Abstract
Although the benefit/risk profile for mRNA COVID‐19 vaccines is recognised as extremely favourable, appendicitis is currently considered an adverse event (AE) of special interest. We describe the case of a 58‐year‐old female who presented with signs and symptoms of appendicitis approximately 48 hours after her first injection of the Pfizer‐BioNTech vaccine. Abdominal ultrasound revealed fluid collection in the right iliac fossa and cecal wall thickening. Following the surgical visit, CT scan with contrast showed a distended appendix with thickened walls, suggestive of acute appendicitis. The patient tested negative to upper respiratory COVID‐19 reverse transcription‐polymerase chain reaction. Clinical trials and observational studies suggest a possible association between appendicitis and COVID‐19 vaccines. Th‐1 driven granulomatous inflammation reported in our case represents an infrequent nonspecific chronic inflammation of the appendix, especially in the setting of delayed or interval appendectomy. In view of the current paediatric vaccination campaign, we recommend monitoring the safety profile and potential gastrointestinal AEs associated with mRNA COVID‐19 vaccines to swiftly manage subjects with gastrointestinal symptoms and prevent potential complications.
Keywords: appendicitis, case report, COVID‐19, histology, pharmacovigilance, vaccine
What is already known about this subject
mRNA COVID‐19 vaccines are recognised as extremely effective and safe.
Post‐marketing surveillance of adverse drug reactions is based more on spontaneous reporting and observational studies than on clinical trials.
Serious adverse events have been suggested as signals in the post‐marketing surveillance of COVID‐19 vaccines.
Appendicitis is currently considered a serious adverse event of special interest.
What this study adds
COVID‐19 vaccines induce strong Th1 immune responses.
A relationship between an increased Th1‐driven response and appendicitis has been suggested.
As in our case, granulomatous inflammation is a common form of nonspecific chronic inflammation of the appendix.
1. INTRODUCTION
More than 2 years into the COVID‐19 pandemic, an unprecedented number of mass vaccination programs are underway in several countries. To date, over 10 billion doses of vaccine have been administered worldwide. 1
Approval studies have shown that both adenovirus‐vectored and messenger RNA (mRNA) COVID‐19 vaccines are efficacious and have a very good safety profile. No clinical trials have as yet reported any serious safety hazards, demonstrating a low incidence of serious adverse events (AEs), defined as any untoward medical occurrence which, at any dose, causes patient death, hospitalisation or its prolongation, or else persistent disability/incapacity. Nevertheless, serious AEs may not always emerge in phase 3 studies due to the small sample size, restrictive inclusion criteria, limited duration of follow‐up and study participants at times differing from the general population.
Appendicitis is an acute gastrointestinal condition that is usually most frequent in younger people. Indeed, it commonly occurs between the ages of 10 and 30, with the highest incidence in children and adolescents. 2 Appendicitis is mentioned in the information fact sheet of the Pfizer‐BioNTech vaccine approved by the FDA after an increase in cases in the vaccine arm of a large clinical trial. 3 However, no causal relationship has been established nor is it labelled for the other vaccines. 4 In view of the recent authorization for administering COVID‐19 vaccines to the paediatric population, especially mRNA vaccines, more attention must be paid to avoid possible complications related to acute appendicitis in this subset.
2. CASE DESCRIPTION
In mid‐January 2021, a 58‐year‐old female academic healthcare professional presented to the emergency department (ED) of Careggi University Hospital (Florence, Italy) with pain in the right iliac fossa (RIF). She reported mild pain in the same site 2 weeks earlier, which had subsided with oral scopolamine and a bland diet. The patient referred to having fever, nausea and vomiting the day before ED admission and that her general practitioner prescribed a penicillin‐based antibiotic (amoxicillin/clavulanate), with no improvement in symptoms. She denied diarrhoea, dyspnoea and dysuria. Furthermore, the patient has never tested positive for COVID‐19 in the past. The patient stated she had received the I dose of Pfizer‐BioNTech vaccine approximately 48 hours prior to ED admission (January 16, 2021).
Her past medical history included left quadrantectomy with radiotherapy for breast cancer and, at the time of ED admission, she was on perindopril/amlodipine therapy for arterial hypertension. The patient confirmed she was a nonsmoker, did not drink alcohol or use recreational drugs, nor was she allergic to any drugs.
At physical examination, the abdomen was flat and soft, but painful on deep palpation in RIF and hypogastrium. Blumberg, Murphy and Giordano signs were negative, and peristalsis was normal. ECG revealed normal heart rate, PR and ST‐T intervals.
Laboratory tests showed normal haemoglobin levels (12.5 g/dL, range 12.0‐16.0), significant leukocytosis (12.1 × 109/L, range 4.0‐10.0), neutrophilia (78.4%, range 37.0‐75.0), high fibrinogen levels (636.0 mg/dL, range 200.0‐400.0) and increased prothrombin time (13.3 seconds, range 9.5‐12.0). Amylase, lipase, urine analysis, liver and renal function tests were normal. The RT‐PCR test for COVID‐19, following hospital protocol, was negative.
An abdominal ultrasound scan was requested for suspected appendicitis, which revealed fluid collection in the RIF and thickening of the cecal wall. CT scan with contrast disclosed a distended appendix (maximum thickness 12 mm) with thickened walls, typical of acute appendicitis. The patient was transferred to an inpatient ward and a video‐laparoscopic appendectomy was performed under full COVID‐19 precautions.
On gross examination, the appendix was swollen, measuring 8 cm in length and 2.5 cm in maximum diameter. Histology showed diffuse acute and chronic inflammatory infiltrates with scattered non‐necrotizing granulomas throughout all layers of the appendiceal wall (Figure 1). Of note, numerous granulomas were identified within the germinal centres of lymphoid follicles. The presence of serosal giant cells surrounded by florid granulation tissue was suggestive of prior appendiceal rupture. No foreign bodies, obstructing lesions or infective microorganisms were detected.
FIGURE 1.
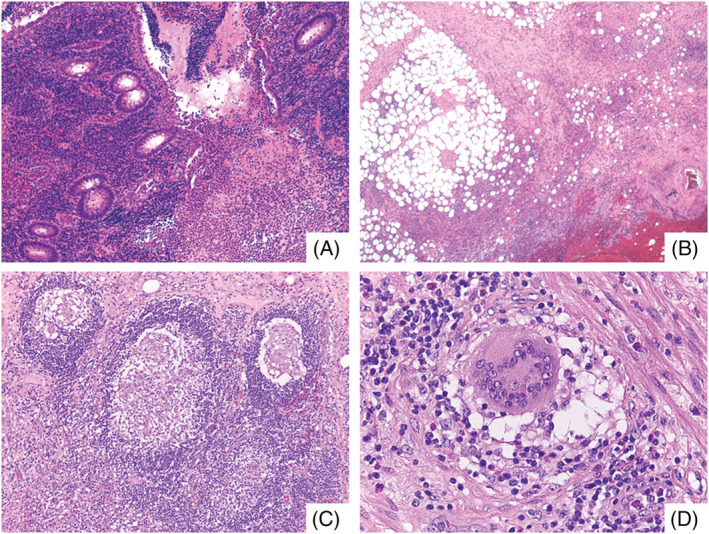
A, Prominent mucosal disease with cryptitis, crypt abscess and erosions. Collections of neutrophils were seen in the lumen (H&E, ×100). B, Subserosal layer of the appendix with fibrosis and abundant acute and chronic inflammation (H&E, ×100). C, Non‐necrotizing epithelioid granulomas within germinal centres of lymphoid follicles (H&E, ×200). D, Scattered foreign‐body type giant cells were also present (H&E, ×400)
The postoperative course was uneventful. After 4 days of hospitalisation (one in the ED and three on the ward), the patient was discharged and prescribed oral amoxicillin/clavulanate, subcutaneous low‐molecular‐weight heparin and oral acetaminophen/codeine as needed.
3. FINAL DIAGNOSIS AND OUTCOME
Final diagnosis of the case was acute appendicitis. Applying the 2018 WHO algorithm to assess the causal relationship between mRNA COVID‐19 vaccine and acute appendicitis, 5 (B1 – Temporal relationship is consistent but there is insufficient definitive evidence that vaccine caused the event).
3.1. Follow‐up
The patient was followed up at home by her general practitioner, with no clinical complications. The patient was administered with the II (February 6, 2021) and III doses (October 27, 2021) of mRNA COVID‐19 vaccine without the occurrence of any other AEs.
4. DISCUSSION
Clinical trials have shown that most COVID‐19 vaccines are efficacious, with a satisfactory safety profile. 6 Trials on the Pfizer‐BioNTech vaccine have revealed a mild imbalance between vaccinated and placebo groups with respect to the number of cases of hypersensitivity reactions, acute myocardial infarction, cerebrovascular accidents and appendicitis. 7 It is unclear, however, whether the risk of appendicitis is higher in the vaccinated than in the general population. Evidence from literature is scarce, with conflicting results, and global longitudinal studies are required to clarify any possible relationship.
Using data from the largest healthcare organisation in Israel, Barda et al 7 noted that such a risk was higher in vaccinated than nonvaccinated patients (risk ratio 1.40, 95% confidence interval [CI] 1.02 to 2.01, risk difference 5.0 events per 100 000 persons, 95% CI 0.3 to 9.9). These authors also examined records from over 240 000 infected subjects to estimate the effects of documented SARS‐CoV‐2 infection on the incidence of the same AEs and found that SARS‐CoV‐2 infection was not associated with any meaningful events, including appendicitis.
Li et al 8 performed a multinational network cohort study to quantify the background incidence rates of 15 pre‐specified AEs that might need evaluation after COVID‐19 vaccination. Based on electronic health records and health claims data from eight countries (Australia, France, Germany, Japan, the Netherlands, Spain, the United Kingdom and the United States), they found that anaphylaxis, Bell's palsy, appendicitis and immune thrombocytopenia were essentially rare in all age groups. Notwithstanding the considerable variability of AE rates within age and sex subgroups, appendicitis was more common in younger populations.
From a pharmacovigilance perspective, Mitchell et al 4 reviewed all cases of appendicitis following administration of COVID‐19 vaccines reported to VigiBase (WHO). The authors observed an apparent increase of such cases within 4 days of vaccination and a possible dose‐response relationship.
Globally, the incidence of appendicitis is approximately 11 cases per 10 000 life years, 9 with the highest incidence in late childhood and early adulthood. The occurrence of appendicitis is low in the neonatal period but most frequent between ages 12 and 18, where it is the leading cause of abdominal surgical emergency.
The exact pathophysiology of appendicitis, as well as any potential causal association with COVID‐19 vaccines, is still unclear. Appendicoliths, lymphoid hyperplasia and lymphadenopathy have been identified as relevant causes of appendicitis. 4 Although lymphadenopathy is listed among AEs of COVID‐19 vaccines, abdominal lymphadenopathies are greatly underreported since they are usually diagnosed only after abdominal imaging. Immune response could also contribute to the onset of appendicitis. COVID‐19 status should also be considered, as a previous infection may enhance immune response to vaccination. 10 Interestingly, COVID‐19 vaccines induce strong Th1 immune responses and a possible relationship has been suggested between an increased Th1‐driven response and appendicitis. In particular, Th1 responses and cytotoxic cells may provide the background for the development of gangrenous and perforated appendicitis, in contrast to the Th2 immune response observed in phlegmonous appendicitis. 11
Granulomatous appendicitis is seen in less than 2% of appendectomy specimens. However, granulomatous inflammation represents a common form of nonspecific chronic inflammation of the appendix, especially in the setting of delayed or interval appendectomy, as in our case. 12 Pathologists should be aware of this inflammatory pattern, and not to misinterpret findings as Crohn's disease, infectious disorders, sarcoidosis or foreign‐body reactions without relevant clinical history. 13
Recently, an observational study aimed to describe US surveillance data collected both through the Vaccine Adverse Event Reporting System (VAERS) and through v‐safe during the first 6 months of the US COVID‐19 vaccination program found a total of 383 cases of appendicitis associated with mRNA vaccines, accounting for 1.3 reports per million doses administered. Based on available real‐world data, appendicitis is currently considered an AE of special interest. 14
At the end of March 2022, Kildegaard et al in Denmark performed a nationwide cohort study to assess the risk of appendicitis after receiving an mRNA COVID‐19 vaccine compared with the risk of appendicitis in unvaccinated individuals. 15 Although authors reported several limitations, they found no association between immunisation with mRNA‐based COVID‐19 vaccines and appendicitis, and they stated that further studies from different settings are needed to clarify this association.
While clinical trials on the efficacy and safety of COVID‐19 vaccines have not yet involved the paediatric population, the European Medicines Agency has recommended extending the use of the Pfizer‐BioNTech vaccine to children aged 5 to 11. 16 Since limited information is available on acute appendicitis following immunisation, especially in children, healthcare providers and national health services should be aware of the potential relationship between COVID‐19 vaccines and acute appendicitis.
5. CONCLUSIONS
The present case highlights the risk of acute appendicitis as an event potentially associated with COVID‐19 vaccines. Considering the ongoing COVID‐19 vaccination campaign aimed at younger individuals, we recommend monitoring safety profiles and potential AEs especially in children so that any gastrointestinal symptoms following immunisation can be rapidly diagnosed and managed.
COMPETING INTERESTS
All authors have no competing interests to disclose.
CONTRIBUTORS
N.L. conceived the original idea. E.M., G.C. and N.L. wrote the manuscript. L.P., R.S. and G.N. performed the morphological analyses. R.B., E.C. and A.V. contributed to the analyses of the results and revised the manuscript for critical content. All authors revised and approved the manuscript.
ACKNOWLEDGMENTS
The authors declare that this research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. Open Access Funding provided by Universita degli Studi di Firenze within the CRUI‐CARE Agreement.
Marconi E, Crescioli G, Bonaiuti R, et al. Acute appendicitis in a patient immunised with COVID‐19 vaccine: A case report with morphological analysis. Br J Clin Pharmacol. 2022;1‐5. doi: 10.1111/bcp.15421
Ettore Marconi, Giada Crescioli, Alfredo Vannacci and Niccolò Lombardi are the co‐first and co‐last authors.
The authors confirm that the principal investigator for this article is Niccolò Lombardi.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Our World In Data . Coronavirus (COVID‐19) Vaccinations. Published 2022. Accessed March 21, 2022. https://ourworldindata.org/covid-vaccinations?country=OWID_WRL
- 2. di Saverio S, Podda M, de Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15(1):27. doi: 10.1186/S13017-020-00306-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. FDA . Comirnaty and Pfizer‐BioNTech COVID‐19 Vaccine. Published 2022. Accessed March 21, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine#additional
- 4. Mitchell J, Yue QY. Appendicitis as a possible safety signal for the COVID‐19 vaccines. Vaccine X. 2021;9:100122. doi: 10.1016/j.jvacx.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO World Health Organization . Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO Classification. Second Edition.; 2018. Accessed March 22, 2022. chrome‐extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl = https%3A%2F%2Fwww.who.int%2Fvaccine_safety%2Fpublications%2Faefi_manual.pdf&clen = 1 956 918&chunk = true.
- 6. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barda N, Dagan N, Ben‐Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078‐1090. doi: 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li X, Ostropolets A, Makadia R, et al. Characterising the background incidence rates of adverse events of special interest for covid‐19 vaccines in eight countries: multinational network cohort study. BMJ. Published online June 14. 2021;n1435. doi: 10.1136/bmj.n1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferris M, Quan S, Kaplan BS, et al. The global incidence of appendicitis. Ann Surg. 2017;266(2):237‐241. doi: 10.1097/SLA.0000000000002188 [DOI] [PubMed] [Google Scholar]
- 10. Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS‐CoV‐2‐infected individuals. Lancet. 2021;397(10279):1057‐1058. doi: 10.1016/S0140-6736(21)00501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubér M, Berg A, Ekerfelt C, Olaison G, Andersson RE. Different cytokine profiles in patients with a history of gangrenous or phlegmonous appendicitis. Clin Exp Immunol. 2005;143(1):117‐124. doi: 10.1111/j.1365-2249.2005.02957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo G, Greenson JK. Histopathology of interval (delayed) appendectomy specimens. Am J Surg Pathol. 2003;27(8):1147‐1151. doi: 10.1097/00000478-200308000-00013 [DOI] [PubMed] [Google Scholar]
- 13. Tucker ON, Healy V, Jeffers M, Keane FBV. Granulomatous appendicitis. Surgeon. 2003;1(5):286‐289. doi: 10.1016/S1479-666X(03)80047-1 [DOI] [PubMed] [Google Scholar]
- 14. Rosenblum HG, Gee J, Liu R, et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID‐19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v‐safe. Lancet Infect Dis. 2022;22(6):802‐812. doi: 10.1016/S1473-3099(22)00054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kildegaard H, Ladebo L, Andersen JH, et al. Risk of appendicitis after mRNA COVID‐19 vaccination in a Danish population. JAMA Intern Med. 2022. Apr 25;e221222. doi: 10.1001/jamainternmed.2022.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. EMA . Comirnaty COVID‐19 vaccine: EMA recommends approval for children aged 5 to 11. Published 2022. Accessed March 21, 2022. https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11#:~:text=EMA's%20human%20medicines%20committee%20(CHMP,children%20aged%2012%20and%20above
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


