Abstract
Background
A national serosurvey of U.S. blood donors conducted in partnership with the Centers for Disease Control and Prevention (CDC) was initiated to estimate the prevalence of SARS‐CoV‐2 infections and vaccinations.
Methods
Beginning in July 2020, the Nationwide Blood Donor Seroprevalence Study collaborated with multiple blood collection organizations, testing labs, and leadership from government partners to capture, test, and analyze approximately 150,000 blood donation specimens per month in a repeated, cross‐sectional seroprevalence survey.
Results
A CDC website (https://covid.cdc.gov/covid-data-tracker/#nationwide-blood-donor-seroprevalence) provided stratified, population‐level results to public health professionals and the general public.
Discussion
The study adapted operations as the pandemic evolved, changing specimen flow and testing algorithms, and collecting additional data elements in response to changing policies on universal blood donation screening and administration of SARS‐CoV‐2 spike‐based vaccines. The national serosurvey demonstrated the utility of serosurveillance testing of residual blood donations and highlighted the role of the blood collection industry in public–private partnerships during a public health emergency.
Abbreviations
- Ab
antibody
- Abs
antibodies
- BCO
blood collection organization
- CDC
United States Centers for Disease Control and Prevention
- COVID‐19
coronavirus disease 2019
- CCP
COVID‐19 convalescent plasma
- ID
identification
- QC
quality control
- REDS‐IV‐P
Recipient Epidemiology and Donor Evaluation Study‐IV‐Pediatric
- RESPONSE
REDS‐IV‐P Epidemiology Surveillance and Preparedness of the Novel SARS‐CoV‐2 Epidemic
- S
spike protein
- S/CO
signal‐to‐cutoff ratio
- TTIMS
Transfusion‐Transmissible Infections Monitoring System
- VRI
Vitalant Research Institute
1. INTRODUCTION
When human transmission of the novel coronavirus SARS‐CoV‐2 first came to worldwide attention in early 2020, there were limited diagnostic tools to track infections. The pandemic evolved rapidly, with global spread and case fatality rates from coronavirus disease 2019 (COVID‐19) exceeding 5% among persons 70 years of age and older. 1 The number of infections outpaced the availability of testing early in the pandemic and quickly overwhelmed available resources for traditional case investigation and contact tracing of current infections. Additionally, people with asymptomatic infection and mild disease, accounting for an estimated one‐third of all cases, were often not tested, contributing further to underdiagnosis of infections. 2 As such, public health officials and clinicians identified a need for novel approaches to estimating the cumulative incidence of SARS‐CoV‐2 infections, including serosurveys. To address the urgent need for reliable information, the U.S. Centers for Disease Control and Prevention (CDC) funded the Nationwide Blood Donor Seroprevalence Study, a repeated cross‐sectional survey monitoring monthly seroprevalence in blood donors across the United States, including Puerto Rico. 3
The serosurvey was an extension of the National Heart, Lung, and Blood Institute‐funded RESPONSE (REDS‐IV‐P Epidemiology Surveillance and Preparedness of the Novel SARS‐CoV‐2 Epidemic) study, which was part of the Recipient Epidemiology and Donor Evaluation Study‐IV‐Pediatric (REDS‐IV‐P) program. RESPONSE conducted monthly cross‐sectional testing for SARS‐CoV‐2 antibodies (Abs) of blood donors in six U.S. metropolitan regions from March to August 2020 to estimate the extent of SARS‐CoV‐2 infections during the early months of the pandemic. 4 The national program, which began while the RESPONSE study was concluding (Figure 1), was funded by CDC and included expanded serosurveillance and other research aims. This publication outlines the operational features of the national serosurvey, including study initiation, inclusion/exclusion criteria and sampling, antibody (Ab) assays and testing algorithms, specimen and data flow, quality control (QC), weighting, and analysis. Given the success of the national serosurvey, this can inform the successful development and implementation of similar large‐scale serosurveillance studies to respond to future pandemics. It is noteworthy that CDC has a long history of working with blood donor organizations in surveillance efforts, with foundational studies initiated in the 1970s on viral hepatitis, in the 1980s on human immunodeficiency virus, and many studies of vector borne infections such as West Nile virus, dengue virus, chikungunya virus, and Zika virus; these collaborations have laid significant groundwork for the approaches described here. 5 , 6
FIGURE 1.
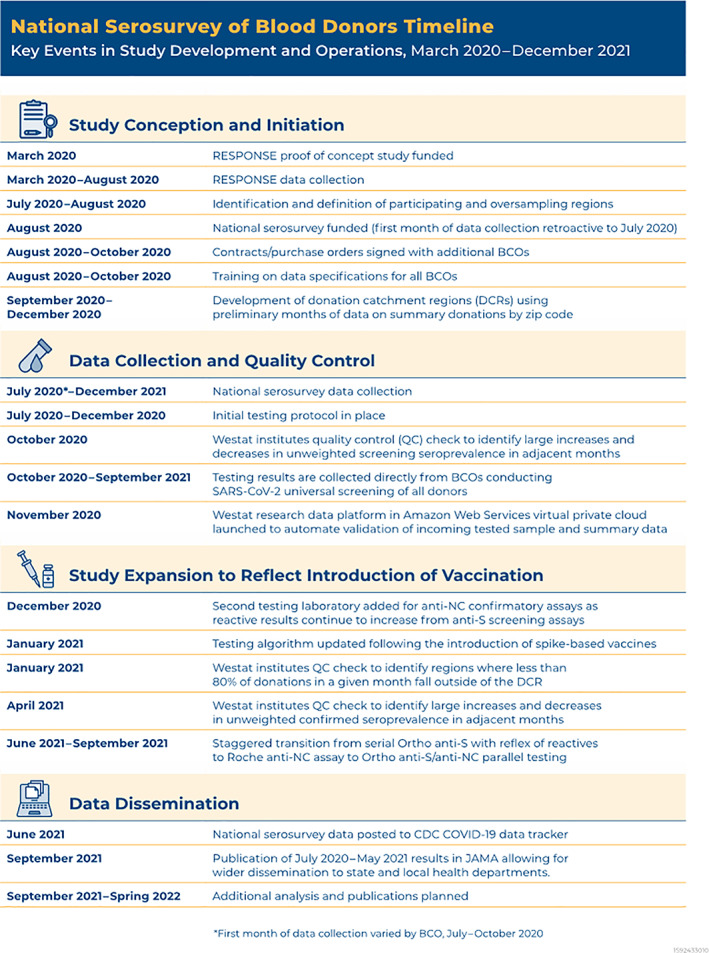
Nationwide blood donor seroprevalence study timeline: key events in study development and operations, March 2020 – Spring 2022 [Color figure can be viewed at wileyonlinelibrary.com]
2. METHODS
2.1. Human subjects research and institutional review board approval
The study was approved by CDC as non‐research public health surveillance based on anonymization of data and routine consent for blood donation testing that includes the use of residual samples for research purposes. The study does not require human‐subject research review nor clearance by the Office of Management and Budget and was conducted consistent with applicable federal law (45 C.F.R. part 46; 21 CFR part 56; 42 USC §241[d], 5 USC §552a, 44 USC §3501) and CDC policy.
2.2. Study initiation
Beginning in the spring of 2020, CDC provided funding to expand the methodology developed by the RESPONSE study to a nationwide network of 17 participating blood collection organizations (BCOs) contributing specimens from 62 blood donor regions. 3 Vitalant Research Institute (VRI) led the effort in collaboration with multiple other BCOs and testing labs. Westat, a research services firm that is the data coordinating center for REDS‐IV‐P, provided additional statistical, data management, and study coordination services. CDC provided leadership and guidance throughout the study.
2.3. Inclusion/exclusion criteria and sampling
All routine blood donations were eligible for inclusion with the exception of COVID‐19 convalescent plasma (CCP) donations to avoid upward bias in seroprevalence estimates due to specific recruitment of previously infected individuals into CCP programs (Figure 2). Most blood donor regions provided 2000 de‐identified specimens per month. Two blood donor regions containing three states each contributed 6000 specimens per month to ensure each state collected at least 1000 specimens. BCOs were instructed to sample donations evenly across at least 3 weeks of every month using pseudorandom or convenience sampling strategies (see Jones et al., eTable 2). 3 Barcoded and blinded study identification (ID) labels, testing tubes, and shipping supplies were provided by VRI to BCOs as needed.
FIGURE 2.
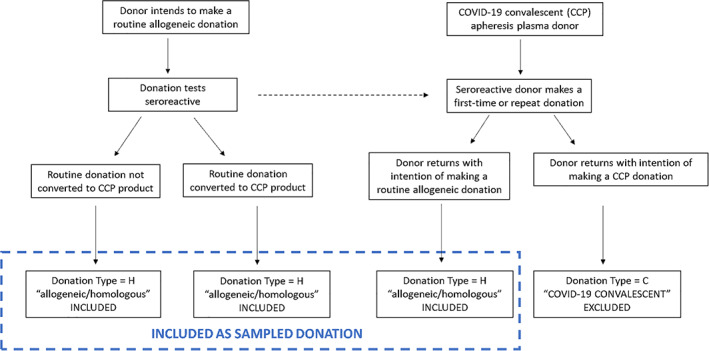
Inclusion criteria for blood donations in the Nationwide Blood Donor Seroprevalence Study. The dotted‐line arrow indicates that blood donors with seroreactive donations may return to make a subsequent donation. [Color figure can be viewed at wileyonlinelibrary.com]
2.4. Race/ethnicity oversampling
One goal of the national serosurvey was to identify health disparities in both community transmission of SARS‐CoV‐2 and the uptake of vaccines. Because racial and ethnic diversity is typically underrepresented in blood donor populations, oversampling 7 was employed in blood donor regions with higher numbers of donors from racial and ethnic minority populations. To identify where oversampling was needed and feasible, donation frequencies by race/ethnicity were examined using existing data from the Transfusion‐Transmissible Infections Monitoring System (TTIMS) in the 17 months prior to the start of the national serosurvey. 8 A list of the states with the highest frequencies of African American or Hispanic donations was developed to help guide final site selections for oversampling. To avoid bias that would be introduced by expanding sampling in selected subgroups only, the entire sample size was increased in the blood donor regions selected for oversampling. In each of 10 blood donor regions chosen for oversampling, monthly samples were increased to 4000 donations.
2.5. Antibody assays and testing algorithms
The initial choice of assays and testing algorithm was developed in RESPONSE and is described by Stone et al. 4 (Figure 3). Briefly, from July to December 2020, specimens that were low reactive (signal‐to‐cutoff ratio [S/CO] 1–10) on the Ortho VITROS Immunodiagnostic Products Anti‐SARS‐CoV‐2 Spike (S) Total Ig assay were tested using the Roche Elecsys Anti‐SARS‐CoV‐2 Nucleocapsid (NC) on cobas assay for confirmation. Specimens with discrepant anti‐S and anti‐NC results were tested for neutralizing Ab activity with a SARS‐CoV‐2 pseudovirus reporter viral particle neutralization assay. 9
FIGURE 3.
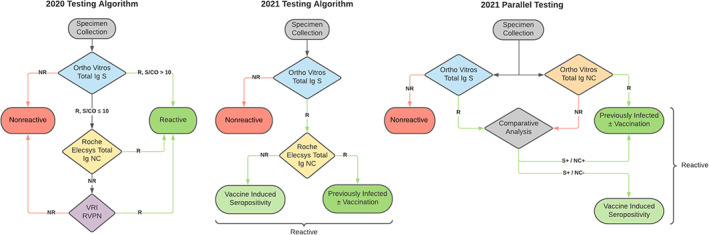
SARS‐CoV‐2 testing algorithms used throughout the course of the Nationwide Blood Donor Seroprevalence Study. R and NR denotes reactive and nonreactive; respectively. S/CO denotes signal to cutoff value for the assay. [Color figure can be viewed at wileyonlinelibrary.com]
Beginning in 2021, following the introduction of S‐based vaccines (Pfizer‐BioNTech, Moderna, and Janssen/J&J), all specimens that were reactive on the anti‐S assay were tested for anti‐NC. With the rapid rollout of SARS‐CoV‐2 vaccines, reflex anti‐NC testing became increasingly important to differentiate infection with or without vaccination (anti‐S/anti‐NC reactivity) from vaccine only‐induced seropositivity (anti‐S reactivity only); 5%–8% of infected donors identified prior to vaccine roll‐out did not develop anti‐NC Abs using the Roche total Ig anti‐NC assay. 4 When anti‐S reactivity rates for the study exceeded 80% in mid‐2021 due to large‐scale vaccination of donors (who had higher rates of vaccination than the general population), the testing algorithm was further modified such that all specimens were tested in parallel with both anti‐S and anti‐NC total Ig assays from Ortho Clinical Diagnostics. This simultaneous testing on a single platform increased accuracy of classification of donors as infected or vaccinated, decreased costs, and shortened the length of time from testing to availability of results. By January 2021, the two largest BCOs implemented a question about past vaccination status at each donation and were able to perform automated extraction and provision of vaccination status data for study purposes using existing study data pipelines.
2.6. Specimen flow
Residual serum was captured for study testing purposes after completion of routine donor screening. Donor serum was either tested directly from the donor serum tube or aliquoted and frozen for transport to a testing laboratory. Specimens were either physically labeled with a blinded study ID or were assigned a blinded study ID electronically. The flow of specimens through the testing algorithm was often complex and varied by BCO. In many cases, specimens were transported multiple times to testing laboratories to complete all required testing (Figure 4).
FIGURE 4.
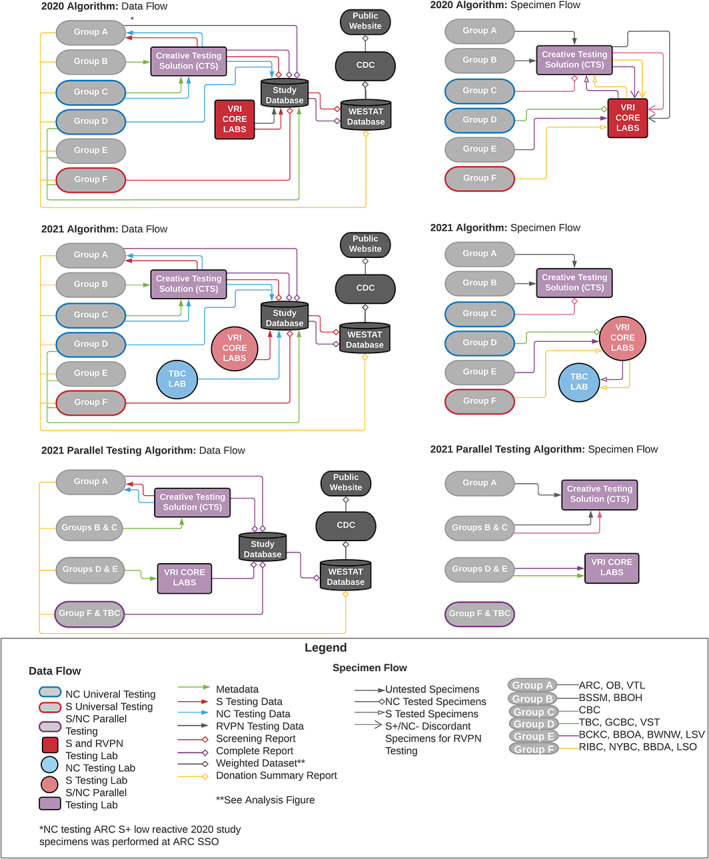
Flow of specimens and data in the Nationwide Blood Donor Seroprevalence Study (see Supplemental Methods) [Color figure can be viewed at wileyonlinelibrary.com]
2.7. Universal screening
Beginning in June 2020, a number of BCOs began including SARS‐CoV‐2 serological testing as part of routine donor testing to identify potential CCP donors and to motivate blood donations at a time of critical blood shortages. This simplified specimen processing and transport because a large portion of study specimens had already been tested using the same anti‐S and/or anti‐NC assays employed in the study. At least one BCO changed its routine donor‐screening assay to align with the national serosurvey. Where present, universal donor screening with study assays reduced the testing workload by removing one or more nodes in the specimen transport network and enabling electronic sampling of the testing results from each blood donor region. In the summer of 2021, all BCOs ended universal screening due to limited ongoing utility and high cost, a change that triggered the need to revert to the original specimen selection and testing flow, again increasing the number of specimens shipped to the various laboratories in the testing network.
2.8. Data flow
Collection of standardized, clean, and analysis‐ready data was critical to producing timely seroprevalence estimates. A standardized data guide (Appendix S2) and data dictionary were developed for the study in collaboration with VRI, Westat, and American Red Cross. The data acquisition systems and data dictionary were initially based on the existing TTIMS study and adapted for the needs of this study. Two file types (tested specimen report and summary donation file) were submitted by BCOs for monthly reporting of study data and are further described in the Supplemental Methods.
2.9. Specimen and data QC
QC procedures were implemented throughout the specimen and data flows. Each BCO and testing laboratory developed procedures to ensure that study specimens submitted were traceable, and that demographic and donation information was correct. Each testing laboratory managed multiple study files at each stage of testing, contributing to a high monthly activity level. VRI and Westat, the data coordinating center, managed and reviewed large volumes of files throughout the study. Specific QC procedures for specimen and data flow are described in the Supplemental Methods.
To accommodate the various data flows, BCOs and testing laboratories were required to write new programs to support the national serosurvey, and in some cases, install new equipment. Given its central role in data collection from multiple points, Westat implemented a research data platform built in a virtual private cloud managed by Amazon Web Services. All file submissions to Westat were made through a data collection client using a secure file transfer protocol.
2.10. Weighting and analysis
Blood donor demographics differ from those of the general population. To extrapolate seroprevalence estimates from the sampled blood donations to the U.S. population, tested specimen data were weighted to reflect the population age, sex, and race/ethnicity distributions of the underlying geographic areas represented by the study, as described in Jones et al. 3 For each geographic blood donor region from which the specimens originated, Westat defined a set of zip codes where more than 90% of blood donors resided using the summary donation file in the month(s) prior to the start of data collection (Figure 5). Specimens from donors with zip codes falling outside of the defined set of zip codes or with missing age, sex, or race/ethnicity were excluded from analysis. Because blood donor regions did not follow standard U.S. administrative boundaries such as state lines, a set of 66 more meaningful study regions was defined using state or metropolitan area borders for the purpose of reporting seroprevalence estimates.
FIGURE 5.
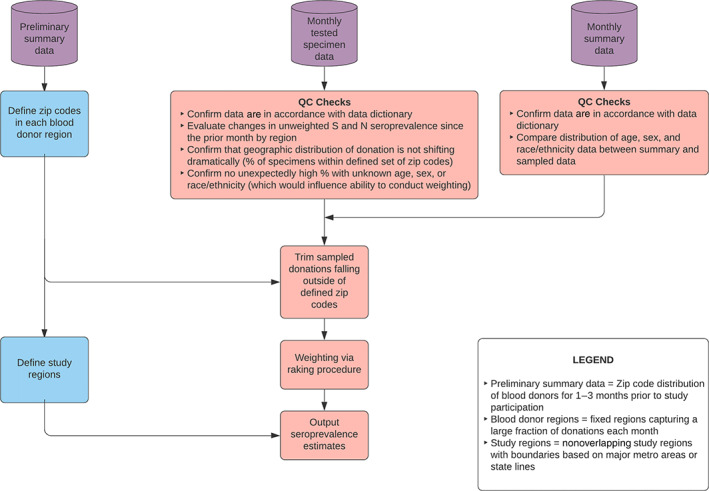
Analysis pipeline flow in the Nationwide Blood Donor Seroprevalence Study (see Supplemental Methods) [Color figure can be viewed at wileyonlinelibrary.com]
3. RESULTS
The Nationwide Blood Donor Seroprevalence Study processed study‐generated and/or existing SARS‐CoV‐2 Ab results and associated demographic data from approximately 150,000 residual blood donor specimens (after analysis exclusion criteria were applied, approximately 135,000 specimens) per month beginning in July 2020 and produced SARS‐CoV‐2 seroprevalence estimates for the 50 U.S. states and Puerto Rico. Each month weighted anti‐S and anti‐NC seroprevalence estimates were produced for 66 study regions, as well as study‐wide, to represent up to 74% of the U.S. population. Each of these estimates was reported overall and by age, sex, and race/ethnicity. Initially, study findings were used by CDC to track community spread by location, age, sex, and race/ethnicity groups. After vaccines became available in December 2020, the study delivered monthly estimates of vaccination status. In the first year of the study, the completion of weighted seroprevalence estimates was delayed 2 to 3 months; however, with the implementation of parallel anti‐S/anti‐NC testing in summer 2021, weighted seroprevalence estimates were ultimately delivered to CDC within approximately 6 weeks following the end of the data collection month.
In June 2021, CDC launched the Nationwide Blood Donor Seroprevalence Survey page on its COVID Data Tracker website, providing seroprevalence estimates to both public health professionals and the general public (Figure 6). Using a Tableau‐generated data visualization tool, users were able to select different views of seroprevalence at monthly time points across the United States and Puerto Rico, including study region‐level estimates as well as results in each study region by age, sex, and race/ethnicity groups. The first published manuscript from the study described the estimated infection‐ and vaccine‐induced SARS‐CoV‐2 seroprevalence. 3
FIGURE 6.
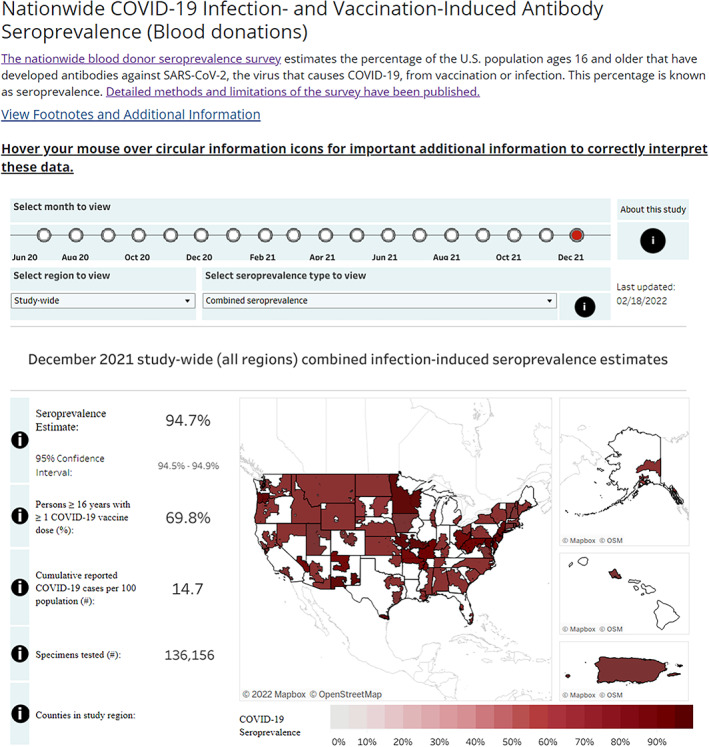
Screen capture of CDC COVID data tracker – Nationwide Blood Donor Seroprevalence Study Page (June 2, 2022) [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Surveillance to ascertain seroprevalence of infection‐ and vaccine‐induced Ab to SARS‐CoV‐2 is a critical component of pandemic response. As the COVID‐19 pandemic unfolded in 2020 and early 2021, the collaborative Nationwide Blood Donor Seroprevalence Study provided retrospective and ongoing adjusted data on the community spread of SARS‐CoV‐2 across the United States. This was the only nationwide, ongoing survey in the United States using the same assays at all sites, enabling geographic, demographic, and temporal comparisons.
Four objectives to be achieved with large‐scale SARS‐CoV‐2 serosurveys include: (1) estimation of the true burden of SARS‐CoV‐2 infections, including mild and asymptomatic infections; (2) tracking the progress of vaccination efforts and differentiation of vaccine‐ and infection‐induced seroprevalence; (3) evaluation of seroprevalence within different population subgroups; and (4) better understanding of the durability of humoral immune responses in the population over time. 10 , 11 , 12 , 13 , 14 , 15
The first objective, determining the proportion of the population that has been infected over time, was the most immediate need when the effort began. Shortly after the emergence of SARS‐CoV‐2, a number of large‐scale, population‐based serological studies with random sampling were undertaken to achieve an accurate estimate of seroprevalence. 16 , 17 , 18 , 19 , 20 , 21 , 22 Although studies employing random sampling of households are representative, they are also resource intensive and therefore have often been limited to narrow geographic areas and/or single points in time. CDC pursued a more practical approach based on the use of residual blood specimens from commercial laboratories 23 and blood donors, 3 the latter of which was the design of this study. Data collected as part of routine donation procedures were anonymized, precluding the need for additional consent beyond that for standard donation, thus enabling estimation of demographically and geographically stratified seroprevalence rates weighted to the general population.
The second objective, tracking vaccine coverage in populations over time, became a critical public health goal starting in 2021. Corresponding with the rapid roll‐out of vaccination, the testing algorithm included reflexive re‐testing of all anti‐S reactive donations with anti‐NC testing, which enabled not only the estimation of the rapidly increasing levels of anti‐S‐only reactivity primarily attributed to vaccination, but also differentiation from combined anti‐S and anti‐NC‐induced seroreactivity due to infection. The inclusion of self‐reported COVID‐19 vaccine status as part of some BCOs' routine donor questionnaire helped refine and validate estimates.
The third objective, evaluation of seroprevalence across demographic subgroups, enabled comparison to CDC‐reported case rates in regional larger populations. Moreover, because serology detects mild and asymptomatic infections, the study achieved more accurate estimates of differential infection penetrance among demographic subgroups. The results allow for the evaluation of regional mitigation policies such as gathering bans and masking mandates in multiple populations. The fourth objective, understanding the persistence of population immunity, is a strength of the study for several reasons as detailed in Stone et al., 4 including the ability to conduct ecologic analyses.
This study had several limitations. Limitations of the study design impacting the accuracy of seroprevalence estimates were described by Jones et al. 3 Briefly, the blood donor population differs from the general population. Despite weighting to account for demographic differences in age, sex, and race/ethnicity, other population differences may remain because certain groups (e.g., persons who are acutely ill or institutionalized) are unable to donate blood. 24 Furthermore, the seroprevalence estimates from the study cover only regions representing an estimated 74% of the U.S. population. From an operations perspective, organizing and managing a study of this magnitude, breadth, and rapidity across multiple organizations and entities presented both expected and unanticipated challenges. Generation of complete weighted estimates competed with the need for more immediate feedback on community spread, especially in the early stages of the pandemic. For example, the rapid release of unadjusted raw screening results might have provided initial real‐time estimates to compare with case reporting measures. However, to avoid potential confusion with multiple sets of seroprevalence estimates, CDC decided to release only final weighted results to state and local health officials. Future national programs would benefit from flexible protocols that anticipate the dissemination of interim and final data.
Even if early release of unweighted screening results had been planned, the initial results still may have been somewhat delayed. The national serosurvey placed extensive demands on BCOs and testing laboratories, most of whom had minimal or no increases in staffing and who sustained increased staff turnover. One to two times per month existing staff were asked to isolate, track, and ship large numbers of specimens between multiple laboratories and prepare, update, and submit accurate electronic records of sample donations and testing results. Coordinating and standardizing specimen and data flows across multiple organizations with differing donor and testing data management platforms and capacity added to the complexity.
There are benefits to making these data accessible to public health professionals and the general public. For example, audiences may be interested in which groups have lower anti‐S reactivity or have higher rates of anti‐NC reactivity, and what these data may suggest about potential need for vaccination campaign efforts or targeted mitigation/education campaigns. Our data are available to the public via a CDC website 25 , 26 and updated regularly on the public‐facing CDC COVID Data Tracker to share this information for such purposes, and local jurisdictions can use local data because data are available at a regional level. However, challenges to validating these data and developing data dissemination materials may delay its release in a timely way to inform public health action practice. Thus, it may sometimes be difficult to use these data to guide decision‐making at the local level.
Extensive QC and data validation systems were required. Inadequate staffing during movement restriction orders and other challenges of the pandemic added to the difficulties. Additionally, operations had to be responsive to the frequently changing conditions of the pandemic and the associated changes in study objectives. Extensive communication was essential. Finally, only minimal disruptions in blood collection processes as a result of executing this program could be tolerated during a time of crisis in blood availability.
Support for organizations that train laboratory personnel to be mobilized in times of public health emergencies would facilitate rapid response. Future preparedness would also benefit from funding to enhance and standardize data handling capacity in the blood collection industry. Lessons learned and suggestions to improve preparedness for similar future epidemic surveillance efforts are presented in Table 1.
TABLE 1.
Considerations to improve preparedness for future epidemic serosurveillance using large‐scale blood donor serosurveys
| Challenges encountered in large‐scale serosurvey | Considerations to improve preparedness for future epidemic serosurveillance |
|---|---|
| No existing national BCO network committed to providing donor data and procuring residual blood specimens to serve epidemic testing needs |
Identify and maintain a national network of BCOs to be prepared to provide donor data and residual blood donation specimens ready to contribute residual specimens when needed. An existing network could quickly determine infection seroprevalence levels regionally and temporally when outbreaks warranting donor serosurveillance occur. Identify appropriate groups to govern the national network, including a national coordinating entity (NCE). NCE to include representatives from the relevant government agencies and the blood collection industry as well as others with complementary expertise. Continuous funding to support the operations of the NCE and additional activities as summarized below. |
| No existing administrative framework for acquisition and testing of residual blood donor specimens |
Establish national guidelines that would enable testing of de‐identified residual blood specimen results for new pathogens. Anticipate appropriate human subjects research requirements, where necessary. Maintain and enhance capacity to establish agreements between individual BCOs, donor testing labs, and the NCE or its delegate to function in response to prioritized outbreaks, including roles and responsibilities for BCOs, testing labs and the NCE. Maintain payment systems for entire program, including specimen acquisition, infectious disease testing for blood safety, testing for relevant serological markers, shipment of specimens to specialized testing laboratories, preparation of data files, and analyses. |
| BCOs and testing labs have varying levels of expertise and capacity to support relevant laboratory and data science needs |
Each BCO and testing lab to define how it can quickly expand laboratory‐trained personnel at the start of a newly identified regional or national outbreak. Each BCO and testing lab to maintain minimum data processing capabilities needed to support rapid implementation and appropriate response consistent with evolving public health needs. Each BCO to develop and maintain operational preparedness, including SOPs and relevant training of BCO staff. Designate and train laboratory personnel located within the testing laboratories of each contributing BCO who, in times of national emergency, could be temporarily re‐assigned to support a dramatically increased volume of specimen acquisition and testing of residual blood donor specimens. |
| No guidelines for generating population estimates from BCO catchment areas |
Establish and maintain population characteristics through regular submission (at least annually) of donation population information for each BCO in the national network. NCE to aggregate statistics on blood donations by zip codes of origin and demographic characteristics of donors. NCE to determine, on a frequent basis, how to pool donations from participating BCOs to generate testing results at the state level and by demographic subgroups. NCE to communicate a list of zip codes in sampling scheme to each BCO. Each BCO to develop and maintain up‐to‐date sampling algorithms and procedures. |
| No guidelines for timely communication of testing results to appropriate public health authorities |
NCE to develop procedures for communication of study data to state and local health authorities. NCE to develop and maintain technology to effectively communicate results. NCE to establish protocols and procedures for communication of preliminary screening data to support real‐time feedback on community spread of novel infectious disease posing substantial risk to the larger population. |
| Flexibility needed in regulatory framework |
Regulatory standards in the blood collection industry should anticipate the need to change processes more quickly in response to an emerging epidemic. For example, BCOs should be able to change their donor intake forms and processes to collect vaccination history or other donor information as needed in the context of a national emergency. NCE to work with regulators and blood collection industry to adopt guidelines granting authority to swiftly adjust procedures during public health emergencies. |
| Limited ability of cross‐sectional approach to track evolution of immunologic responses over time within individual donors | Establish “immunological observatory” of repeat donor cohorts with longitudinal specimens to compliment cross sectional serosurveillance. Serial serological data from repeat donations from donors with various categories of previous infection/vaccination provides the opportunity to identify and track incident infections and characterize Ab waning, vaccine breakthrough infections, and boosting following reinfections. |
A major strength of this study was the strong partnership between CDC and the blood collection industry in a study of national scope that enabled rapid scale‐up and agile response to the evolving pandemic. The study was a practical, relatively efficient approach to estimate cumulative incidence of SARS‐CoV‐2 infections with results made publicly available through an interactive website maintained by CDC.
Large‐scale serosurveillance is an essential tool in the management of pandemics. The World Health Organization underscored the need for serosurveillance in its global roadmap for the management of COVID‐19. 27 In 2021, the Bipartisan Policy Center called for the integration of data from all types of patient care, including blood banks, 28 into a modern disease surveillance system better positioned to respond to and mitigate the consequences of this and potential future pandemics. 29 Maintaining key infrastructure developed by this successful public–private collaboration as a tool for future preparedness would facilitate rapid delivery of timely seroprevalence estimates. Future manuscripts describing the results of the Nationwide Blood Donor Seroprevalence Study are planned, including analyses of self‐reported vaccine history and of seroprevalence patters in a cohort of repeat blood donors.
5. CONCLUSIONS
Amidst a rapidly evolving pandemic, significant logistical challenges, and a complex network of specimen and data flows, a national serosurvey based on residual blood donor samples provided demographic and geographically weighted prevalence estimates of SARS‐CoV‐2 infection and vaccination rates nationwide and over time. Features of this public–private program could be maintained on an ongoing basis to promote pandemic preparedness.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention and Agency for Toxic Substances and Disease Registry (CDC).
Supporting information
Appendix S1: Supplemental Methods
Appendix S2: Data Guide
ACKNOWLEDGMENTS
The Nationwide Blood Donor Seroprevalence Study is the responsibility of the following persons: Centers for Disease Control and Prevention: J. Jones, M. Miller, B. Biggerstaff, T. Benoit, and N. Thornburg, Atlanta, GA. Vitalant Research Institute: M.P. Busch and M. Stone, San Francisco, CA. Westat: S.M. Mathew, J. Opsomer, and R. Fink, Rockville, MD. Blood Collection Organizations (BCOs): American Red Cross, Gaithersburg, MD, S. Stramer, E. Notari, and P. Saa; Banco de Sangre de Servicios Mutuos, San Juan, PR, G. Latoni; Blood Bank of Alaska, Anchorage, M. Ritter; Blood Bank of Hawaii, Honolulu, HI, K. Nguyen; Blood Works Northwest, Seattle, WA, M. Destree; Carter BloodCare, Bedford, TX, M. Sayers; Gulf Coast Regional Blood Center, Houston, Texas, S. Rossman; LifeServe, Des Moines, Iowa, S. Sime; LifeSouth Community Blood Centers, Gainesville, Florida, C. Lough; New York Blood Center Enterprise, New York, NY, D. Strauss and D. Kessler; OneBlood, St. Petersburg, FL, R Reik and C. Engert; The Blood Center, New Orleans, Louisiana, B. Weales; Vitalant, San Francisco, CA and Scottsdale, AZ, B. Custer and R. Vassallo; Versiti, Milwaukee, Wisconsin, D. Bougie and A. Mast. Testing Laboratories: Creative Testing Solutions, Temple, AZ, P. Williamson, S. Cyrus, and V. Green; Innovative Blood Resources, St Paul, Minnesota, J. Gorlin; Gulf Coast Regional Blood Center, Houston, Texas, S. Rossman; Rhode Island Blood Center, Providence, RI, J. Alberigo and D. Strauss; The Blood Center, New Orleans, Louisiana, B. Weales and R. Chatelain; Vitalant Research Institute, San Francisco, CA, C. Di Germanio and G. Simmons. Consultant: S. Kleinman, University of British Columbia, Victoria, British Columbia, Canada. The authors would like to acknowledge Dane Freeman and Micaela Siraj of Georgia Tech Research Institute; the many staff at CDC, VRI, Westat, American Red Cross, and the participating BCOs and testing laboratories; and the blood donors whose specimens were analyzed in this study.
Fink RV, Fisher L, Sulaeman H, Dave H, Levy ME, McCann L, et al. How do we …form and coordinate a national serosurvey of SARS‐CoV‐2 within the blood collection industry? Transfusion. 2022;62(7):1321–1333. 10.1111/trf.16943
Funding information Centers for Disease Control and Prevention, Grant/Award Number: 75D30120C08170
REFERENCES
- 1. Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS‐CoV‐2 with SARS‐CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oran DP, Topol EJ. The proportion of SARS‐CoV‐2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174(5):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, et al. Estimated US infection‐ and vaccine‐induced SARS‐CoV‐2 seroprevalence based on blood donations, July 2020‐May 2021. JAMA. 2021;326:1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone M, Di Germanio C, Wright DJ, Sulaeman H, Dave H, Fink RV, et al. Use of U.S. blood donors for national serosurveillance of SARS‐CoV‐2 antibodies: basis for an expanded national donor serosurveillance program. Clin Infect Dis. 2022;74:871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glynn SA, Busch MP, Dodd RY, Katz LM, Stramer SL, Klein HG, et al. Emerging infectious agents and the nation's blood supply: responding to potential threats in the 21st century. Transfusion. 2013;53(2):438–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen LR, Cassetti MC. Learning about Zika virus epidemiology and diagnostics from blood donor studies. Lancet Infect Dis. 2020;20(12):1357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilheimer LT, Klein RJ. Data and measurement issues in the analysis of health disparities. Health Serv Res. 2010;45(5 Pt 2):1489–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steele WR, Dodd RY, Notari EP, Xu M, Nelson D, Kessler DA, et al. Prevalence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus in United States blood donations, 2015 to 2019: the Transfusion‐Transmissible Infections Monitoring System (TTIMS). Transfusion. 2020;60(10):2327–39. [DOI] [PubMed] [Google Scholar]
- 9. Di Germanio C, Simmons G, Kelly K, Martinelli R, Darst O, Azimpouran M, et al. SARS‐CoV‐2 antibody persistence in COVID‐19 convalescent plasma donors: dependency on assay format and applicability to serosurveillance. Transfusion. 2021;61(9):2677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Donnici C, et al. Global seroprevalence of SARS‐CoV‐2 antibodies: a systematic review and meta‐analysis. PLoS One. 2021;16(6):e0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lerner AM, Eisinger RW, Lowy DR, Petersen LR, Humes R, Hepburn M, et al. The COVID‐19 serology studies workshop: recommendations and challenges. Immunity. 2020;53(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid‐19 ‐ studies needed. N Engl J Med. 2020;382(13):1194–6. [DOI] [PubMed] [Google Scholar]
- 14. Lourenço J, Paton R, Thompson C, Klenerman P, Gupta S. Fundamental principles of epidemic spread highlight the immediate need for large‐scale serological surveys to assess the stage of the SARS‐CoV‐2 epidemic. medRxiv. 2020. [Google Scholar]
- 15. Chen X, Chen Z, Azman AS, Deng X, Sun R, Zhao Z, et al. Serological evidence of human infection with SARS‐CoV‐2: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9(5):e598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai CC, Wang JH, Hsueh PR. Population‐based seroprevalence surveys of anti‐SARS‐CoV‐2 antibody: an up‐to‐date review. Int J Infect Dis. 2020;101:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pollan M, Perez‐Gomez B, Pastor‐Barriuso R, Oteo J, Hernan MA, Perez‐Olmeda M, et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet. 2020;396(10250):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ward H, Cooke GS, Atchison C, Whitaker M, Elliott J, Moshe M, et al. Prevalence of antibody positivity to SARS‐CoV‐2 following the first peak of infection in England: serial cross‐sectional studies of 365,000 adults. Lancet Reg Health Eur. 2021;4:100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murhekar MV, Bhatnagar T, Selvaraju S, Rade K, Saravanakumar V, Vivian Thangaraj JW, et al. Prevalence of SARS‐CoV‐2 infection in India: findings from the national serosurvey, May‐June 2020. Indian J Med Res. 2020;152(1 & 2):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hallal PC, Hartwig FP, Horta BL, Silveira MF, Struchiner CJ, Vidaletti LP, et al. SARS‐CoV‐2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8(11):e1390–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haq M, Rehman A, Ahmad J, Zafar U, Ahmed S, Khan MA, et al. SARS‐CoV‐2: big seroprevalence data from Pakistan—is herd immunity at hand? Infection. 2021;49(5):983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tadesse EB, Endris AA, Solomon H, Alayu M, Kebede A, Eshetu K, et al. Seroprevalence and risk factors for SARS‐CoV‐2 infection in selected urban areas in Ethiopia: a cross‐sectional evaluation during July 2020. Int J Infect Dis. 2021;111:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ, et al. Seroprevalence of antibodies to SARS‐CoV‐2 in 10 sites in the United States, March 23‐May 12, 2020. JAMA Intern Med. 2020;180(12):1576–1586. [DOI] [PubMed] [Google Scholar]
- 24. Li Z, Lewis B, Berney K, Hallisey E, Williams AM, Whiteman A, et al. Social vulnerability and rurality associated with higher SARS‐CoV‐2 infection‐induced seroprevalence: a nationwide blood donor study, United States, July 2020 ‐ June 2021. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nationwide Blood Donor Seroprevalence Survey infection‐induced seroprevalence estimates. https://data.cdc.gov/Laboratory-Surveillance/Nationwide-Blood-Donor-Seroprevalence-Survey-Infec/mtc3-kq6r
- 26. Nationwide Blood Donor Seroprevalence Survey combined infection‐ and vaccination‐induced seroprevalence estimates. https://data.cdc.gov/Laboratory-Surveillance/Nationwide-Blood-Donor-Seroprevalence-Survey-Combi/wi5c-cscz
- 27. A coordinated global research roadmap: 2019 Novel Coronavirus. World Health Organization. March 2020.
- 28. Saeed S, Uzicanin S, Lewin A, Lieshout‐Krikke R, Faddy H, Erikstrup C, et al. Current challenges of severe acute respiratory syndrome coronavirus 2 seroprevalence studies among blood donors: a scoping review. Vox Sang. 2022;117(4):476–487. [DOI] [PubMed] [Google Scholar]
- 29. Positioning America's Public Health System for the Next Pandemic. Bipartisan Policy Center, June 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplemental Methods
Appendix S2: Data Guide


