or IC did not indicate the same in our study. However, the difference can also be due to the device itself, calibration issues, and condition of measurements, as compared with that employed in the current study. 24 It is noted that the mean EEVCO2 energy estimation was quite close to the EE by IC, with a small bias of only 118 kcal. Reliability between EEVCO2 and EE by IC was good, suggesting that EECO2 energy estimation can be used as the best alternative to EE by IC. 12 , 25
There are a few strengths of this study. This is the largest study using IC to date in this group of mechanically ventilated patients with COVID‐19 done during the trying times of the pandemic. We were able to include a specific group of patients with COVID‐19 with the same immunopathology (ARDS), as compared with a varied group of critically ill patients.
The study did have certain limitations. We used the RQ as a fixed value for the calculation of REE, with the help of the VCO2 measurement. Because of logistical reasons, calculating RQ (using an individual ratio of carbohydrate, fat, and protein) from the prescribed diet was not done. This could have led to inherent inaccuracy while using the VCO2 for calculating EE. Nevertheless, many experts do recommend the EE derived from VCO2, assuming an RQ of 0.85, rather than applying predictive equations to reduce overfeeding and underfeeding. 26 This study was performed with one type of mechanical ventilator by establishing a steady state and hence may not be comparable with other ventilators. This is a single‐center study, and a larger multicenter study will help to validate our results. We did not study the REE after the patient was extubated and shifted to the wards. This would have also helped to understand the EE in the recovery period.
CONCLUSION
The energy estimated with EEVCO2 correlated better with IC values than energy derived from weight‐based calculations. Our data suggest that the use of simple predictive equations may potentially lead to overfeeding in mechanically ventilated patients with COVID‐19.
AUTHOR CONTRIBUTIONS
Sanjith Saseedharan contributed to the conception and design of the study; Radha R. Chada, Sanjith Saseedharan, Vaijayanti Kadam, Annapurna Chiluka, and Balakrishna Nagalla contributed to the acquisition, analysis, and interpretation of data and critically revised the manuscript; Radha R. Chada and Sanjith Saseedharan drafted the manuscript. All the authors gave final approval. All authors revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
CONFLICTS OF INTEREST
None declared.
Saseedharan S, Chada RR, Kadam V, Chiluka A, Nagalla B. Energy expenditure in COVID‐19 mechanically ventilated patients: a comparison of three methods of energy estimation. J Parenter Enteral Nutr. 2022;1‐8. 10.1002/jpen.2393
REFERENCES
- 1. Lal A, Mishra AK, Sahu KK. CT chest findings in coronavirus disease‐19 (COVID‐19). J Formos Med Assoc. 2020;119(5):1000‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu P‐C, Cassiere H, DeRosa S, Bocchieri K, Yar S, Hartman A. Hypermetabolism and coronavirus disease 2019. JPEN J Parenter Enteral Nutr. 2020;44(7):1234‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uno C, Maeda K, Wakabayashi H, et al. Nutritional status change and activities of daily living in elderly pneumonia patients admitted to acute care hospital: a retrospective cohort study from the Japan Rehabilitation Nutrition Database. Nutrition. 2020;71(x 110613). [DOI] [PubMed] [Google Scholar]
- 4. Tan LY, Komarasamy TV, RMT Balasubramaniam V. Hyperinflammatory immune response and COVID‐19: a double edged sword. Front Immunol. 2021;12:742941. 10.3389/fimmu.2021.742941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gualtieri P, Falcone C, Romano L, et al. Body composition findings by computed tomography in SARS‐CoV‐2 patients: increased risk of muscle wasting in obesity. Int J Mol Sci. 2020;21(13):4670. 10.3390/ijms21134670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506‐517. [DOI] [PubMed] [Google Scholar]
- 7. Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full‐energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med. 2011;39(5):967‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singer P, Anbar R, Cohen J, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37(4):601‐609. [DOI] [PubMed] [Google Scholar]
- 9. Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502‐509. [DOI] [PubMed] [Google Scholar]
- 10. Weijs PJM, Looijaard GPM, Beishuizen A, Girbes ARJ, Oudemans‐van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non‐septic mechanically ventilated critically ill patients. Crit Care. 2014;18(6):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singer P, Pichard C, Heidegger CP, Wernerman J. Considering energy deficit in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2010;13(2):170‐176. [DOI] [PubMed] [Google Scholar]
- 12. Stapel SN, de Grooth HJ, Alimohamad H, et al. Ventilator‐derived carbon dioxide production to assess energy expenditure in critically ill patients: proof of concept. Crit Care. 2015;19:370. 10.1186/s13054-015-1087-2. PMID: 26494245; PMCID: PMC4619027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whittle J, Molinger J, MacLeod D, Haines K, Wischmeyer PE, LEEP‐COVID Study Group . Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID‐19. Crit Care. 2020;24(1):581. 10.1186/s13054-020-03286-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singer P. Nutritional and metabolic management of COVID‐19 intensive care patients. J Intensive Med. 2021;1(1):31‐34. ISSN 2667‐100X 10.1016/j.jointm.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enter Nutr. 2016;40(2):159‐211. [DOI] [PubMed] [Google Scholar]
- 16. Barazzoni R, Bischoff SC, Breda J, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS‐CoV‐2 infection. Clin Nutr. 2020;39(6):1631‐1638. 10.1016/j.clnu.2020.03.022. Jun Epub 2020 Mar 31. P MID: 32305181; PMCID: PMC7138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta NM, Smallwood CD, Joosten KF, Hulst JM, Tasker RC, Duggan CP. Accuracy of a simplified equation for energy expenditure based on bed‐ side volumetric carbon dioxide elimination measurement—a two‐center study. Clin Nutr. 2015;34(1):151‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rousing ML, Hahn‐Pedersen MH, Andreassen S, Pielmeier U, Preiser J‐C. Energy expenditure in critically ill patients estimated by population‐based equations, indirect calorimetry and CO2‐based indirect calorimetry. Ann. Intensive Care. 2016;6(1):16. 10.1186/s13613-016-0118-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oshima T, Graf S, Heidegger CP, Genton L, Pugin J, Pichard C. Can calculation of energy expenditure based on CO2 measurements replace indirect calorimetry? Crit Care. 2017;21(1):13. 10.1186/s13054-016-1595-8 Erratum in: Crit Care. 2017 Apr 12;21(1):95. PMID: 28107817; PMCID: PMC5251283.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Acute Respiratory Distress Syndrome Network , Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301‐1308. [DOI] [PubMed] [Google Scholar]
- 21. Mackinnon A. A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter‐rater agreement. Comput Biol Med. 2000;30(3):127‐134. [DOI] [PubMed] [Google Scholar]
- 22. Martindale RG, Patel JJ, Taylor B, Arabi YM, Warren M, McClave SA. Nutrition therapy in critically ill patients with coronavirus disease (COVID‐19). JPEN J Parenter Enter Nutr. 2020;44(7):1174‐1184. 10.1002/jpen.1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapple LS, Tatucu‐Babet OA, Lambell KJ, Fetterplace K, Ridley EJ. Nutrition guidelines for critically ill adults admitted with COVID‐19: Is there consensus? Clin Nutr ESPEN. 2021;44:69‐77. 10.1016/j.clnesp.2021.05.003 Epub 2021 May 25 PMID: 34330515; PMCID: PMC8146268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allingstrup MJ, Kondrup J, Perner A, Christensen PL, Jensen TH, Henneberg SW. Indirect calorimetry in mechanically ventilated patients: a prospective, randomized, clinical validation of 2 devices against a gold standard. JPEN J Parenter Enteral Nutr. 2017;41(8):1272‐1277. 10.1177/0148607116662000 Epub 2016 Aug 3. PMID: 27488830. [DOI] [PubMed] [Google Scholar]
- 25. Pielmeier U, Andreassen S. VCO2 calorimetry is a convenient method for improved assessment of energy expenditure in the intensive care unit. Crit Care. 2016;20(1):224. 10.1186/s13054-016-1397-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stapel SN, Elbers PW, Straaten HM. VCO2‐derived energy expenditure: do not throw the baby out with the bath water! Crit Care. 2017;21(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]


