Abstract
Glutamate enhances the yield of exotoxin A (ETA), which is induced by iron limitation, from Pseudomonas aeruginosa. We tested the possibility that glutamate affects growth during iron restriction. We confirmed that iron limitation caused early entry into stationary phase but had no effect on the exponential growth rate. We showed that glutamate, as well as citrate and isocitrate, partially overcame this growth limitation. Glutamate had no effect on toxA (ETA-encoding) transcription, which implies that glutamate primarily increases the number of toxin-producing cells. In contrast, citrate and isocitrate diminished toxA transcription. Since glutamate, citrate, and isocitrate stimulated growth, we suspected a block in the citric acid cycle. Iron limitation reduced the activity of the iron-containing aconitase 12-fold but had no effect on isocitrate dehydrogenase activity, which was assayed as a control. There is a reciprocal relationship between aconitase activity and ETA synthesis, and this correlation does not appear to be coincidental because aconitase-specific effectors affect ETA synthesis. We tested whether a metabolic block is sufficient to induce ETA synthesis, but an aconitase-specific inhibitor diminished ETA production, which argues against this possibility. Finally, we present preliminary evidence that iron limitation may reversibly and posttranslationally inactivate aconitase in vivo. In summary, the environmental factors that stimulate ETA synthesis are related: glutamate bypasses an iron limitation-dependent metabolic block that causes entry into stationary phase. We speculate that one or more of the aconitases in P. aeruginosa may contribute to the control of virulence factor synthesis.
Pseudomonas aeruginosa is a gram-negative soil bacterium that can transiently colonize animal hosts. Host defenses are usually sufficient to prevent infections, but when these defenses are compromised (e.g., after burns, during immunosuppressive therapy, or in wounds), P. aeruginosa becomes an opportunistic pathogen that can cause serious life-threatening infections. During such infections, P. aeruginosa secretes or carries on its outer membrane several virulence factors that contribute to its pathogenicity. These include pili, ADP-ribosyltransferase toxins (exotoxin A [ETA] and exoenzyme S), rhamnolipids, hemolytic and nonhemolytic phospholipases, endotoxin, proteases, and the exopolysaccharide alginate (10). Of these, ETA is the most toxic (26).
Most of the known regulators of ETA synthesis control the synthesis of RegA, which has been proposed to interact with RNA polymerase holoenzyme and thereby facilitate open promoter complex formation (36, 37). Transcription of regA requires the products of vfr and pvdS. Vfr is a Pseudomonas homologue of the cyclic AMP receptor protein (38), and pvdS codes for an alternate sigma factor (31) which is also required for synthesis of the iron chelator pyoverdine (6). Finally, the iron-sensing ferric uptake regulator (Fur) represses pvdS transcription (31). Although several genes have been implicated in the control of ETA synthesis, there are some significant gaps in our knowledge. For example, a site for an activator of toxA (ETA-encoding) expression has been identified, but the activator and the factors that control its activity are not known (35). In fact, no protein that interacts with the toxA promoter region has been definitively identified.
Iron limitation is a major stimulus for ETA synthesis (3). Iron-dependent regulation is mediated, in part, by Fur, which controls PvdS synthesis. However, residual control by iron availability in Pseudomonas fur mutants suggests the existence of a Fur-independent mechanism of iron regulation (1). Two other environmental factors stimulate ETA synthesis: entry into stationary phase (2) and high levels of certain amino acids, such as glutamate (25). The relationships between these factors are poorly understood. Our primary purpose was to analyze the physiological function of supplemental amino acids. We made the new observations that glutamate, citrate, and isocitrate partially mimic the growth-stimulatory effect of exogenous iron; that iron limitation resulted in a reduction in the activity of aconitase, an iron-sulfur protein, thus accounting for the growth stimulation by glutamate; and that there was a reciprocal correlation between aconitase activity and ETA synthesis. We discuss the broader significance of the amino acid supplementation and the circumstantial evidence that a bacterial aconitase may have a regulatory function similar to that of the human iron-regulatory protein 1 (IRP-1) (32).
(This work is presented as partial fulfillment for the requirements for the Ph.D. degree at the University of Texas at Dallas for G. Somerville.)
MATERIALS AND METHODS
Bacterial strains.
The P. aeruginosa strains used in this study were the prototrophic strain PA103 (27) and PA103-CM1, which contains a chromosomal toxA-lacZ fusion. The latter strain was constructed as follows. The toxA promoter region was removed from pRC362 (5) as a 760-bp PvuII-BamHI fragment and ligated into the SmaI and BamHI sites in the polylinker of pRS414 (34), which has lacZ immediately downstream of the BamHI site. The first 24 bp of the toxA gene were joined in frame to the N terminus of lacZ by linearizing the plasmid at the unique BamHI site, filling in with the Klenow fragment of Escherichia coli DNA polymerase, and religating. The toxA-lacZ fusion was removed as a 2.7-kb EcoRI-SacI fragment, which replaced the corresponding fragment of pUJ9 (8). This plasmid has a T7 terminator at the end of lacZ and allows excision of the toxA-lacZ fusion as a NotI fragment. The 4.2-kb NotI fragment was inserted into the transposon vector pUT, which contains the mini-Tn5 streptomycin/spectinomycine resistance gene cassette, and the plasmid was transformed into E. coli S17-1(λpir) (19). The donor E. coli strain was grown overnight at 37°C; the recipient strain, PA103, was grown at 43°C. Mobilization of the toxA-lacZ-containing pUT derivative was achieved by mixing the two strains in a 1:3 ratio (donor to recipient), collecting the cells on a 0.45-μm-pore-size filter, and allowing the cells to mate on an LB plate at 32°C. The next day the cells were eluted and an appropriate dilution was plated on LB plates with streptomycin (500 μg/ml). This selects against E. coli, which is not resistant to such a high concentration of streptomycin, and selects for the transconjugates. This procedure selects for cells with a random insertion of the fusion onto the chromosome—the fusion does not replace the wild-type toxA gene. The P. aeruginosa strain designated PA103-CM1 was chosen based on a quantitative assay for β-galactosidase that showed iron regulation.
Media and growth conditions.
Pseudomonas cultures were grown in either 2xYT (29), deferrated trypticase soy broth dialysate (DTSB) (20), or Pseudomonas isolation agar (Difco) at 32°C. DTSB was supplemented with 1 mM MgSO4 and with other compounds as noted. Liquid cultures were shaken at 250 rpm. The flask-to-medium volume ratio was always 20:1. The removal of iron from DTSB was not quantitative but was sufficient to limit growth (see Results). Most of the time, growth ceased at the same cell density, but there was some variation from batch to batch of medium. Occasionally the iron chelation was so efficient that it was necessary to add 1 μM FeSO4 to obtain sufficient growth.
Biochemical assays.
Aconitase activity was assayed by the method of Gruer and Guest (17). Briefly, 1.5-ml cultures were harvested at the indicated times, resuspended in Tris-citrate (20 mM, pH 8.0), and ultrasonically disrupted. The suspension was then centrifuged for 5 min at 12,000 rpm to remove cell debris. The cell-free lysate was then assayed for aconitase activity. Enzyme activity was determined spectrophotometrically by monitoring the formation of cis-aconitate from isocitrate at 240 nm according to the method of Kennedy et al. (21).
Isocitrate dehydrogenase activity was assayed by the method of Williamson and Corkey (39). Cell-free lysates were prepared as described above for the aconitase assays. Enzymatic activity was assayed spectrophotometrically at 30°C by monitoring the formation of NADPH in the presence of isocitrate.
β-Galactosidase activity was determined by the method of Miller (30).
Protein concentrations were determined by the method of Lowry et al. (28), using bovine serum albumin as the standard.
The variations in results from cells grown with different batches of media was much greater than for cells grown with the same batch of medium. This variation probably resulted from slight differences in iron content of these media, despite efforts to ensure that the iron-limited medium was prepared in the same way each time. Slight differences in a limiting nutrient can obviously have dramatic effects on growth. Because of this variability, the results presented were from one experiment, with assays done in triplicate, unless otherwise noted. The variability reported is for these triplicate determinations. All experiments were done at least twice, and in cases where cells had been grown in different batches of media, the results were always in agreement.
RESULTS
Effect of glutamate during iron-restricted growth.
The first studies on ETA synthesis showed that the optimal medium was a broth that contained glycerol and albumin (7, 25). Because albumin and several amino acids stimulated growth, it was concluded that toxigenic medium was nitrogen limiting (25). It was subsequently shown that iron limitation induced ETA synthesis (2, 3). Glutamate is still routinely added to iron-limited toxigenic medium, but its function, if any, has not been reexamined. It is possible that glutamate counters an effect of iron limitation.
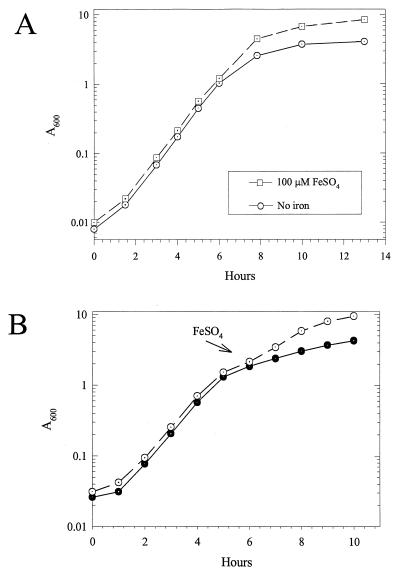
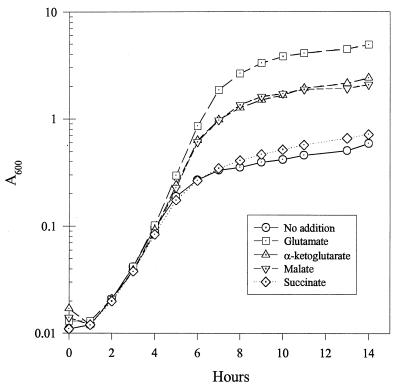
To test this possibility, we first compared the growth of P. aeruginosa PA103 in DTSB medium, which is iron restricted, with growth in iron-supplemented DTSB. The final cell density was much lower with limiting iron, but the growth rates were independent of iron availability (Fig. 1A). These results confirm previous results (3, 12, 13). We will refer to the low-iron-dependent entry into stationary phase as iron limitation or iron restriction. Adding iron to an iron-depleted culture immediately stimulated growth (Fig. 1B), which implies that the effects of iron limitation are readily reversible, at least at this stage of growth. Glutamate significantly increased the cell yield of iron-limited cells by delaying the time when growth slowed (Fig. 2 and Table 1).
FIG. 1.
Iron-restricted growth of PA103. (A) Growth of PA103 in DTSB without supplemental iron or with 100 μM FeSO4. (B) Parallel cultures were grown in iron-limited DTSB for 6 h. Supplemental iron [100 μM Fe(NH4)2(SO4)2] was added to one (○) but not the other (●).
FIG. 2.
Additions to DTSB affect the growth yield but not the growth rate. PA103 cultures were grown in DTSB with no supplement or with the indicated supplement at 50 mM.
TABLE 1.
Additions to DTSB affect growth and the production of β-galactosidase from a toxA-lacZ fusion
| Supplement(s) | A600 at 14 h | Mean β-galactosidase activity (U/mg of protein) ± SD |
|---|---|---|
| None | 1.16 | 1,144 ± 75 |
| Glutamate | 4.66 | 1,376 ± 38 |
| Glutamate + FeSO4 | 7.15 | 328 ± 6 |
| Citrate | 3.39 | 643 ± 15 |
| Isocitrate | 3.91 | 451 ± 2 |
| Malate | 1.96 | 1,625 ± 21 |
| Succinate | 0.94 | 1,292 ± 69 |
These results imply that limiting iron supports optimal growth until iron is essentially depleted, which then causes entry into stationary phase. The point at which cell growth ceased varied from batch to batch of medium. Iglewski and coworkers have reported iron-limited entry into stationary phase at a cell density that was 10 times higher than we observed, although this result varied significantly from report to report (3, 12, 13). The variability probably results from differences in the efficiency of iron extraction during medium preparation. From the effect of iron concentration on growth, we estimate that 1 μM FeSO4 supports a cell density that increases the A600 by 0.6. This is probably an overestimate because some medium components bypass the effects of iron limitation (described below). Nonetheless, growth without exogenous iron ceased at an A600 of about 0.6 (Fig. 2). From this, we estimate that about 1 μM iron remains in DTSB after iron extraction. At such low levels, small variations in iron content will have significant effects on growth. However, these batch variations had no effect on the pattern of results, and they do not affect our conclusions.
Function of glutamate.
It was originally proposed that glutamate overcomes a deficiency of nitrogen (25). However, it is also possible that glutamate provides carbon and energy, as it does for Haemophilus influenzae, which lacks several citric acid cycle enzymes (11). To distinguish between these possibilities, we examined the effects of several citric acid cycle intermediates on the growth of iron-restricted cells (Table 1). Stimulation of growth by nonnitrogenous intermediates would imply that iron restriction imposes a carbon or energy limitation. Isocitrate and citrate increased the growth yield almost as well as glutamate (Table 1). Malate and α-ketoglutarate stimulated growth but not as well as glutamate, whereas succinate did not affect growth (Fig. 2 and Table 1). None of these supplements altered the growth rate (Fig. 2). Since nonnitrogenous compounds stimulated growth, we conclude that glutamate primarily provides carbon or energy. The fact that glutamate is the best growth supplement suggests either that the provision of nitrogen stimulates growth slightly or that glutamate is transported more efficiently than the other compounds.
The effect of glutamate on specific toxA (ETA-coding) expression has not been examined since iron limitation was shown to induce ETA. It is possible that glutamate simply increases the number of toxin-producing cells without affecting expression. To test this possibility, we assessed toxA expression by measuring β-galactosidase from strain PA103-CM1, which contains a toxA-lacZ fusion on the chromosome (Table 1). Iron reduced β-galactosidase activity and ETA (measured by a direct immunological assay) fourfold (not shown), which implies that the major control is transcriptional and that β-galactosidase accurately reflects toxA expression. These results confirm previous observations (2, 3, 14).
Glutamate had little effect on β-galactosidase (Table 1). We conclude that glutamate does not control toxA expression; i.e., it does not contribute to the induction signal. Therefore, the only effect of glutamate is to stimulate the growth of toxin-producing cells.
In contrast, the other growth-stimulating supplements did affect toxA expression. Isocitrate and citrate reduced β-galactosidase activity two- to threefold, whereas malate increased β-galactosidase activity 50% (Table 1). These results suggest that some growth-stimulating compounds can affect the inducing signal.
Iron limitation and aconitase activity.
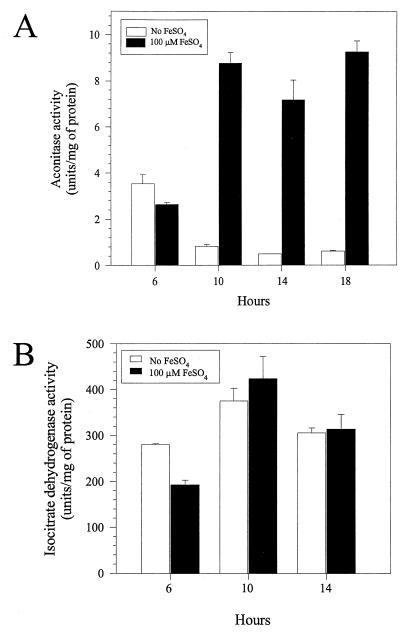
Since some citric acid cycle intermediates, but especially citrate, isocitrate, and glutamate, enhanced growth, we suspected that iron limitation inactivated a citric acid cycle enzyme that is required for the synthesis of α-ketoglutarate, the precursor for glutamate. Therefore, we examined the effects of iron limitation on two citric acid cycle enzymes, aconitase and isocitrate dehydrogenase (Fig. 3).
FIG. 3.
Effects of iron restriction on aconitase (A) and isocitrate dehydrogenase (B) activities over time in PA103 cultures grown in iron-limited DTSB or DTSB with 100 μM FeSO4.
Aconitase catalyzes the conversion of citrate to isocitrate via cis-aconitate, and a labile [4Fe-4S] cluster is required for catalytic activity. In eucaryotes, aconitase activity is sensitive to iron availability (9, 21). Therefore, we considered the possibility that iron limitation impairs aconitase activity in P. aeruginosa. There was a fourfold drop in aconitase activity when iron-limited cultures of PA103 entered stationary phase. In contrast, there was a threefold increase in aconitase activity from iron-replete cultures (Fig. 3A). The net effect was an approximately 12-fold difference in aconitase activity between iron-limited and iron-replete cells.
We also assayed isocitrate dehydrogenase, which catalyzes the reaction after the aconitase reaction: the NADP-dependent oxidation of isocitrate to α-ketoglutarate. It does not contain iron, and its activity should not be affected by iron availability. Nonetheless, its inactivation could also conceivably account for the growth stimulation by glutamate. However, neither iron availability nor entry into stationary phase adversely affected its activity (Fig. 3B). We conclude that iron limitation specifically reduces aconitase activity.
Reciprocal relationship between aconitase activity and ETA synthesis.
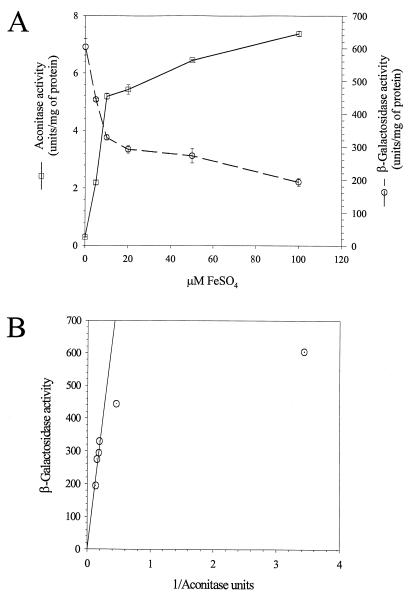
Iron availability affects both aconitase activity and ETA synthesis. Therefore, we examined how closely these two activities correlated as a function of exogenous iron. Cells were grown for 14 h and then assayed for aconitase and β-galactosidase from PA103-CM1. A reciprocal correlation between aconitase and β-galactosidase activities was readily apparent (Fig. 4A). Replotting β-galactosidase activity as a function of the reciprocal of aconitase activity shows a linear relationship, which breaks down only when β-galactosidase activity is nearly maximal (Fig. 4B). In other words, aconitase activity is low when toxA is expressed.
FIG. 4.
Transcription of toxA inversely correlates with aconitase activity in PA103. (A) Aconitase activity (□) and toxA expression (β-galactosidase activity from a strain with a toxA-lacZ fusion) (○) in cultures of PA103-CM1 grown for 14 h in increasing concentrations of FeSO4. (B) Replot of the data from panel A, showing the inverse correlation between aconitase activity and toxA transcription.
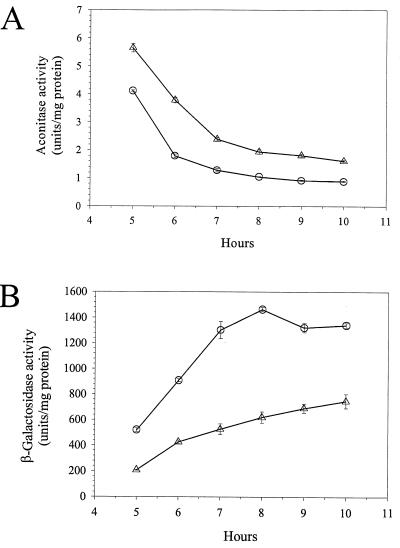
Such a correlation is not particularly surprising, since all reactions sensitive to iron availability should be affected once iron is depleted. If the correlation is coincidental, then the factors that specifically affect aconitase activity should have absolutely no influence on ETA synthesis. Therefore, we examined the effect of supplemental isocitrate on aconitase activity. Isocitrate is a product of the aconitase reaction, and its presence may prevent the reduction in aconitase activity, possibly by stabilizing the protein. We compared aconitase activities at several times for cells grown with either isocitrate or glutamate. Isocitrate stabilized aconitase activity about twofold (Fig. 5A). Isocitrate had the opposite effect on β-galactosidase activity, reducing its activity two- to threefold (Fig. 5B). These results further confirm the reciprocal correlation between aconitase activity and ETA synthesis. They also suggest that the reciprocal correlation between aconitase activity and ETA synthesis might not be coincidental.
FIG. 5.
Isocitrate stabilizes aconitase and reduces toxA expression. (A) Aconitase activities of PA103-CM1 cultures grown in DTSB supplemented with 50 mM glutamate (○) or isocitrate (▵). (B) β-Galactosidase activity of cultures from panel A.
A metabolic block in the citric acid cycle is not sufficient to induce ETA synthesis.
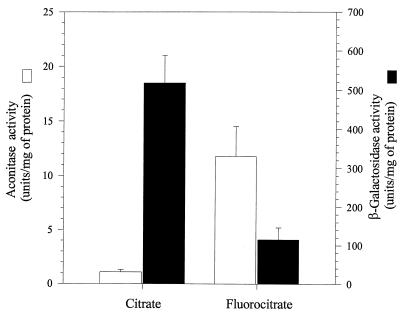
A possible explanation for the reciprocal relationship between aconitase activity and ETA synthesis is that an iron limitation-dependent metabolic block in the citric acid cycle is sufficient to stimulate ETA synthesis. To test this possibility, we examined the effect of fluorocitrate, an aconitase-specific inhibitor, on ETA synthesis. Aconitase converts fluorocitrate to fluoro-cis-aconitate and then to 4-hydroxy-trans-aconitate, which binds tightly, but not covalently, to aconitase. The latter intermediate inhibits aconitase activity. At the same time that 4-hydroxy-trans-aconitate inhibits aconitase, it also stabilizes the [4Fe-4S] cluster and thus prevents aconitase’s conversion into the enzymatically inactive [3Fe-4S] form (23). The inhibition by 4-hydroxy-trans-aconitate is reversible; therefore, when cell extracts are prepared and aconitase is saturated with substrate, aconitase activity can be measured. This leads to the apparently paradoxical situation in which an increase in aconitase activity from a crude extract, which results from stabilization of the [4Fe-4S] cluster, implies a proportional inhibition of aconitase activity in vivo. If fluorocitrate stimulates ETA synthesis, we would conclude that a metabolic block induces ETA synthesis. This result was not observed.
Cells were grown for 10 h in DTSB supplemented with 50 mM citrate instead of 50 mM glutamate, in order to minimize the effects of the subsequent medium shift. Cells were then resuspended and incubated for 4 h in DTSB without glutamate but with either 2 mM citrate or 2 mM fluorocitrate. The latter incubation permits ETA synthesis and supports a fourfold increase in cell density. Fluorocitrate increased aconitase activity measured from extracts 12-fold (Fig. 6). This increase probably results from two factors: stabilization of the Fe-S center, which inhibits activity in vivo, and new synthesis of aconitase. The greatly elevated activity may result from fluorocitrate-dependent inhibition, which stimulates compensatory synthesis of more aconitase. In either case, inhibition of aconitase by fluorocitrate must be invoked to explain the increase in activity. The 12-fold increase in aconitase activity is accompanied by a four-fold reduction in β-galactosidase activity (Fig. 6). These results show that the metabolic block imposed by fluorocitrate impairs ETA synthesis. This is contrary to the expectation of the metabolic block hypothesis, which predicts an increase in ETA synthesis. Therefore, these results suggest that a metabolic block is not sufficient to induce ETA synthesis.
FIG. 6.
Fluorocitrate stabilizes aconitase and inhibits toxA expression. PA103-CM1, which contains a chromosomal toxA-lacZ fusion, was grown for 10 h in DTSB supplemented with citrate. The cells were then harvested and resuspended in prewarmed DTSB supplemented with either 2 mM citrate or 2 mM fluorocitrate. These cultures were grown for 4 h and then assayed for β-galactosidase and aconitase activities. The data represents the averages of two independent experiments with each assay done in triplicate, with the standard deviation for each also presented.
Evidence for posttranslational aconitase inactivation.
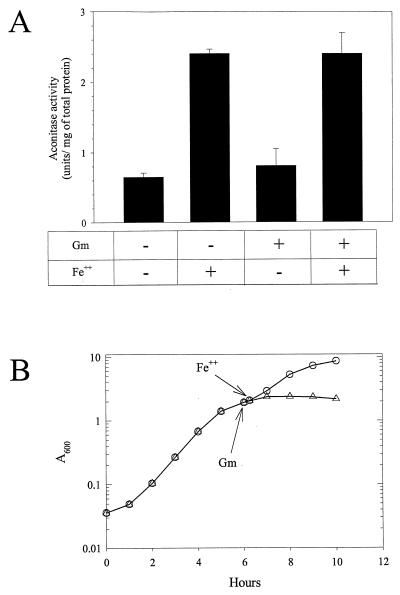
Two explanations could account for the diminished aconitase activity from iron-restricted cells. First, iron depletion could prevent aconitase synthesis, and subsequent cell growth could dilute aconitase activity. Alternately, iron restriction could posttranslationally inactivate aconitase by loss of iron from its essential [4Fe-4S] cluster. If iron depletion reversibly inactivates aconitase, then addition of iron to iron-limited cells would reactivate aconitase. To test this possibility, we incubated cells in the presence of a protein synthesis inhibitor, which should prevent an increase in newly synthesized aconitase. Figure 7B shows the growth of iron-limited cells to which exogenous iron was added. Without gentamicin, growth resumed at a faster rate, which implies that iron is being transported into the cell under the conditions of the experiment. With gentamicin, growth ceased, which implies substantial, although perhaps not complete, inhibition of protein synthesis. Under these conditions, exogenous iron increased aconitase activity fourfold (Fig. 7A).
FIG. 7.
In vivo reactivation of aconitase. (A) PA103 was grown in iron-limited DTSB for 6 h (A600 of ∼2.0), at which time the culture was split into four equal volumes. Two of the cultures were treated with 20 μg/ml of gentamicin, (Gm), and two were untreated. The cultures were incubated for an additional 15 min, and then one treated and one untreated culture were supplemented with Fe(NH4)2(SO4)2 to a concentration of 100 μM. The cultures were incubated for an additional 45 min, harvested, and assayed for aconitase activity. Without gentamicin, the cell density increased by about 15% without iron and 30% with iron. The data represent the averages from two independent cultures done on different days. The assays were done in triplicate, with error bars indicating the standard deviations. (B) Growth of iron-limited PA103 with (▵) or without gentamicin (○). Both cultures were supplemented with 100 μM Fe(NH4)2(SO4)2 15 min after addition of gentamicin to one culture.
It is tempting to conclude that iron limitation reversibly and posttranslationally inactivates aconitase. Such a conclusion would be consistent with the extraordinary sensitivity of the E. coli aconitase to oxygen radicals (18), which suggests that the [4Fe-4S] cluster is accessible and readily disassembled. It is also consistent with the effects of iron limitation on the homologous aconitases in eucaryotes. However, regardless of how effective the protein synthesis inhibitor is, even if protein synthesis is undetectable, it could still be argued that ribosomes preferentially translate aconitase mRNA. Therefore, only biochemical experiments can definitively establish that the reduction in aconitase activity results from reversible inactivation. Our initial attempts to reactivate aconitase in vitro have failed, but these experiments were performed in an aerobic environment, which may prevent reactivation. Therefore, we consider the conclusion that iron reactivates aconitase posttranslationally to be tentative. The major physiological conclusions of this report depend only on the fact that aconitase activity is reduced, not on the mechanism of the reduction.
DISCUSSION
Glutamate and virulence factor synthesis.
Our primary objective was to analyze glutamate’s effect on ETA synthesis, which was first observed 25 years ago (25). We found that glutamate delayed the entry of iron-limited cells into stationary phase. Several citric acid cycle intermediates, especially citrate and isocitrate, had a similar effect. These results suggested that iron limitation impaired citric acid cycle metabolism, and we found that iron limitation specifically reduced aconitase activity. The growth stimulation by citrate, which is the substrate for aconitase, does not contradict this conclusion, since citrate may either stabilize aconitase or increase the reaction rate of residual aconitase.
Glutamate has no apparent effect on toxA expression. Nonetheless, factors such as glutamate that stimulate the growth of toxin-producing cells may determine the outcome of an infection. This may be especially true for pathogens that produce toxins that are induced by iron limitation. If the iron-free form of aconitase regulates virulence factor synthesis (a possibility that is discussed below), then the moment the regulatory function of aconitase is activated is the same moment that metabolism through the citric acid cycle is impaired. In this case, total toxin production would be proportional to the extent that the metabolic block is circumvented, assuming that nothing interferes with the induction signal.
Aconitases in E. coli and P. aeruginosa.
E. coli has two, and possibly three, aconitases. AcnB is the major aconitase during exponential growth (16). A second aconitase, AcnA, is induced during stress in stationary phase, and genetic evidence suggests that its induction requires iron (17). A double mutant without AcnA and AcnB still has residual activity, which suggests that there may be a third aconitase (16). Analysis of the partially complete P. aeruginosa genome sequence suggests that there are three aconitase-specifying genes: two are homologous to E. coli acnA, and one is homologous to E. coli acnB. The multiplicity of aconitases can explain some of the results presented in this report if the P. aeruginosa aconitases are similar to the E. coli enzymes. For example, the 12-fold difference in total aconitase activity between iron-replete and iron-limited cells might be the result of differential effects on two different aconitases. Iron restriction might cause a fourfold reduction in the activity of the exponential phase aconitase and, in addition, failure to induce the iron-inducible stationary phase aconitase. This would also explain the extent of the apparent iron reactivation of aconitase from iron-limited cells. In the absence of the iron-inducible stationary phase aconitase, iron would be expected to increase aconitase activity only fourfold, and this is exactly what is observed (Fig. 7A).
Aconitase and virulence factor synthesis in other pathogens.
The amino acid sequences of aconitases are highly conserved (15, 32), which might suggest that conditions that reduce aconitase activity in one organism probably do so in other organisms as well. Since oxidative stress, which inactivates E. coli aconitase activity (18), and iron restriction are frequently encountered by pathogens, it is possible that such environments are generally associated with a reduction in aconitase activity. If so, then the stimulatory effect of amino acids and citric acid cycle intermediates on virulence factor synthesis may be more general than is currently recognized. For example, since the same amino acids that stimulate ETA synthesis also stimulate cholera toxin synthesis in Vibrio cholerae (4), cholera toxin synthesis may also negatively correlate with aconitase activity. If this is true, then agents that stabilize aconitase, such as isocitrate and fluorocitrate, may also impede virulence factor synthesis in other pathogens.
Iron, aconitase, and ETA synthesis.
Iron is essential for most bacteria, and complex and diverse mechanisms have evolved to sense and respond to iron restriction. Iron limitation was first implicated in microbial virulence over 50 years ago (33), and since then has been shown to be important for many pathogens.
Iron availability regulates gene expression in bacteria and eucaryotes. The best-characterized iron-sensing regulator in P. aeruginosa (and other bacteria) is Fur. An iron-Fur complex normally represses gene expression. For example, it represses pvdS, which codes for a sigma factor that is required for regA and toxA (ETA) expression (24, 31). In eucaryotes, the best-characterized iron-sensing regulators are the cytoplasmic aconitases. These aconitases, such as the human IRP-1, bind to mRNA and can either inhibit translation or impede degradation (for reviews, see references 18 and 22). Binding to mRNA becomes possible after iron limitation results in disassembly of the [4Fe-4S] cluster and aconitase inactivation (9, 21). In other words, it is the iron-free form of the eucaryotic aconitases that regulates gene expression. Bacterial aconitases are homologous to IRP-1. The homology of E. coli AcnA to IRP-1 led to the suggestion that it may have a regulatory function (32). This conjecture has yet to be proven.
It is tempting to speculate that one or more of the P. aeruginosa aconitases may have a regulatory function. Such a hypothesis may be the simplest explanation for the absolute reciprocal correlation between aconitase activity and ETA synthesis. It could be argued that this correlation is coincidental. If so, then aconitase-specific effectors should not have affected ETA synthesis. However, two different aconitase-specific effectors, isocitrate and fluorocitrate, significantly suppressed ETA synthesis. Both of these effectors appear to stabilize aconitase, but isocitrate stimulates aconitase activity whereas fluorocitrate inhibits activity. These results imply that aconitase activity in vivo does not correlate with ETA synthesis. Instead, they suggest that stabilization of iron-containing aconitase per se impairs ETA synthesis and that iron-free aconitase activates toxA expression. However, the physiological results presented in this report cannot prove this hypothesis. Proof would require a combination of genetics and biochemistry. We are actively testing this hypothesis.
ACKNOWLEDGMENT
This work was supported by National Institute of General Medical Sciences grant GM47965.
REFERENCES
- 1.Barton H A, Johnson Z, Cox C D, Vasil A I, Vasil M L. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol Microbiol. 1996;21:1001–1017. doi: 10.1046/j.1365-2958.1996.381426.x. [DOI] [PubMed] [Google Scholar]
- 2.Bjorn M J, Iglewski B H, Ives S K, Sadoff J C, Vasil M L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978;19:785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorn M J, Sokol P A, Iglewski B H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979;138:193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callahan L T, Richardson S H. Biochemistry of Vibrio cholerae virulence. III. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973;7:567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S T, Jordan E M, Wilson R B, Draper R K, Clowes R C. Transcription and expression of the exotoxin A gene of Pseudomonas aeruginosa. J Gen Microbiol. 1987;133:3081–3091. doi: 10.1099/00221287-133-11-3081. [DOI] [PubMed] [Google Scholar]
- 6.Cunliffe H E, Merriman T R, Lamont I L. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J Bacteriol. 1995;177:2744–2750. doi: 10.1128/jb.177.10.2744-2750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBell R M. Production of exotoxin A by Pseudomonas aeruginosa in a chemically defined medium. Infect Immun. 1979;24:132–138. doi: 10.1128/iai.24.1.132-138.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emptage M H, Dreyers J L, Kennedy M C, Beinert H. Optical and EPR characterization of different species of active and inactive aconitase. J Biol Chem. 1983;258:11106–11111. [PubMed] [Google Scholar]
- 10.Fick R B. Pseudomonas aeruginosa: the microbial hyena and its role in disease: an introduction. In: Fick R B, editor. Pseudomonas aeruginosa, the opportunist: pathogenesis and disease. Boca Raton, Fla: CRC Press; 1993. pp. 1–5. [Google Scholar]
- 11.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 12.Frank D W, Iglewski B H. Kinetics of toxA and regA mRNA accumulation in Pseudomonas aeruginosa. J Bacteriol. 1988;170:4477–4483. doi: 10.1128/jb.170.10.4477-4483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank D W, Storey D G, Hindahl M S, Iglewski B H. Differential regulation by iron of regA and toxA transcript accumulation in Pseudomonas aeruginosa. J Bacteriol. 1989;171:5304–5313. doi: 10.1128/jb.171.10.5304-5313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant C C, Vasil M L. Analysis of transcription of the exotoxin A gene of Pseudomonas aeruginosa. J Bacteriol. 1986;168:1112–1119. doi: 10.1128/jb.168.3.1112-1119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruer M J, Artymiuk P J, Guest J R. The aconitase family: three structural variations on a common theme. Trends Biochem Sci. 1997;22:3–6. doi: 10.1016/s0968-0004(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 16.Gruer M J, Bradbury A J, Guest J R. Construction and properties of aconitase mutants of Escherichia coli. Microbiology. 1997;143:1837–1846. doi: 10.1099/00221287-143-6-1837. [DOI] [PubMed] [Google Scholar]
- 17.Gruer M J, Guest J R. Two genetically-distinct and differentially-regulated aconitases (AcnA and AcnB) in Escherichia coli. Microbiology. 1994;140:2531–2541. doi: 10.1099/00221287-140-10-2531. [DOI] [PubMed] [Google Scholar]
- 18.Hentze M W, Kuhn L C. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iglewski B H, Sadoff J C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy M C, Emptage M H, Dreyer J L, Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983;258:11098–11105. [PubMed] [Google Scholar]
- 22.Klausner R D, Harford J B. cis-trans models for post-transcriptional gene regulation. Science. 1989;246:870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- 23.Lauble H, Kennedy M C, Emptage M H, Beinert H, Stout C D. The reaction of fluorocitrate with aconitase and the crystal structure of the enzyme-inhibitor complex. Proc Natl Acad Sci USA. 1996;93:13699–13703. doi: 10.1073/pnas.93.24.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leoni L, Ciervo A, Orsi N, Visca P. Iron-regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J Bacteriol. 1996;178:2299–2313. doi: 10.1128/jb.178.8.2299-2313.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973;128:506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- 26.Liu P V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974;130(Suppl):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- 27.Liu P V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966;116:481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- 28.Lowry O H, Rosebrough N J, Farr L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 31.Ochsner U A, Johnson Z, Lamont I L, Cunliffe H E, Vasil M L. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol Microbiol. 1996;21:1019–1028. doi: 10.1046/j.1365-2958.1996.481425.x. [DOI] [PubMed] [Google Scholar]
- 32.Prodromou C, Artymiuk P J, Guest J R. The aconitase of Escherichia coli. Nucleotide sequence of the aconitase gene and amino acid sequence similarity with mitochondrial aconitases, the iron-responsive-element-binding protein and isopropylmalate isomerases. Eur J Biochem. 1992;204:599–609. doi: 10.1111/j.1432-1033.1992.tb16673.x. . (Erratum, 207:1129.) [DOI] [PubMed] [Google Scholar]
- 33.Schade A L, Caroline L. Raw hen egg white and the role of iron in growth inhibition of Shigella dysenteriae, Staphylococcus aureus, Escherichia coli, and Saccharomyces cerevisiae. Science. 1944;100:14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- 34.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 35.Tsaur M L, Clowes R C. Localization of the control region for expression of exotoxin A in Pseudomonas aeruginosa. J Bacteriol. 1989;171:2599–2604. doi: 10.1128/jb.171.5.2599-2604.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker S L, Hiremath L S, Galloway D R. ToxR (RegA) activates Escherichia coli RNA polymerase to initiate transcription of Pseudomonas aeruginosa toxA. Gene. 1995;154:15–21. doi: 10.1016/0378-1119(94)00870-x. [DOI] [PubMed] [Google Scholar]
- 37.Walker S L, Hiremath L S, Wozniak D J, Galloway D R. ToxR (RegA)-mediated in vitro transcription of Pseudomonas aeruginosa toxA. Gene. 1994;150:87–92. doi: 10.1016/0378-1119(94)90863-x. [DOI] [PubMed] [Google Scholar]
- 38.West S E, Sample A K, Runyen-Janecky L J. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J Bacteriol. 1994;176:7532–7542. doi: 10.1128/jb.176.24.7532-7542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson J R, Corkey B E. Assays of Intermediates of the citric acid cycle and related compounds by fluorometric enzyme methods. Methods Enzymol. 1969;13:434–513. [Google Scholar]