Abstract
Clinical outcomes in solid organ transplant (SOT) recipients with breakthrough COVID (BTCo) after two doses of mRNA vaccination compared to the non-immunocompromised/immunosuppressed (ISC) general population, are not well described. In a cohort of adult patients testing positive for COVID-19 between December 10, 2020 and April 4, 2022, we compared the cumulative incidence of BTCo in a non-ISC population to SOT recipients (overall and by organ type) using the National COVID Cohort Collaborative (N3C) including data from 36 sites across the United States. We assessed the risk of complications post-BTCo in vaccinated SOT recipients versus SOT with unconfirmed vaccination status (UVS) using multivariable Cox proportional hazards and logistic regression. BTCo occurred in 4776 vaccinated SOT recipients over a median of 149 days (IQR 99–233), with the highest cumulative incidence in heart recipients. The relative risk of BTCo was greatest in SOT recipients (relative to non-ISC) during the pre-Delta period (HR 2.35, 95% CI 1.80–3.08). The greatest relative benefit with vaccination for both non-ISC and SOT cohorts was in BTCo mortality (HR 0.37, 95% CI 0.36–0.39 for non-ISC; HR 0.67, 95% 0.57–0.78 for SOT relative to UVS). While the relative benefit of vaccine was less in SOT than non-ISC, SOT patients still exhibited significant benefit with vaccination.
KEYWORDS: allograft type, breakthrough, cardiac, COVID-19, heart, infection, kidney, liver, lung, MACE, MARCE, mortality, outcome, SARS-CoV-2, solid organ transplantation, vaccination
Abbreviations: AKI, acute kidney injury; BMI, body mass index; BTCo, breakthrough COVID-19; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HR, hazard ratio; MACE, major adverse cardiac event; MARCE, major adverse renal or cardiac event; N3C, national COVID cohort collaborative; NCATS, National Center for Advancing Translational Sciences; Non-ISC, non-immunosuppressed/immunocompromised; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SOT, solid organ transplant; UVS, unconfirmed vaccination status; VAX2, 2 mRNA vaccine doses or 1 Johnson & Johnson dose; VAX3, VAX2 with an additional dose of any vaccine type
1. INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in an unprecedented strain on social, economic, and health care systems, with solid organ transplant (SOT) recipients being among the most vulnerable. The incidence of COVID-19 in SOT recipients is ~15-fold higher than in the general immunocompetent population1 and SOT patients appear to be at higher risk of severe outcomes.1, 2, 3 In the pre-vaccine COVID era, SOT recipients were noted to have a case fatality rate of ~20%,3, 4, 5 versus 0.8%–2% risk in the general population6 and in the 90 days following COVID-19 diagnosis, >40% of SOT recipients experience a major adverse renal or cardiac event (MARCE).7 The risk of post-COVID outcomes differs by organ type, with heart and kidney recipients at the highest risk of MARCE, and lung and heart recipients at the highest risk of organ rejection.8 , 9
COVID-19 vaccines, which developed rapidly over the first year of the pandemic, have proved remarkably effective in the general population, including the elderly and those with comborbidities10, 11, 12; however, the benefit of vaccination in immunosuppressed SOT has been questioned. Although SOT recipients appear to mount an adequate humoral response to natural SARS-CoV-2 infection, poor anti-spike antibody responses to mRNA vaccination in SOT recipients have been reported (e.g., 46%–97.4% non-response after the second dose).13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 While some studies have demonstrated evidence of increased cellular immunity post-vaccination,13 , 24 , 25 others have shown significant impairment of both humoral and cellular immune responses in SOT recipients.15 , 18 This has led to significant changes in CDC guidance for immunosuppressed patients.26
Because no threshold has been established for the minimum neutralizing antibody titer required for protective immunity, studies examining clinical outcomes post-vaccination in immunosuppressed populations are paramount. Yet, most studies of vaccine efficacy in SOT recipients to date have examined the immune response to vaccination, with a much smaller proportion examining clinical outcomes occurring with breakthrough COVID (BTCo) infection. In a recent study of 226 kidney transplant recipients who received BNT162b2 mRNA vaccination, 16% (n = 37) experienced BTCo infections (vs. 22% of unvaccinated controls). There was no difference in COVID-19 severity in the vaccinated patients versus unvaccinated controls in terms of hospitalization or mortality rates; this did not differ in those who had received two mRNA vaccine doses or one Johnson & Johnson dose (VAX2) versus those with only a single dose.13 Another study of >18 000 VAX2 SOT recipients from 17 centers across the United States demonstrated a mortality rate of 9.3% in 151 BTCo infections; an 82-fold increased risk of BTCo infection and a 485-fold higher risk of mortality in VAX2 SOT recipients than in the VAX2 general US population.27 Finally, recent work by Ravanan in the United Kingdom Health System noted a higher mortality rate (12%) after SARS-CoV-2 infection in unvaccinated SOT versus those vaccinated with either Pfizer (two doses) or Astra Zeneca (n = 143, mortality in 7.7%).9 A more in depth analysis of complications following BTCo has yet to be performed.
While the protective benefits of vaccination are attenuated in immunocompromised populations, other studies have demonstrated significant benefit in vaccinated versus unvaccinated individuals (e.g., 77% reduction in COVID-19-associated hospitalization rates [vs. a 90% reduction in the vaccinated immunocompetent population]),28 and a reduction in severe COVID-19 when BTCo occurred.29 However, vaccine effectiveness varies among immunocompromised subgroups with SOT being the highest risk for BTCo.28 , 29 Specific outcomes in SOT patients with BTCo have not been explored in detail.
The National COVID Cohort Collaborative (N3C) is the largest database on COVID-19 in the United States. As of May 6, 2022 (release 76), N3C contains longitudinal Electronic Health Record data on >4.9 million SARS-CoV-2 infected patients and >8.1 million uninfected controls from 72 data providers. A recent study using data from the N3C demonstrated a high risk of BTCo infection in patients with immune dysfunction (including, but not limited to SOT recipients) with an incidence rate of 15.7 per 1000 person months among VAX2 SOT recipients over a median of ~3 months.29 However, adverse outcomes after BTCo infection were not examined in detail, nor stratified by immunocompromised subgroup. Therefore, in a N3C follow-up study, we aim to examine the risk of developing BTCo and the rate and severity of adverse outcomes occurring in SOT recipients with BTCo after VAX2 versus COVID disease occurring in an unvaccinated SOT control group (overall, and by organ type). To date, this will be the largest study of BTCo in SOT recipients examining clinical outcomes by organ type. For comparison of vaccine efficacy, we will also examine COVID-19 outcomes in the VAX2 versus unvaccinated non-immunosuppressed/immunocompromised (non-ISC) general population. Although event numbers and follow-up time are limited, where available, we will also examine outcomes in a smaller subgroup of SOT and non-ISC patients with BTCo following VAX2 and an additional vaccine dose (VAX3).
2. METHODS
N3C is a centralized repository containing longitudinal electronic health record data from SARS-CoV-2-infected persons in the United States. N3C includes a broad category of patients with limited inclusion criteria for incoming data; specifically COVID-19 positivity or suspected positivity by laboratory testing or diagnostic codes for both inpatient and outpatient encounters.30 The incoming data come from four primary data models—OMOP, PCORnet, TriNetX, and ACT—harmonized into the OMOP 5.3.1 data model and made available within a secure enclave for analysis at the patient and encounter level.31 The design, data collection, sampling approach, and data harmonization methods used by the N3C have been described previously.32 , 33
2.1. Design
Using the N3C Enclave, we conducted a cohort study of adult SOT patients (>18 years) in the United States with a diagnosis of COVID-19 between December 10, 2020 and April 4, 2022, with data extracted on May 6, 2022. The COVID diagnosis was based on having a positive result from one of a set of a priori—defined tests including real-time polymerase chain reaction, antigen testing, and International Classification of Diseases diagnostic codes as previously reported.29 , 32 , 33 We excluded those with a diagnosis based on antibody test results alone due to potential for confounding based on prior vaccination. As a comparator group, we examined all adult non-ISC patients (excluding any patients with a diagnosis of auto-immune rheumatologic disease, prior bone marrow transplant, human immunodeficiency virus, multiple sclerosis, or malignant neoplasm) as described previously,29 captured in the Enclave with first positive test for COVID-19 over the same period, Figure S1.
2.2. Exposure
The primary exposure was COVID-19 vaccination status. As per our earlier analysis, we had data on the three SARS-CoV-2 vaccines with Food and Drug Administration authorization (two mRNA vaccines from Pfizer-BioNTech [BNT162b2] and Moderna [mRNA-1273], and one viral vector vaccine from Johnson & Johnson/Janssen [JNJ-784336725]). Acknowledging recent recommendations suggesting a third mRNA dose in immunosuppressed patients, for the purposes of this study, we defined vaccination as being ≥14 days post two doses for mRNA vaccines, one dose for Johnson & Johnson/Janssen vaccine, or two doses for other vaccines (VAX2), and VAX3 as being ≥14 days post a booster dose of any of the above vaccine preparations following VAX2.29 The control group consisted of patients with no record of COVID-19 vaccination; given the nature of data reporting in the N3C on vaccination (Figures S2 and S3) and the possibility that these patients may have been vaccinated through the end of the study period, and their vaccine status not fully captured in the Enclave, the control group was defined as those with unconfirmed vaccine status (UVS), as opposed to being unvaccinated. COVID-19 diagnosis occurring at least 14 days after VAX2 was considered BTCo infection.
2.3. Outcomes
Our analysis aimed to explore outcomes in those with BTCo (prior vaccination) versus in non-BTCo (COVID with no prior vaccination) separately in SOT recipients and the non-ISC general population.
The cumulative incidence of BTCo infection in the 6 months post-VAX2 in the non-ISC population and in the SOT cohort by organ type (kidney, liver, lung, or heart) was demonstrated using cumulative incidence curves per 1000 persons. Time to BTCo infection was assessed during the study period. Patient time was censored at: (1) 14 days after third vaccine dose (or second vaccine dose following J&J), (2) death or transfer to hospice, and (3) end of study period (April 4, 2022) or latest data partner reporting date.
Outcomes after BTCo included MARCE defined as a composite of acute kidney injury (AKI) with or without dialysis, acute myocardial infarction, angina, stent occlusion/thrombosis, stroke, transient ischemic attack, congestive heart failure or death from any cause,8 major adverse cardiac events (MACE) in isolation, renal failure requiring dialysis or AKI not requiring dialysis, death from any cause, hospitalization, and severe COVID-19 (including need for ventilation, extra corporeal membrane oxygenation [ECMO], or death) in the 90 days following COVID-19 diagnosis. In the SOT population, we also examined the outcomes of acute rejection and graft loss. Time from COVID diagnosis to each outcome was administratively censored at: (1) end of risk period (90 days), (2) May 6, 2022, or latest data partner reporting date.
2.4. Data collection
In addition to the primary exposure, we included information on variables associated with the outcomes of interest including age, sex, race, time since transplant, type of organ transplant (kidney, liver, heart, lung, multi-organ), comorbidities (chronic kidney disease [CKD], hypertension, diabetes, chronic obstructive pulmonary disease [COPD]/asthma, cancer, coronary artery disease, congestive heart failure, peripheral vascular disease, liver disease, and obesity [body mass index, BMI, >30 kg/m2]), and immunosuppression (anti-thymocyte globulin [ATG] induction, Basiliximab induction, and maintenance therapy with prednisone, tacrolimus, cyclosporine, or mycophenolic acid). We also included whether patients had a diagnosis of COVID-19 prior to vaccination (or prior to study initiation in the UVS control group). Finally, we included information regarding the SARS-CoV-2 variant dominance period defined as pre-Delta wave (December 10, 2020–June 19, 2021), Delta wave (June 20, 2021–December 19, 2021), and Omicron wave (≥December 20, 2021).34 Concept sets defining all standardized vocabulary used for medications, laboratories, procedures, and outcomes, are available in Table S1.35 For the primary analysis, complete case analysis was performed. An indicator variable for missingness was created for any variable with >10% missing data and included as an adjustment variable in multivariable analyses. This only applied to BMI, which had high missingness.
2.5. Analysis
Descriptive statistics were used to report baseline characteristics for all SOT and non-ISC patients included in the study, stratified by vaccination status (UVS, VAX2, and VAX3 where available). Median time from the second dose for mRNA vaccines and first dose for Johnson & Johnson/Janssen vaccines to the diagnosis of COVID-19 was determined in SOT and non-ISC patients with BTCo.
Separately for SOT and non-ISC patients in each vaccination category, we examined the proportion of patients developing each primary and secondary outcome in the 90 days after a diagnosis of COVID-19. The association between vaccination status and 90-day risk of each outcome was evaluated separately in SOT and non-ISC cohorts using multivariable Cox proportional hazards models adjusting for the covariates indicated above, including COVID variant dominance period (with time since transplant, organ type, and immunosuppression regimen in the SOT group) to determine cause-specific hazard ratios, or multivariable logistic regression for the binary outcomes of hospitalization or need for ECMO/ventilation/death.
2.6. Secondary analyses
The analyses described above were repeated using organ-specific cohorts ([i] kidney, [ii] liver, [iii] lung, and [iv] heart transplant recipients separately) instead of all SOT to examine each outcome after BTCo. For this secondary analysis, patients with combined transplants were excluded from the organ-specific cohorts.
2.7. Sensitivity analysis
We repeated our primary and secondary analysis censoring at 180 days after VAX2 for BTCo.
Our study protocol received Institutional Review Board (IRB) approval from the University of Nebraska Medical Center and Johns Hopkins University and the N3C Data Access Committee prior to analysis. All statistical analyses were performed using R within the N3C Enclave, in accordance with N3C privacy requirements.
3. RESULTS
3.1. Breakthrough COVID infection risk
Over the study period, following either VAX2 or VAX3, BTCo occurred in 4776 (24%) SOT recipients and 419 433 (21%) non-ISC patients, Tables 1 and 2. Median time from vaccination to BTCo was 149 days (IQR 99–233) in the SOT cohort and 201 days (IQR 112–258) in the non-ISC cohort (p-value <.001). 8193 SOT recipients and 1 343 841 non-ISC patients with UVS were also diagnosed with COVID over the study period (Tables 1 and 2). Characteristics of the SOT and non-ISC patients who received VAX2 are shown in Table S2.
TABLE 1.
COVID-19 infection in solid organ transplant recipients by vaccination status
| Variable | Unconfirmed vaccine status, N = 8193 | Primary series (VAX2)a, N = 2724 | VAX3a, N = 2052 |
|---|---|---|---|
| Sex | |||
| Male | 4823 (59%) | 1563 (57%) | 1205 (59%) |
| Female | 3370 (41%) | 1161 (43%) | 847 (41%) |
| Age | |||
| 18–45 | 2291 (28%) | 645 (24%) | 391 (19%) |
| 46–65 | 3926 (48%) | 1274 (47%) | 981 (48%) |
| >65 | 1976 (24%) | 805 (30%) | 680 (33%) |
| Race/Ethnicity | |||
| White | 4640 (57%) | 1520 (56%) | 1255 (61%) |
| Black/African American | 1550 (19%) | 623 (23%) | 439 (21%) |
| Hispanic/Latino | 1114 (14%) | 351 (13%) | 189 (9.2%) |
| Other/Unknown | 889 (11%) | 230 (8.4%) | 169 (8.2%) |
| Comorbidities | |||
| CKD | 5889 (72%) | 2038 (75%) | 1583 (77%) |
| Hypertension | 6781 (83%) | 2345 (86%) | 1772 (86%) |
| Diabetes | 4658 (57%) | 1626 (60%) | 1265 (62%) |
| COPD/Asthma | 1468 (18%) | 572 (21%) | 492 (24%) |
| Cancer | 1332 (16%) | 497 (18%) | 438 (21%) |
| CAD | 2312 (28%) | 851 (31%) | 695 (34%) |
| CHF | 2461 (30%) | 869 (32%) | 647 (32%) |
| PVD | 1776 (22%) | 665 (24%) | 553 (27%) |
| Liver | 1246 (15%) | 445 (16%) | 335 (16%) |
| Obesity | |||
| BMI >30 | 2779 (34%) | 877 (32%) | 683 (33%) |
| Missing | 1299 (16%) | 506 (19%) | 329 (16%) |
| Transplant status | |||
| Kidney | 5415 (66%) | 1752 (64%) | 1296 (63%) |
| Liver | 1836 (22%) | 577 (21%) | 385 (19%) |
| Lung | 769 (9.4%) | 292 (11%) | 371 (18%) |
| Heart | 1020 (12%) | 394 (14%) | 279 (14%) |
| Pancreas | 67 (0.8%) | 26 (1.0%) | 21 (1.0%) |
| Multiple | 839 (10%) | 290 (11%) | 274 (13%) |
| Time since transplant | |||
| <6 months | 1250 (15%) | 290 (11%) | 148 (7.2%) |
| 6–24 months | 2043 (25%) | 692 (25%) | 575 (28%) |
| >24 months | 4900 (60%) | 1742 (64%) | 1329 (65%) |
| Maintenance immunosuppression | |||
| Prednisone | 5680 (69%) | 1862 (68%) | 1517 (74%) |
| Tacrolimus | 5916 (72%) | 1880 (69%) | 1591 (78%) |
| Cyclosporine | 726 (8.9%) | 252 (9.3%) | 152 (7.4%) |
| MMF | 5662 (69%) | 1819 (67%) | 1530 (75%) |
| Induction immunosuppression | |||
| ATG | 715 (8.7%) | 243 (8.9%) | 215 (10%) |
| Basiliximab | 388 (4.7%) | 139 (5.1%) | 176 (8.6%) |
| Time period | |||
| Pre-Delta (<June 20, 2021) | 2399 (29%) | 210 (7.7%) | <20b |
| Delta (June 20, 2021–December 19, 2021) | 2407 (29%) | 1244 (46%) | <380b |
| Omicron (≥December 20, 2021) | 3387 (41%) | 1270 (47%) | 1674 (82%) |
Infections occurred ≥14 days following COVID-19 vaccine.
N3C privacy policies require censoring small cell counts (<20) and obfuscating adjacent cells to prevent back-calculating for all summary statistics.
TABLE 2.
COVID-19 infection in non-immunocompromised patients by vaccination status
| Variable | Unconfirmed vaccine status, N = 1 343 841 | Primary series (VAX2)a, N = 338 252 | VAX3a, N = 81 181 |
|---|---|---|---|
| Sex | |||
| Male | 615 261 (46%) | 129 403 (38%) | 30 100 (37%) |
| Female | 728 580 (54%) | 208 849 (62%) | 51 081 (63%) |
| Age | |||
| 18–45 | 786 274 (59%) | 167 232 (49%) | 31 786 (39%) |
| 46–65 | 388 757 (29%) | 113 768 (34%) | 27 725 (34%) |
| >65 | 168 810 (13%) | 57 252 (17%) | 21 670 (27%) |
| Race/Ethnicity | |||
| White | 856 821 (64%) | 238 589 (71%) | 59 262 (73%) |
| Black/African American | 155 436 (12%) | 34 525 (10%) | 6729 (8.3%) |
| Hispanic/Latino | 140 423 (10%) | 36 360 (11%) | 7358 (9.1%) |
| Other/Unknown | 191 161 (14%) | 28 778 (8.5%) | 7832 (9.6%) |
| Comorbidities | |||
| CKD | 34 783 (2.6%) | 13 194 (3.9%) | 4342 (5.3%) |
| Hypertension | 222 952 (17%) | 80 662 (24%) | 23 685 (29%) |
| Diabetes | 120 566 (9.0%) | 43 859 (13%) | 12 708 (16%) |
| COPD/Asthma | 107 209 (8.0%) | 34 011 (10%) | 9539 (12%) |
| Cancer | 41 892 (3.1%) | 16 145 (4.8%) | 6238 (7.7%) |
| CAD | 44 909 (3.3%) | 16 575 (4.9%) | 5478 (6.7%) |
| CHF | 36 506 (2.7%) | 11 703 (3.5%) | 3544 (4.4%) |
| PVD | 32 610 (2.4%) | 12 930 (3.8%) | 4149 (5.1%) |
| Liver | 10 680 (0.8%) | 3539 (1.0%) | 923 (1.1%) |
| Obesity | |||
| BMI >30 | 243 837 (18%) | 62 050 (18%) | 15 653 (19%) |
| Missing | 801 923 (60%) | 206 491 (61%) | 43 608 (54%) |
| Time period | |||
| Pre-Delta (<June 20, 2021) | 392 000 (29%) | 6505 (1.9%) | 89 (0.1%) |
| Delta (June 20, 2021–December 19, 2021) | 495 743 (37%) | 137 794 (41%) | 6670 (8.2%) |
| Omicron (≥December 20, 2021) | 456 098 (34%) | 193 953 (57%) | 74 422 (92%) |
Infections occurred ≥14 days following COVID-19 vaccine.
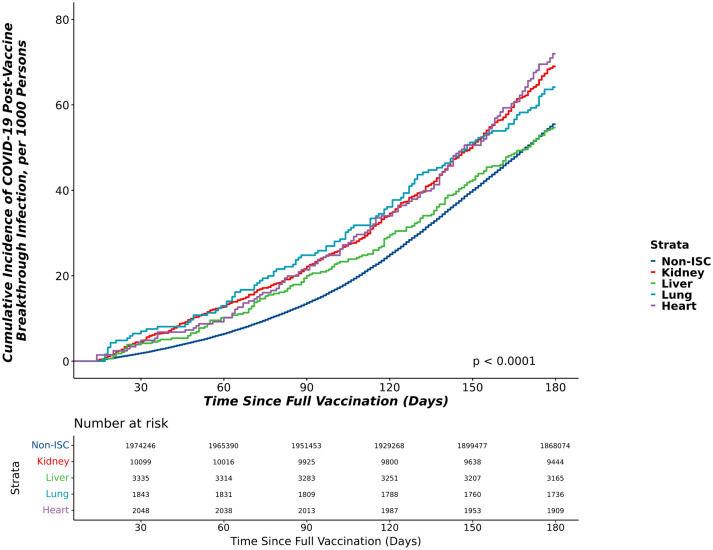
The 180-day cumulative incidence of BTCo post-VAX2 in the non-ISC population and in the SOT cohort by organ type (kidney, liver, lung, or heart) is shown in Figure 1 and combined (all SOT) in Figure S4. Heart recipients had the highest cumulative incidence of BTCo. Uncensored cumulative incidence showed similar distributions, Figure S5. The adjusted risk of BTCo among VAX2 and VAX2 boosted non-ISC and SOT recipients is shown in Table 3 overall, and by period of time. Overall, SOT status (all organ types) was independently associated with risk of BTCo (hazard ratio [HR] 1.76, 95% confidence interval [CI] 1.67–1.85 relative to non-ISC). In an analysis adjusting for specific organ type, lung recipients were highest risk for BTCo (HR 2.11, 95% CI 1.91–2.33) and liver recipients were lowest risk (HR 1.39, 95% CI 1.28–1.52) relative to non-ISC. Relative to non-ISC, SOT recipients were highest risk of BTCo early in the pandemic, with the relative risk attenuating over time (SOT vs. non-ISC: HR 2.35, 95% CI 1.80–3.08 during the pre-Delta wave; HR 1.67, 95% CI 1.53–1.81 during the Delta wave; HR 1.51, 95% CI 1.41–1.61 during the Omicron wave), Table 3.
FIGURE 1.
The cumulative incidence of COVID-19 breakthrough infection in the 6 months postVAX2 in the non-ISC population and in the SOT cohort by organ type. ISC, immunosuppressed/immunocompromised.
TABLE 3.
Multivariable Cox proportional hazard model for the adjusted risk of breakthrough COVID-recipients overall and by COVID-19 period
| Variable | All periods, HR (95% CI) | All periods (organ specific), HR (95% CI) | Pre-Delta wave, HR (95% CI) | Delta wave, HR (95% CI) | Omicron wave, HR (95% CI) |
|---|---|---|---|---|---|
| Vaccination status | |||||
| VAX2 | Ref | Ref | – | – | – |
| VAX3 | 0.33 (0.33, 0.33) | 0.33 (0.33, 0.33) | – | – | – |
| Pre-vaccination COVID infection | 0.18 (0.18, 0.19) | 0.18 (0.18, 0.19) | – | – | – |
| Sex | |||||
| Male | 0.89 (0.89, 0.90) | 0.89 (0.89, 0.90) | 0.97 (0.93, 1.02) | 0.94 (0.93, 0.95) | 0.86 (0.85, 0.87) |
| Female | Ref | Ref | Ref | Ref | Ref |
| Age (median) | |||||
| 18–45 | Ref | Ref | Ref | Ref | Ref |
| 46–65 | 0.94 (0.94, 0.95) | 0.95 (0.94, 0.95) | 1.12 (1.06, 1.20) | 1.12 (1.10, 1.13) | 0.81 (0.80, 0.82) |
| >65 | 0.83 (0.82, 0.84) | 0.83 (0.82, 0.84) | 1.30 (1.21, 1.39) | 1.24 (1.22, 1.26) | 0.63 (0.62, 0.63) |
| Race/Ethnicity | |||||
| White | Ref | Ref | Ref | Ref | Ref |
| Black/African American | 0.82 (0.81, 0.82) | 0.81 (0.81, 0.82) | 1.10 (1.02, 1.20) | 0.54 (0.53, 0.55) | 1.02 (1.00, 1.03) |
| Hispanic/Latino | 0.75 (0.75, 0.76) | 0.75 (0.75, 0.76) | 1.16 (1.07, 1.25) | 0.54 (0.53, 0.55) | 0.91 (0.90, 0.92) |
| Other/Unknown | 0.61 (0.60, 0.62) | 0.61 (0.60, 0.62) | 1.18 (1.09, 1.27) | 0.53 (0.52, 0.54) | 0.78 (0.77, 0.79) |
| Comorbidities | |||||
| CKD | 1.01 (0.99, 1.02) | 1.01 (0.99, 1.02) | 1.06 (0.96, 1.17) | 1.05 (1.02, 1.08) | 1.01 (0.99, 1.03) |
| Hypertension | 1.03 (1.02, 1.03) | 1.03 (1.02, 1.03) | 0.91 (0.86, 0.98) | 1.01 (1.00, 1.03) | 1.05 (1.04, 1.06) |
| Diabetes | 1.15 (1.14, 1.16) | 1.15 (1.14, 1.16) | 1.15 (1.07, 1.23) | 1.13 (1.11, 1.15) | 1.09 (1.07, 1.10) |
| COPD/Asthma | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.03) | 1.03 (0.95, 1.11) | 1.00 (0.98, 1.02) | 1.05 (1.04, 1.07) |
| Cancer | 0.82 (0.81, 0.83) | 0.82 (0.81, 0.83) | 1.19 (1.10, 1.29) | 0.84 (0.82, 0.86) | 0.85 (0.83, 0.86) |
| CAD | 0.96 (0.95, 0.98) | 0.96 (0.95, 0.98) | 0.99 (0.90, 1.09) | 0.98 (0.95, 1.00) | 0.99 (0.97, 1.01) |
| CHF | 0.93 (0.92, 0.95) | 0.93 (0.91, 0.95) | 1.22 (1.10, 1.35) | 0.95 (0.92, 0.97) | 0.97 (0.95, 0.99) |
| PVD | 0.81 (0.80, 0.82) | 0.81 (0.80, 0.82) | 0.80 (0.73, 0.88) | 0.89 (0.87, 0.91) | 0.86 (0.84, 0.87) |
| Liver | 0.85 (0.82, 0.87) | 0.86 (0.84, 0.89) | 1.13 (0.96, 1.32) | 0.87 (0.83, 0.92) | 0.88 (0.85, 0.91) |
| Obesity | |||||
| BMI > 30 | 1.19 (1.18, 1.20) | 1.19 (1.18, 1.20) | 1.06 (1.00, 1.13) | 1.23 (1.21, 1.25) | 1.12 (1.10, 1.13) |
| Missing | 3.40 (3.37, 3.42) | 3.40 (3.37, 3.42) | 1.33 (1.26, 1.41) | 3.01 (2.97, 3.05) | 2.62 (2.59, 2.65) |
| Solid organ transplant | 1.76 (1.67, 1.85) | 2.35 (1.80, 3.08) | 1.67 (1.53, 1.81) | 1.51 (1.41, 1.61) | |
| Organ type | |||||
| Kidney | 1.81 (1.70, 1.91) | ||||
| Liver | 1.39 (1.28, 1.52) | ||||
| Lung | 2.11 (1.91, 2.33) | ||||
| Heart | 1.83 (1.66, 2.02) | ||||
| Maintenance immunosuppression | |||||
| Prednisone | 1.10 (1.09, 1.11) | 1.10 (1.09, 1.11) | 0.92 (0.86, 0.99) | 1.11 (1.09, 1.12) | 1.09 (1.08, 1.11) |
| Tacrolimus | 0.88 (0.84, 0.92) | 0.89 (0.85, 0.93) | 0.81 (0.62, 1.05) | 0.81 (0.74, 0.87) | 0.92 (0.87, 0.98) |
| Cyclosporine | 0.89 (0.85, 0.93) | 0.89 (0.85, 0.93) | 1.06 (0.83, 1.35) | 0.88 (0.81, 0.94) | 0.90 (0.84, 0.95) |
| MMF | 1.27 (1.20, 1.34) | 1.25 (1.18, 1.33) | 1.76 (1.31, 2.36) | 1.14 (1.03, 1.25) | 1.20 (1.12, 1.29) |
| Induction immunosuppression | |||||
| ATG | 1.03 (0.94, 1.14) | 1.02 (0.91, 1.13) | 1.02 (0.65, 1.62) | 1.17 (0.99, 1.38) | 0.89 (0.78, 1.01) |
| Basiliximab | 1.17 (1.04, 1.31) | 1.00 (0.87, 1.14) | 1.11 (0.65, 1.88) | 0.94 (0.77, 1.16) | 1.07 (0.92, 1.23) |
3.2. Outcomes among those with breakthrough COVID infection
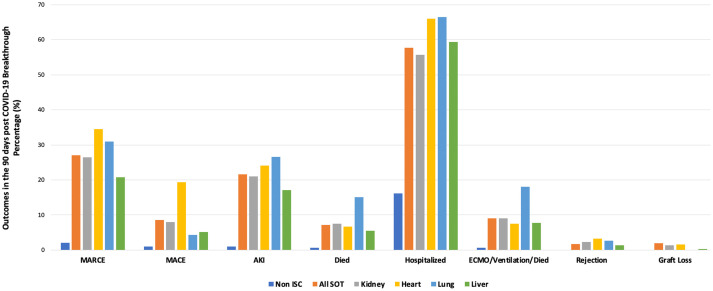
Among those who experienced COVID-19 infection over the study period, 90-day MARCE occurred in 3.6% of non-ISC and 33.9% of SOT UVS patients. In VAX2 patients, 90-day MARCE occurred in 2.1% of non-ISC patients and 27.0% of SOT, Table S3a. The relative risk of MARCE was 12.9-fold higher in SOT versus non-ISC with VAX2, but 20.0% lower than SOT with UVS. The crude rate of each adverse outcome (MARCE, MACE, AKI, death, hospitalization, need for ECMO, ventilation or death, rejection, and graft loss) in VAX2 patients with BTCo is shown in Figure 2, separately for non-ISC patients (excluding rejection and graft loss), all SOT, and individually by kidney, liver, lung, and heart organ type. The crude rates of each outcome in non-ISC and SOT recipients with VAX2 versus UVS are shown in Table S3. Irrespective of vaccination status, SOT were higher risk for each post-COVID complication than non-ISC patients; 90-day mortality was 10.3-fold higher in VAX2 SOT than non-ISC. VAX2 heart transplant recipients were at the highest risk for MARCE, MACE, organ rejection, and graft loss, whereas lung recipients were higher risk for AKI, death, hospitalization, and combined ECMO/ventilation/death.
FIGURE 2.
The proportion of non-immunosuppressed, all solid organ transplant, and organ specific transplant (kidney, liver, lung, heart) patients who experienced adverse outcomes in the 90 days after COVID-19 infection in those with two doses of mRNA or one dose of Johnson & Johnson (VAX2). ISC, immunosuppressed/immunocompromised; SOT, solid organ transplant; MARCE, major adverse renal or cardiac event; MACE, major adverse cardiac event; AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation.
3.3. Outcomes among those with COVID infection by vaccination status
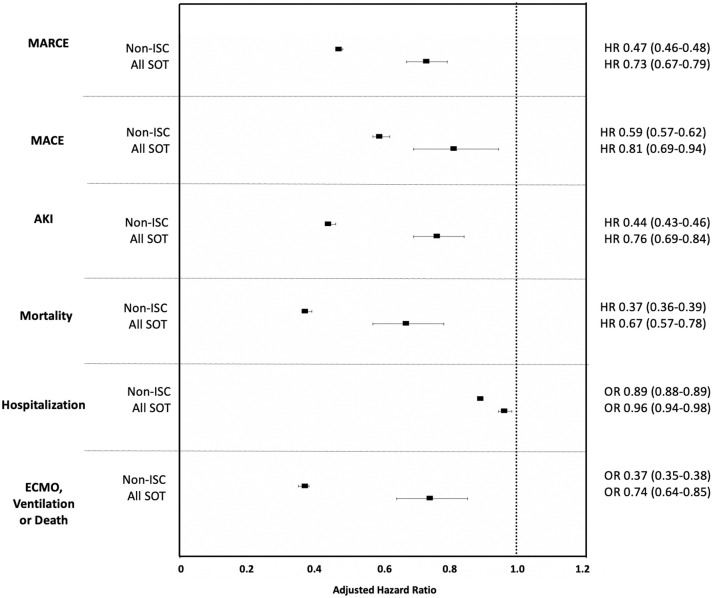
The adjusted hazard ratio (aHR) for each outcome in VAX2 non-ISC patients and SOT recipients with COVID-19, relative to UVS non-ISC and SOT, respectively, is shown in Figure 3. Figure S6 depicts the aHR for each outcome in the smaller subset of non-ISC and SOT patients with VAX3 and adequate follow-up time, relative to UVS. Table S4 also depicts the aHR associated with VAX2 and in those with data, VAX3. The benefit of vaccination in reducing risk of each complication was greater in the non-ISC cohort, but SOT recipients also achieved significant benefit with vaccination for all outcomes compared to those with UVS. The greatest relative benefit with VAX2 for both non-ISC and SOT cohorts was in reducing COVID-19 mortality (HR 0.37, 95% CI 0.36–0.39 for non-ISC and HR 0.67, 95% CI 0.57–0.78 for SOT relative to UVS). An organ-specific breakdown of vaccine efficacy in reducing each outcome is shown in Figure S7; the benefit of vaccination versus UVS did not differ significantly by organ type.
FIGURE 3.
The adjusted relative hazard ratios for adverse outcomes after COVID-19 infection in VAX2 non-ISC patients and SOT recipients relative to those with unconfirmed vaccination status. ISC, immunosuppressed/immunocompromised; SOT, solid organ transplant; MARCE, major adverse renal or cardiac event; MACE, major adverse cardiac event; AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; OR, odds ratio.
4. DISCUSSION
SOT recipients have been shown to have a blunted humoral response to mRNA vaccination against COVID-19,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 however, studies examining clinical outcomes in vaccinated SOT recipients with BTCo are limited. Here, we present the largest study to date of outcomes following BTCo infection in SOT recipients; over 4700 vaccinated SOT recipients with BTCo (defined as having received at least two doses of an mRNA vaccine or one dose of J&J). While the benefit of vaccination was attenuated in SOT recipients versus in non-ISC controls, VAX2 SOT still had a significantly lower risk of all outcomes post-COVID than those with UVS. This was particularly true for more severe outcomes; the composite of needing ECMO, ventilation, or death was reduced by 26% in VAX2 SOT, and isolated mortality was reduced by 33% relative to those with UVS. Additionally, we show that breakthrough infection occurred earlier in SOT than in non-ISC patients and vaccinated SOT experienced more adverse complications than vaccinated non-ISC populations; 90-day mortality was 10.3-fold higher in VAX2 SOT than non-ISC.
The Center for Disease Control and American Society of Transplantation currently recommend SOT recipients receive three mRNA vaccine doses, two doses of J&J, or 1 dose mRNA + 1 dose J&J, all with an additional mRNA booster.26 , 36 Studies have demonstrated a favorable humoral response to the third BNT162b2 vaccine dose in SOT recipients with the proportion of patients with detectable anti-SARS-CoV-2 antibodies increasing from 40% after the second dose to 68% 4 weeks after the third dose.37 In our study, we demonstrate a median time from VAX2 to BTCo infection of 5.0 months in the SOT cohort and 6.7 months in the non-ISC cohort. This difference suggests a potential benefit of a shorter interval for booster doses in the SOT population and aligns with CDC recommendations.
We have previously shown that in the pre-vaccine era, the outcomes after COVID-19 infection vary by SOT type, with heart and kidney recipients at the greatest risk for most complications.8 A later study examined the differential antibody response to a second dose of the mRNA vaccine in SOT recipients by organ type, and demonstrated the lowest seroconversion rate in heart (18.8%), followed by kidney (45.5%), and liver (69.4%) recipients. However, the total SOT included in the study was only 226, and lung transplant recipients were not included, with no assessment of clinical outcomes following vaccination.38 After VAX2, earlier studies have shown an immune response (either humoral or cellular) in 65% of kidney recipients (n = 117), 87% of heart recipients (n = 46), and 93% of liver recipients (n= 58) with an isolated humoral response demonstrated in only 29.9%, 57%, and 71%, respectively.25
We show for the first time that there are significant differences in the cumulative incidence of BTCo infection by organ type, with lung recipients at the highest adjusted risk and liver recipients lowest. Furthermore, we examine clinical outcomes after BTCo in a vaccinated non-ISC cohort, among all SOT, and by individual organ type. The risk of MARCE, MACE, organ rejection, and graft loss was highest in heart recipients with BTCo, whereas lung recipients were highest risk for AKI, death, hospitalization, and the composite of ECMO/ventilation/death. Conversely, the risk of all outcomes (except hospitalization) in the vaccinated non-ISC cohort was low (<3%).
Despite the demonstrated benefit, albeit attenuated, with VAX2 in the SOT population, immunosuppressed transplant recipients remain at significant risk for adverse COVID-attributable outcomes. Therefore, it is important that vaccinated SOT recipients continue to practice behavior modifications to minimize exposure risk, including masking, handwashing, and social distancing. Vaccination of close contacts should be prioritized, similar to influenza vaccination recommendations.39 Strategies to improve immune response may also be considered. These may include vaccination of waitlisted transplant candidates, recognizing that those with organ failure may have less humoral immunity than healthy individuals,18 , 23 avoiding vaccination in the early transplant period when immunosuppression levels are highest may be considered,40 or holding mycophenolic acid at the time of vaccination to enhance the vaccine response.41 , 42 However, these approach have not been systematically studied and the potential risks and benefit of each requires further study.
This is the largest study to examine clinical outcomes after BTCo infection in vaccinated SOT recipients (compared with UVS SOT recipients and vaccinated non-ISC patients) and the first to explore organ specific clinical outcomes after BTCo. However, there are limitations. Due to the nature of inclusion criteria for the N3C, we did not have access to a representative cohort of patients without COVID-19. We were therefore unable to determine if the incidence of BTCo in vaccinated SOT recipients was reduced relative to the unvaccinated cohort or how it compared to the reduction in BTCo infection in vaccinated non-ISC patients. We could only examine differences in complication rates by vaccine status in those who were diagnosed with COVID-19 in the N3C database, which may represent a different subgroup less likely to mount any immune response to vaccination. While we demonstrate a reduction in adverse outcomes in the VAX2 SOT (and non-ISC populations), this risk reduction would be expected to be greater if our cohort was incepted at the time of vaccination (rather than at BTCo) to account for the anticipated reduction in the risk of even acquiring BTCo in the vaccinated versus non-vaccinated populations. Furthermore, not being able to confirm our control group as unvaccinated is another limitation as it could have been contaminated with a small subset of unrecognized vaccination. However, this would only attenuate the benefit demonstrated with vaccination versus the UVS control group. Therefore, there is the potential that the benefit we demonstrate in this study may underestimate the true benefit of vaccination in SOT patients. Nevertheless, we would expect the risk of undocumented vaccination to be similar in the SOT and non-ISC cohorts, and thus the comparison of relative risk reduction with vaccination between the two groups is reasonable. We did not have any information regarding seroconversion or antibody titers in either group after vaccination to correlate with clinical outcomes, nor did we have data regarding the culprit SARS-Co-V-2 variants. This information is not collected in a consistent fashion across patient populations, however, we did adjust for the temporal periods that have been shown to correlate with the pre-Delta, Delta, and Omicron COVID-19 variants. Finally and most importantly, four doses of an mRNA vaccination are now standard of care for reducing BTCo risk in SOT.26 Given insufficient follow-up time to accumulate events, our study primarily examines BTCo outcomes after what is currently considered incomplete vaccination (two doses of a two dose series or one dose of J&J). We include subgroup analyses of a smaller cohort of patients with VAX3 dosing, however, given low event rates in the SOT cohort with BTCo following VAX3, we could not perform reliable organ-specific analyses. While future studies will further explore risk reduction in SOT and non-ISC populations with BTCo infection after a third +/− fourth mRNA vaccine dose, we feel the current study still contributes important information to the growing body of literature examining COVID vaccination in SOT. Reliance on post-vaccination anti-SARS-CoV-2 antibody titers alone to predict COVID-attributable risk may not be sufficient given the abysmal humoral response demonstrated in SOT recipient post-vaccine, yet we demonstrated reduction in serious COVID complications after only a two-dose vaccine regimen in this population. The benefit would be expected to be even greater following a third and/or fourth vaccine dose. Despite the poor serologic vaccine response in SOT, our study reiterates the importance of vaccination in SOT.
In conclusion, in the largest study of BTCo in SOT to date, we demonstrate that while COVID-19 VAX2 in the SOT population is not as effective at reducing adverse outcomes from BTCo as in the non-ISC population, there are still marked benefits. COVID-19 infection occurred at a median of 5.0 months post-VAX2, which may influence decisions around third dose timing. The effect a third dose has on minimizing adverse outcomes after BTCo requires study when sufficient data and follow-up time are available. Finally, although this study demonstrates moderate benefit in reducing major complications after BTCo in SOT recipients, immunosuppressed patients must remain vigilant of their risk and continue to minimize exposure.
ACKNOWLEDGMENTS
All research team members relied upon Data Use Agreements (DUAs) executed between their home institutions and National Center for Advancing Translational Sciences (NCATS) for access to N3C. National Institute of Health’s (NIH) National COVID Cohort Collaborative (N3C) Data Utilization Request Approval committee approved the data utilization request of this project (RP-CA3365). Each author’s home institution executed DUAs for participation in N3C. National Institute of Health’s (NIH) National COVID Cohort Collaborative (N3C) Data Utilization Request Approval committee approved the data utilization request of this project (RP-CA3365). Each author’s home institution executed Data Use Agreements for participation in N3C. All research team members relied upon Data Use Agreements executed between their home institutions and National Center for Advancing Translational Sciences (NCATS) for access to N3C. Our study protocol was approved by the N3C Data Access Committee prior to analysis. NACTS reviewed all data elements prior to extraction. The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources.
AO and EF were supported by CTSA award No. UL1TR002649 from the National Center for Advancing Translational Sciences. AJA was supported by U54GM104942-05S2 and U54GM115458 from the National Institute of General Medical Sciences, which funds the West Virginia Clinical & Translational Science Institute and the Great Plains IDeA Clinical & Translational Research Network. The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave https://covid.cd2h.org and N3C Attribution and Publication Policy v 1.2-2020-08-25b supported by NCATS U24 TR002306. RBM is supported by BMX003272 and the Dr. Dennis Ross Research Fund in Nephrology, University of Nebraska.
This research was possible because of the patients whose information is included within the data and the organizations (see covid.cd2h.org) and scientists who have contributed to the ongoing development of this community resource. The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
We gratefully acknowledge contributions from the following N3C core teams (leads designated with asterisks): (1) CD2H Principal Investigators and N3C Lead Investigators: Melissa A. Haendel*, Christopher G. Chute*, Anita Walden; (2) NCATS CD2H and N3C Science Officer: Kenneth R. Gersing; (3) NCATS CD2H and N3C Program Officer: Leonie Misquitta; (4) NCATS N3C Leadership Team: Joni L. Rutter*, Kenneth R. Gersing*, Penny Wung Burgoon, Samuel Bozzette, Mariam Deacy, Christopher Dillon, Rebecca Erwin-Cohen, Nicole Garbarini, Valery Gordon, Michael G. Kurilla, Emily Carlson Marti, Sam G. Michael, Leonie Misquitta, Lili Portilla, Clare Schmitt, Meredith Temple-O’Connor; (5) Workstream, subgroup and administrative leaders: Melissa A. Haendel*, Tellen D. Bennett, Christopher G. Chute, David A. Eichmann, Justin Guinney, Warren A. Kibbe, Hongfang Liu, Philip R.O. Payne, Emily R. Pfaff, Peter N. Robinson, Joel H. Saltz, Heidi Spratt, Justin Starren, Christine Suver, Adam B. Wilcox, Andrew E. Williams, Chunlei Wu; (6) Key liaisons at data partner sites; (7) Regulatory staff at data partner sites; (8) Individuals at the sites who are responsible for creating the datasets and submitting data to N3C; (9) Data Ingest and Harmonization Team: Christopher G. Chute*, Emily R. Pfaff*, Davera Gabriel, Stephanie S. Hong, Kristin Kostka, Harold P. Lehmann, Richard A. Moffitt, Michele Morris, Matvey B. Palchuk, Xiaohan Tanner Zhang, Richard L. Zhu; (10) Phenotype Team (Individuals who create the scripts that the sites use to submit their data, based on the COVID and Long COVID definitions): Emily R. Pfaff*, Benjamin Amor, Mark M. Bissell, Marshall Clark, Andrew T. Girvin, Stephanie S. Hong, Kristin Kostka, Adam M. Lee, Robert T. Miller, Michele Morris, Matvey B. Palchuk, Kellie M. Walters; (11) N3C Community Project Management and Operations Team: Anita Walden*, Will Cooper, Patricia A. Francis, Rafael Fuentes, Alexis Graves, Julie A. McMurry, Andrew J. Neumann, Shawn T. O’Neil, Usman Sheikh, Elizabeth Zampino; (12) Analytics Team (Individuals who build the Enclave infrastructure, help create codesets, variables, and help Domain Teams and project teams with their datasets): Benjamin Amor*, Mark M. Bissell, Katie Rebecca Bradwell, Andrew T. Girvin, Amin Manna, Nabeel Qureshi; (13) Publication Committee Team: Mary Morrison Saltz*, Christine Suver*, Christopher G. Chute, Melissa A. Haendel, Julie A. McMurry, Andréa M. Volz, Anita Walden, Carolyn Bramante, Jeremy Richard Harper, Wenndy Hernandez, Farrukh M Koraishy, Federico Mariona, Amit Saha, Satyanarayana Vedula.
We acknowledge support from many grants; the content is solely the responsibility of the authors and does not necessarily represent the official views of the N3C Program, the NIH or other funders. In addition, access to N3C Data Enclave resources does not imply endorsement of the research project and/or results by NIH or NCATS.
The following institutions whose data are released or pending: Available: Advocate Health Care Network—UL1TR002389: The Institute for Translational Medicine (ITM), Boston University Medical Campus—UL1TR001430: Boston University Clinical and Translational Science Institute, Brown University—U54GM115677: Advance Clinical Translational Research (Advance-CTR), Carilion Clinic—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia, Charleston Area Medical Center—U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI), Children’s Hospital Colorado—UL1TR002535: Colorado Clinical and Translational Sciences Institute, Columbia University Irving Medical Center—UL1TR001873: Irving Institute for Clinical and Translational Research, Duke University—UL1TR002553: Duke Clinical and Translational Science Institute, George Washington Children’s Research Institute—UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN), George Washington University—UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN), Indiana University School of Medicine—UL1TR002529: Indiana Clinical and Translational Science Institute, Johns Hopkins University—UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research, Loyola Medicine—Loyola University Medical Center, Loyola University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM), Maine Medical Center—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network, Massachusetts General Brigham—UL1TR002541: Harvard Catalyst, Mayo Clinic Rochester—UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS), Medical University of South Carolina—UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR), Montefiore Medical Center—UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore, Nemours—U54GM104941: Delaware CTR ACCEL Program, NorthShore University HealthSystem—UL1TR002389: The Institute for Translational Medicine (ITM), Northwestern University at Chicago—UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS), OCHIN—INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks, Oregon Health & Science University—UL1TR002369: Oregon Clinical and Translational Research Institute, Penn State Health Milton S. Hershey Medical Center—UL1TR002014: Penn State Clinical and Translational Science Institute, Rush University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM), Rutgers, The State University of New Jersey—UL1TR003017: New Jersey Alliance for Clinical and Translational Science, Stony Brook University—U24TR002306, The Ohio State University—UL1TR002733: Center for Clinical and Translational Science, The State University of New York at Buffalo—UL1TR001412: Clinical and Translational Science Institute, The University of Chicago—UL1TR002389: The Institute for Translational Medicine (ITM), The University of Iowa—UL1TR002537: Institute for Clinical and Translational Science, The University of Miami Leonard M. Miller School of Medicine—UL1TR002736: University of Miami Clinical and Translational Science Institute, The University of Michigan at Ann Arbor—UL1TR002240: Michigan Institute for Clinical and Health Research, The University of Texas Health Science Center at Houston—UL1TR003167: Center for Clinical and Translational Sciences (CCTS), The University of Texas Medical Branch at Galveston—UL1TR001439: The Institute for Translational Sciences, The University of Utah—UL1TR002538: Uhealth Center for Clinical and Translational Science, Tufts Medical Center—UL1TR002544: Tufts Clinical and Translational Science Institute, Tulane University—UL1TR003096: Center for Clinical and Translational Science, University Medical Center New Orleans—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center, University of Alabama at Birmingham—UL1TR003096: Center for Clinical and Translational Science, University of Arkansas for Medical Sciences—UL1TR003107: UAMS Translational Research Institute, University of Cincinnati—UL1TR001425: Center for Clinical and Translational Science and Training, University of Colorado Denver, Anschutz Medical Campus—UL1TR002535: Colorado Clinical and Translational Sciences Institute, University of Illinois at Chicago—UL1TR002003: UIC Center for Clinical and Translational Science, University of Kansas Medical Center—UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute, University of Kentucky—UL1TR001998: UK Center for Clinical and Translational Science, University of Massachusetts Medical School Worcester—UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS), University of Minnesota—UL1TR002494: Clinical and Translational Science Institute, University of Mississippi Medical Center—U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR), University of Nebraska Medical Center—U54GM115458: Great Plains IDeA-Clinical & Translational Research, University of North Carolina at Chapel Hill—UL1TR002489: North Carolina Translational and Clinical Science Institute, University of Oklahoma Health Sciences Center—U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI), University of Rochester—UL1TR002001: UR Clinical & Translational Science Institute, University of Southern California—UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI), University of Vermont—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network, University of Virginia—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia, University of Washington—UL1TR002319: Institute of Translational Health Sciences, University of Wisconsin-Madison—UL1TR002373: UW Institute for Clinical and Translational Research, Vanderbilt University Medical Center—UL1TR002243: Vanderbilt Institute for Clinical and Translational Research, Virginia Commonwealth University—UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research, Wake Forest University Health Sciences—UL1TR001420: Wake Forest Clinical and Translational Science Institute, Washington University in St. Louis—UL1TR002345: Institute of Clinical and Translational Sciences, Weill Medical College of Cornell University—UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center, West Virginia University—U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI)
Submitted: Icahn School of Medicine at Mount Sinai—UL1TR001433: ConduITS Institute for Translational Sciences, The University of Texas Health Science Center at Tyler—UL1TR003167: Center for Clinical and Translational Sciences (CCTS), University of California, Davis—UL1TR001860: UCDavis Health Clinical and Translational Science Center, University of California, Irvine—UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS), University of California, Los Angeles—UL1TR001881: UCLA Clinical Translational Science Institute, University of California, San Diego—UL1TR001442: Altman Clinical and Translational Research Institute, University of California, San Francisco—UL1TR001872: UCSF Clinical and Translational Science Institute.
Pending: Arkansas Children’s Hospital—UL1TR003107: UAMS Translational Research Institute, Baylor College of Medicine—None (Voluntary), Children’s Hospital of Philadelphia—UL1TR001878: Institute for Translational Medicine and Therapeutics, Cincinnati Children’s Hospital Medical Center—UL1TR001425: Center for Clinical and Translational Science and Training, Emory University—UL1TR002378: Georgia Clinical and Translational Science Alliance, HonorHealth—None (Voluntary), Loyola University Chicago—UL1TR002389: The Institute for Translational Medicine (ITM), Medical College of Wisconsin—UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin, MedStar Health Research Institute—UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS), MetroHealth—None (Voluntary), Montana State University—U54GM115371: American Indian/Alaska Native CTR, NYU Langone Medical Center—UL1TR001445: Langone Health’s Clinical and Translational Science Institute, Ochsner Medical Center—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center, Regenstrief Institute—UL1TR002529: Indiana Clinical and Translational Science Institute, Sanford Research—None (Voluntary), Stanford University—UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education, The Rockefeller University—UL1TR001866: Center for Clinical and Translational Science, The Scripps Research Institute—UL1TR002550: Scripps Research Translational Institute, University of Florida—UL1TR001427: UF Clinical and Translational Science Institute, University of New Mexico Health Sciences Center—UL1TR001449: University of New Mexico Clinical and Translational Science Center, University of Texas Health Science Center at San Antonio—UL1TR002645: Institute for Integration of Medicine and Science, Yale New Haven Hospital—UL1TR001863: Yale Center for Clinical Investigation.
DATA AVAILABILITY STATEMENT
All diagnostic, medication, procedure, and laboratory concepts used in this study are available in Table S1. Raw code (R, Python, SQL) is available upon request. N3C is a public resource maintained by NCATS to support COVID-19 research. To access patient-level data from the N3C consortium, institutions must have a signed Data Use Agreement executed with NCATS and investigators must complete mandatory training along with submitting a Data Use Request to N3C. Investigators can request access to the Enclave here.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. AV has done consultancy work and received funding for a fellowship project grant through Paladin Labs Inc. AG has received educational funds from Mallinckrodt Pharmaceuticals and has served as PI for studies by Mallinckrodt Pharmaceuticals and CSL Behring. RBM reports grant funding from VericiDX, honoraria from Olaris Inc, personal fees from Vitaerris as member of the IMAGINE Trial Steering committee, and personal fees from American Journal of Transplantation as Deputy Editor of the journal, outside the submitted work. MGI reports research support, paid to Northwestern University, from AiCuris, GlaxoSmithKline, Janssen, and Shire; he is a paid consultant for Adagio, AlloVir, Celltrion, Cidara, Genentech, Roche, Janssen, Shionogi, and Viracor Eurofins; he is also a paid member of DSMBs from Janssen, Merck, SAB Biotherapeutics, Sequiris, Takeda, and Vitaeris; he also receives royalties from UpToDate. MGI is supported, in part, by NCATS grant UL1TR001422.
Footnotes
Michael G. Ison and Roslyn B. Mannon are co-senior authors.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Appendix S1 Supporting information
REFERENCES
- 1.Elias M, Pievani D, Randoux C, et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31(10):2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020;73:e4090–e4099. doi: 10.1093/cid/ciaa1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021;35(1):100588. doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastor-Barriuso R, Perez-Gomez B, Hernan MA, et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. BMJ. 2020;371:m4509. doi: 10.1136/bmj.m4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinson AJ, Agarwal G, Dai R, et al. COVID-19 in solid organ transplantation: results of the national COVID cohort collaborative. Transplant Direct. 2021;7(11):e775. doi: 10.1097/TXD.0000000000001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinson AJ, Dai R, Agarwal G, et al. Sex and organ-specific risk of major adverse renal or cardiac events in solid organ transplant recipients with COVID-19. Am J Transplant. 2022;22(1):245–259. doi: 10.1111/ajt.16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UKregistry linkage analysis. Transplantation. 2021;105(11):e263–e264. doi: 10.1097/TP.0000000000003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt AA, Omer SB, Yan P, Shaikh OS, Mayr FB. SARS-CoV-2 vaccine effectiveness in a high-risk National Population in a real-world setting. Ann Intern Med. 2021;174(10):1404–1408. doi: 10.7326/M21-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reischig T, Kacer M, Vlas T, et al. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am J Transplant. 2021;22:801–812. doi: 10.1111/ajt.16902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(8):1173. doi: 10.1016/j.cmi.2021.04.028. e1-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rincon-Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):eabj1031. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 16.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):e150175. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174(9):1336–1338. doi: 10.7326/M21-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021;21(10):3496–3499. doi: 10.1111/ajt.16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. Jama. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galmiche S, Luong Nguyen LB, Tartour E, et al. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin Microbiol Infect. 2021;28(2):163–177. doi: 10.1016/j.cmi.2021.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolb T, Fischer S, Müller L, et al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360. 2021;2:1491–1498. doi: 10.34067/KID.0003512021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021;21(12):3971–3979. doi: 10.1111/ajt.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prevention CfDCa. COVID-19 vaccines for moderately or severely immunocompromised people. February 16, 2022. Accessed February 17, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html?s_cid=11707:covid%20booster%20immunocompromised:sem.ga:p:RG:GM:gen:PTN:FY22
- 27.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105(11):e265–e266. doi: 10.1097/TP.0000000000003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of two-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults-nine states, January-September 2021. Am J Transplant. 2022;22(1):306–314. doi: 10.1111/ajt.16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2021;182:153. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GitHub. National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition. 2021. Accessed March 17, 2021. https://github.com/National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition
- 31.Melissa H, Christopher C, Kenneth G. The National COVID Cohort Collaborative (N3C): rationale, infrastructure, and deployment. J Am Med Inform Assoc. 2020;28(3):427–443. doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28(3):427–443. doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett TD, Moffitt RA, Hajagos JG, et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open. 2021;4(7):e2116901. doi: 10.1001/jamanetworkopen.2021.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prevention CfDCa . U.S. Department of Health & Human Services; 2022. COVID data tracker: monitoring variant proportions. Accessed May 2, 2022. https://covid.cdc.gov/covid-data-tracker/#variantproportions. [Google Scholar]
- 35.E. F. Marce 2021. Github Repository; 2021.
- 36.Transplantation ASo. Joint statement about COVID-19 vaccination in organ transplant candidates and recipients. March 13, 2022. Accessed May 10, 2022. https://www.myast.org/sites/default/files/03-13-22%20ISHLT-AST-ASTS%20joint%20society%20guidance%20vaccine_v9.pdf
- 37.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahav G, Lustig Y, Lavee J, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: a prospective cohort study. EClinicalMedicine. 2021;41:101158. doi: 10.1016/j.eclinm.2021.101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heldman MR, Limaye AP. SARS-CoV-2 vaccines in kidney transplant recipients: will they be safe and effective and how will we know? J Am Soc Nephrol. 2021;32(5):1021–1024. doi: 10.1681/ASN.2021010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duchini A, Goss JA, Karpen S, Pockros PJ. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16(3):357–364. doi: 10.1128/CMR.16.3.357-364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deborska-Materkowska D, Kaminska D. The immunology of SARS-CoV-2 infection and vaccines in solid organ transplant recipients. Viruses. 2021;13(9):1–40. doi: 10.3390/v13091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stock PG, Henrich TJ, Segev DL, Werbel WA. Interpreting and addressing suboptimal immune responses after COVID-19 vaccination in solid-organ transplant recipients. J Clin Invest. 2021;131(14):e151178. doi: 10.1172/JCI151178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
All diagnostic, medication, procedure, and laboratory concepts used in this study are available in Table S1. Raw code (R, Python, SQL) is available upon request. N3C is a public resource maintained by NCATS to support COVID-19 research. To access patient-level data from the N3C consortium, institutions must have a signed Data Use Agreement executed with NCATS and investigators must complete mandatory training along with submitting a Data Use Request to N3C. Investigators can request access to the Enclave here.