Abstract
We aimed to assess longitudinal changes in clinical indexes of corona disease 2019 (Covid‐19) patients with mild pulmonary infection during 5 days of remdesivir therapy and determine the effect of age and gender on remdesivir adverse effects (AE). Patients' clinical data including inflammatory markers, liver and renal function tests, and heart rate (HR) were extracted from medical records. Linear mixed model (LMM) was used to analyze longitudinal changes in patients' clinical indexes. Gender and age were inserted in LMM as covariates to find their correlation with AE and clinical indexes. Of 84 patients, 35 patients met our criteria for the study. There were significant increases in mean levels of white blood cell (WBC; p = 0.005), alanine aminotransferase (ALT; p = 0.001), aspartate aminotransferase (p = 0.001), blood urea nitrogen (BUN; p = 0.001), and creatinine (p = 0.006), whereas mean levels of erythrocyte sedimentation rate (p = 0.005), C‐reactive protein (p = 0.001), alkaline phosphatase (p = 0.001), and potassium (p = 0.003) decreased significantly. Estimated glomerular filtration rate (p = 0.001) and HR (p = 0.001) showed a notable decline over the course of treatment. LMM analysis showed that mean changes in WBC (β = 0.94, p = 0.029), creatinine (β = 0.12, p = 0.020), and HR (β = 6.47, p = 0.008) were greater in males than in females. Also, age of patients had a significant effect on the mean changes of WBC (β = −0.02, p = 0.023), sodium (β = −0.06, p = 0.010), BUN (β = 0.23, p = 0.001), and HR (β = −0.29, p = 0.001). Despite no renal and liver dysfunction, Covid‐19 patients with mild pulmonary infection may develop some remdesivir AE and attributed side effects might be affected by gender and age of patients.
Keywords: adverse effects, Covid‐19, linear mixed model, remdesivir
1. INTRODUCTION
Corona disease 2019 (Covid‐19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is a viral infection of the respiratory tract typified with fever, dry cough, fatigue, and worsening dyspnea. 1 During the current pandemic, several treatment protocols for Covid‐19 including chloroquine/hydroxychloroquine, favipiravir, lopinavir/ritonavir, and azithromycin have been tested and used. 2 Remdesivir, an adenosine triphosphate (ATP) analog that inhibits viral RNA polymerase, has been approved by the US Food and Drug Administration (FDA) for hospitalized patients with Covid‐19. 3 , 4 Despite the beneficial effects of remdesivir in Covid‐19 treatment, hepatotoxicity and nephrotoxicity have been reported as adverse effects (AEs) in Covid‐19 patients receiving intravenous remdesivir because of accumulation of sulfobutylether‐β‐cyclodextrin (SBECD) carrier. 5
Despite the emergent and widespread use of remdesivir for the Covid‐19 treatment, some AE including cardiac, renal, and liver complications have been reported in patients receiving remdesivir. 6 Among the remdesivir AEs, anemia, infusion site reactions, increased liver enzymes, cutaneous rash, kidney injuries, and hypotension are the most common. According to the FDA Emergency Use Authorization, a loading dose of 200 mg (5 mg/kg) once in a day in patients ≥ 40 kg and 100 mg from Day 2–5 (2.5 mg/kg) has been proposed. Clinical investigations on patients with normal kidney function indicated that remdesivir and its active metabolite, remdesivir triphosphate, are predominantly (74%) eliminated by the kidneys. 7 , 8
Moreover, transaminase elevations have been observed in Covid‐19 patients receiving remdesivir and, therefore, liver function tests (LFTs) must be monitored daily, and remdesivir is discontinued in individuals with alanine aminotransferase (ALT) more than five times the upper limit of normal healthy individuals. 9 Regarding cardiovascular complications, sinus bradycardia, hypotension, T‐wave abnormalities, atrial fibrillation (AF), and a prolonged QT interval are the most common AEs. 10
It has been shown that remdesivir is adequately tolerated but does not provide significant therapeutic effects in seriously COVID‐19 patients. Furthermore, there is a lack of clinical evidence to evaluate the appearance of remdesivir AE and outcomes in men and women and different age groups. 11 The goal of the current study is to investigate longitudinal changes in clinical indexes of hospitalized Covid‐19 patients with mild pulmonary infection during a 5‐day course for remdesivir therapy.
2. PATIENTS AND METHODS
2.1. Study design and subjects
This retrospective longitudinal study was conducted on Covid‐19 hospitalized patients receiving intravenous remdesivir in the regular ward between June and September 2021. The protocol of the study was approved by the ethics and research review committee of the Iran University of Medical Sciences (IR. IUMS. REC.1400.072). All participants gave written informed consent, and the study was carried out following the guidelines of the Declaration of Helsinki. Inclusion criteria to enter in the study were patients who had no history of kidney and liver dysfunction, estimated glomerular filtration rate (eGFR) cutoffs of 30 or 50 ml/min per 1.73 m2, elevated LFT less than five times the upper limit of normal, oxygen saturation above 90%, and pulmonary infection of <20% in lung high‐resolution computed tomography. The estimated sample size was 36 calculated based on Type I error (5%), power (80%), an effect size of 2.9, and change SD 6 for ALT before and after remdesivir reported by Aiswarya et al. 12 All patients were treated according to protocol with dexamethasone 6 mg daily, remdesivir (a loading dose of 200 mg on day 1 followed by 100 mg for 4 days), and enoxaparin 60 mg daily. Demographic data and medical records of patients were extracted from Hospital Information System and analyzed.
2.2. Clinical indexes
To follow Covid‐19 disease inflammatory state during 5 days remdesivir therapy, white blood cell (WBC) count, C‐reactive protein (CRP), and erythrocyte sedimentation rate (ESR) were assessed in the patients. Also, remdesivir AEs on the function of the liver, kidney, and heart were monitored through checking clinical indexes including aspartate transaminase (AST), ALT, alkaline phosphatase (ALP) for liver function, blood urea nitrogen (BUN), creatinine, eGFR, sodium, and potassium for kidney function, and heart rate (HR) on the first, third, and fifth days of remdesivir therapy.
2.3. Statistical analysis
Linear mixed model (LMM) is one of the longitudinal data analysis methods that describes the relationship between a response variable and other explanatory variables that have been obtained along with the response. We used LMM to assess mean changes of clinical indexes in patients over the time of remdesivir therapy. Age and gender were inserted in the model as covariates to analyze their effects on the mean changes in clinical indexes. Restricted maximum likelihood with slope and intercept random effect was used to estimate all coefficients in the model. Continuous variables are presented as mean ± SD and categorical variables are presented as frequency (%). All statistical analyses were done using IBM SPSS version 24.0 (IBM, Inc.). p < 0.05 were considered significant.
3. RESULT
3.1. Characteristics of Covid‐19 patients
As depicted in Figure 1, among 84 Covid‐19 patients who were admitted for remdesivir therapy, 35 patients met our inclusion criteria and enter into the study. Demographic and clinical characteristics of Covid‐19 patients are summarized in Table 1. The mean age of patients was 56.22 ± 17.86 years and 57.1% of them were women. Dyspnea (77%) and dry cough (88.6%) were the most common symptoms of the disease and 62.9% of patients received oral Favipiravir before starting remdesivir therapy.
Figure 1.
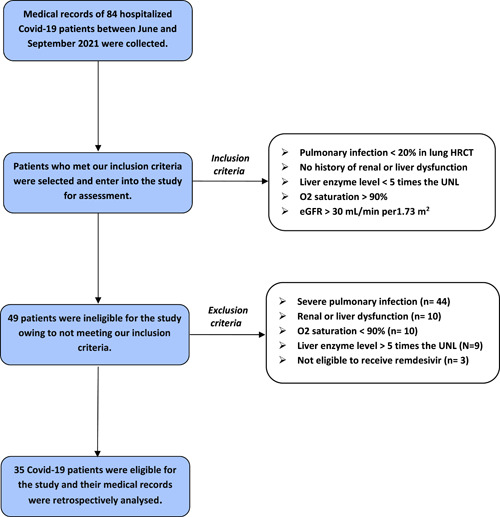
Flowchart of the studied patients. eGFR, estimated glomerular filtration rate; HRCT, high‐resolution computed tomography; UNL, upper normal limit.
Table 1.
Characteristics of Covid‐19 patients
| Age (year)a | 56.22 ± 17.86 |
|---|---|
| Gender (n, %)b | |
| Male | 15 (42.9) |
| Female | 20 (57.1) |
| Fever (n, %)b | 14 (40) |
| Dyspnea (n, %)b | 27 (77.1) |
| Dry cough (n, %)b | 31 (88.6) |
| HTN (n, %)b | 11 (31.4) |
| DM (n, %)b | 6 (17.1) |
| Favipiravir consumption (n, %)b | 22 (62.9) |
| Spo2 (%)a | 92.22 ± 2.30 |
Abbreviations: Covid‐19, corona disease 2019; DM, diabetes mellitus; HTN, hypertension.
Continues data are presented as mean ± SD.
Categorical data are presented as frequency (percentage).
3.2. Remdesivir AE in Covid‐19 patients during treatment
As represented in Table 2, mean WBC increased significantly (male: p = 0.006, female: p = 0.001), whereas mean ESR (male: p = 0.003, female: p = 0.001) and CRP (male: p = 0.001, female: p = 0.002) showed a notable decline through remdesivir therapy. Figure 2 shows changes in mean WBC, CRP, and ESR index for male and female Covid‐19 patients in more detail during 5 days of remdesivir injection.
Table 2.
Clinical indexes of Covid‐19 patients over the course of remdesivir therapy
| Clinical index | Reference range | Gender | Days of remdesivir therapy | p | ||
|---|---|---|---|---|---|---|
| 1st | 3rd | 5th | ||||
| WBC | 4.5–11 (×10*3/micL) |
M F |
5.30 ± 1.84 4.63 ± 1.53 |
7.41 ± 2.25 6.23 ± 1.84 |
6.94 ± 2.28 6.05 ± 1.06 |
0.006 0.001 |
| ESR | Up to 15 (mm/h) |
M F |
41.27 ± 26.44 44.50 ± 20.58 |
41.01 ± 29.78 34.81 ± 17.48 |
22.0 ± 12.66 30.60 ± 14.85 |
0.003 0.001 |
| CRP | Up to 6 (mg/L) |
M F |
27.11 ± 26.99 22.34 ± 18.54 |
16.57 ± 15.53 14.58 ± 15.04 |
10.88 ± 11.91 10.49 ± 10.66 |
0.001 0.002 |
| ALT | Up to 40 (U/L) |
M F |
32.73 ± 19.60 43.10 ± 23.68 |
51.60 ± 31.05 49.21 ± 28.69 |
61.22 ± 31.87 55.30 ± 30.07 |
0.001 0.001 |
| AST | Up to 37 (U/L) |
M F |
37.86 ± 8.77 43.51 ± 15.54 |
47.86 ± 11.35 55.50 ± 18.59 |
51.93 ± 15.76 60.95 ± 18.98 |
0.001 0.001 |
| ALP | 65–305 (U/L) |
M F |
198.94 ± 65.25 234.60 ± 115.08 |
134.93 ± 43.47 152.10 ± 58.75 |
126.40 ± 27.75 128.20 ± 58.41 |
0.001 0.001 |
| Sodium | 135–145 (mEq/L) |
M F |
135.67 ± 3.31 137.80 ± 4.12 |
138.47 ± 3.74 137.30 ± 2.72 |
137.87 ± 3.81 138.80 ± 1.98 |
0.026 0.195 |
| Potassium | 3.5–5.5 (mEq/L) |
M F |
4.07 ± 0.19 3.90 ± 0.37 |
3.91 ± 0.17 3.69 ± 0.29 |
3.90 ± 0.16 3.63 ± 0.21 |
0.017 0.004 |
| BUN | 14–45 (mg/dl) |
M F |
29.67 ± 12.52 27.99 ± 9.78 |
40.33 ± 14.48 36.60 ± 9.12 |
41.01 ± 6.47 38.31 ± 5.98 |
0.001 0.001 |
| Creatinine | 0.7–1.4 (mg/dl) |
M F |
1.06 ± 0.25 0.86 ± 0.15 |
1.05 ± 0.21 0.83 ± 0.17 |
1.04 ± 0.20 1.0 ± 0.16 |
0.848 0.001 |
| eGFR | ≥60 ml/min/1.73 m2 |
M F |
81.07 ± 22.18 79.31 ± 18.88 |
79.53 ± 19.74 75.90 ± 17.30 |
78.73 ± 17.19 62.05 ± 14.37 |
0.907 0.001 |
| Hear rate | 60–100 beats/min |
M F |
81.13 ± 12.19 77.30 ± 10.30 |
62.33 ± 12.14 60.85 ± 6.23 |
58.13 ± 14.06 51.15 ± 4.34 |
0.001 0.001 |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Covid‐19, corona disease 2019; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; F, female; M, male; WBC, white blood cell.
Continues data are presented as mean ± SD.
Figure 2.
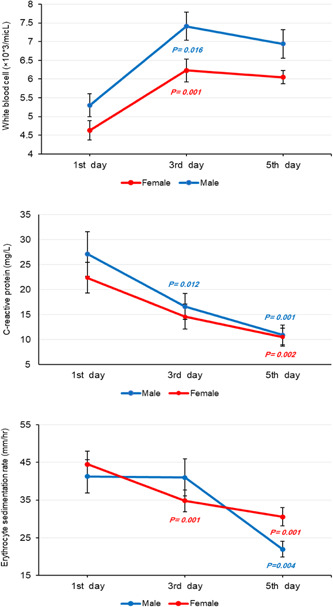
Changes in mean white blood cell (WBC), C‐reactive protein (CRP), and erythrocyte sedimentation rate (ESR) index estimated by linear mixed model (LMM) analysis on the first, third, and fifth day of remdesivir therapy over a 5‐day course of treatment in corona disease 2019 (Covid‐19) patients (reference: first day).
Regarding remdesivir AE, we found a significant rise in mean levels of ALT (male: p = 0.001, female: p = 0.001) and AST (male: p = 0.001, female: p = 0.001), and a considerable decline in mean ALP (male: p = 0.001, female: p = 0.001) during treatment. Figure 3 illustrates changes in mean ALT, AST, and ALP in further detail over the course of remdesivir therapy. Mean sodium in males showed a notable increase (p = 0.026), whereas mean potassium in both males (p = 0.017) and females (p = 0.004) decreased significantly. However, sodium and potassium values remained within the normal range during treatment. Also, mean BUN in both genders (p = 0.001) increased notably, while just females experienced a significant rise in creatinine levels (p = 0.001). Figure 4 provides more information about changes in mean levels of BUN, creatinine, sodium, and potassium on the first, third, and fifth day of remdesivir injection.
Figure 3.
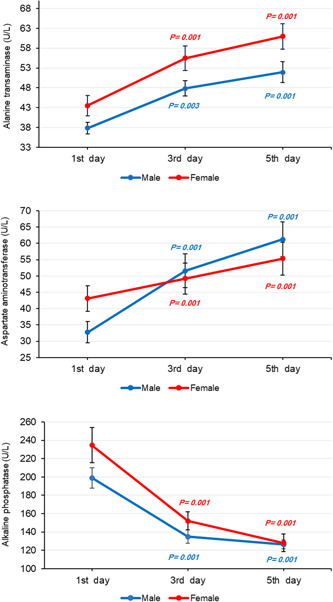
Changes in mean alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) index estimated by linear mixed model (LMM) analysis on the first, third, and fifth day of remdesivir therapy over a 5‐day course of treatment in corona disease 2019 (Covid‐19) patients (reference: first day).
Figure 4.
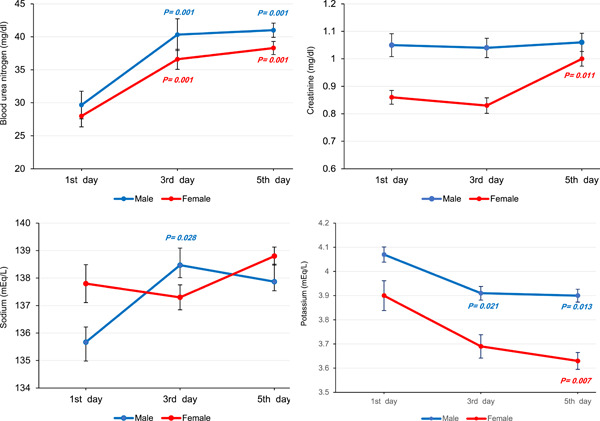
Changes in mean blood urea nitrogen (BUN), creatinine, sodium, and potassium index estimated by linear mixed model (LMM) analysis on the first, third, and fifth day of remdesivir therapy over a 5‐day course of treatment in corona disease 2019 (Covid‐19) patients (reference: first day).
Furthermore, females experienced a notable decrease in eGFR (p = 0.001) and mean HR of patients decreased significantly (male: p = 0.001, female: p = 0.001) during remdesivir therapy but HR gradually restored to normal after drug discontinuation. Figure 5 presents changes in mean eGFR and HR in more detail over the course of treatment.
Figure 5.
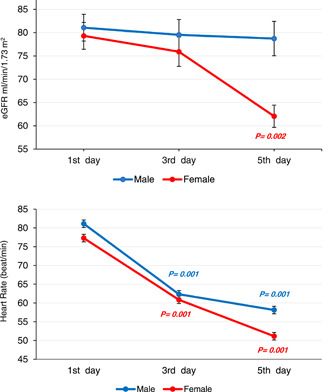
Changes in mean estimated glomerular filtration rate (eGFR) and heart rate (HR) index estimated by linear mixed model (LMM) analysis on the first, third, and fifth day of remdesivir therapy over a 5‐day course of treatment in corona disease 2019 (Covid‐19) patients (reference: first day).
3.3. Relationship of remdesivir AE with age and gender of patients
As described in the Section 2.3, age and gender were inserted in the LMM as covariates to find their effects on changes in clinical indexes in patients treated with remdesivir. Our results are summarized in Table 3 and indicate that gender as a covariate was significantly related to the changes in patients' indexes. Mean changes in WBC (β = 1.03, p = 0.029), creatinine (β = 0.15, p = 0.009), and HR (β = 6.22, p = 0.015) attributed to the remdesivir were greater for men than women. In addition, age of patients had a significant effect on the mean changes of WBC (β = −0.02, p = 0.023), sodium (β = −0.06, p = 0.026), BUN (β = 0.23, p = 0.001), and HR (β = −0.29, p = 0.001) over the time of remdesivir therapy.
Table 3.
Effects of gender and age on remdesivir AEs assessed by LMM analysis
| Variables | Mean ± SE | Gender | Age |
|---|---|---|---|
| WBC | Male = 6.57 ± 0.33 | β = 1.03 | β = −0.02 |
| Female = 5.63 ± 0.30 | p = 0.029 | p = 0.023 | |
| ESR | Male = 29.83 ± 5.52 | β = −2.69 | β = −0.13 |
| Female = 40.30 ± 4.81 | p = 0.629 | p = 0.404 | |
| CRP | Male = 17.80 ± 2.84 | β = 1.54 | β = −0.062 |
| Female = 16.26 ± 2.65 | p = 0.770 | p = 0.676 | |
| ALT | Male = 48.09 ± 6.81 | β = −1.42 | β = 0.09 |
| Female = 49.51 ± 6.08 | p = 0.882 | p = 0.735 | |
| AST | Male = 46.29 ± 3.49 | β = −7.60 | β = 0.05 |
| Female = 53.01 ± 3.16 | p = 0.143 | p = 0.708 | |
| ALP | Male = 151.47 ± 15.73 | β = −21.62 | β = 0.43 |
| Female = 173.09 ± 13.03 | p = 0.310 | p = 0.478 | |
| Sodium | Male = 137.78 ± 0.56 | β = 0.15 | β = −0.06 |
| Female = 137.64 ± 0.49 | p = 0.847 | p = 0.026 | |
| Potassium | Male = 3.90 ± 0.04 | β = 0.12 | β = 0.001 |
| Female = 3.78 ± 0.03 | p = 0.060 | p = 0.597 | |
| BUN | Male = 35.52 ± 1.54 | β = 0.73 | β = 0.23 |
| Female = 34.78 ± 1.46 | p = 0.623 | p = 0.001 | |
| Creatinine | Male = 1.04 ± 0.04 | β = 0.15 | β = −0.02 |
| Female = 0.91 ± 0.03 | p = 0.009 | p = 0.460 | |
| eGFR | Male = 84.04 ± 4.08 | β = 10.79 | β = −0.16 |
| Female = 71.15 ± 3.66 | p = 0.051 | p = 0.294 | |
| Heart rate | Male = 68.55 ± 1.76 | β = 6.22 | β = − 0.29 |
| Female = 62.10 ± 1.53 | p = 0.015 | p = 0.001 |
Abbreviations: AE, adverse effect; ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; LMM, linear mixed model; WBC, white blood cell.
4. DISCUSSION
In this single‐center study, clinical outcomes of 35 Covid‐19 patients with mild pulmonary infection treated with remdesivir were evaluated. We did not find serious AEs attributed to the remdesivir therapy but bradycardia with renal and liver dysfunction were seen in the Covid‐19 patients. In addition, age and gender of patients had significant effects on some AEs of remdesivir.
The clinical syndrome caused by Covid‐19 infection primarily affects respiratory tracts, but other organs including the heart, gastrointestinal system, and liver may also be affected. 13 Recent reports have shown that some patients with the severe form of the Covid‐19 infection experience an intensified immune response similar to cytokine release syndrome and multisystem inflammatory syndrome. 14 Consistent with us, a prior study by Stoeckle et al. 15 have shown that CRP levels decrease significantly after remdesivir therapy in none intubated Covid‐19 patients, indicating that remdesivir mitigates inflammatory response in a certain group of patients but not in all. Moreover, women patients in the study had lower CRP levels than men that may be due to the lower viral load level, higher number of CD4+ T cells, and higher levels of antibodies in women. 16 Interestingly, producing interleukin‐6, which actively participates in the inflammatory response to Covid‐19 infection is lower in women than in men and often correlated with better longevity. 17 Prior studies on Covid‐19 patients have reported that 37–69% of Covid‐19 patients experience at least one abnormal ALT and AST on hospital admission, whereas 93% have at least one abnormal LFT throughout the disease. 18 Furthermore, increased LFT is among the most common AEs attributed to the remdesivir reported in patients hospitalized with Covid‐19 infection. 19 The exact mechanisms of liver injury caused by Covid‐19 infection have not completely understood. However, direct viral infection, indirect host‐immune responses, and treatment drug toxicity could damage liver and induce hepatotoxicity. 20 From the cellular point of view, remdesivir has been demonstrated to be toxic to hepatocytes, and the FDA has cautioned about the incidence of elevated ALT and AST in patients receiving remdesivir, as observed in our patients. 9 , 21 Furthermore, nephrotoxicity attributed to the remdesivir has been reported in Covdid‐19 patients that may be a consequence of mitochondrial toxicity in renal tubular epithelial cells and accumulation of remdesivir's active metabolite or SBECD. 5 , 22 Although patients in our study had normal renal and LFT before remdesivir therapy, decreased eGFR and increased ALT and AST were observed during a 5‐day course of treatment. These results are in parallel to findings of recent randomized control trials 23 , 24 and our data indicated that renal function may be affected by age and gender of patients treated with remdesivir. Taken together, it is necessary to emphasize that remdesivir is not the only factor involved in the nephrotoxicity or hepatotoxicity in Covid‐19 patients and both Covid‐related and non‐Covid‐related risk factors such as older age and gender may be contributed. 5 , 25
Some Covid‐19 patients on remdesivir may exhibit sinus bradycardia, hypotension, T‐wave abnormalities, and a prolonged QT interval. It has been shown that remdesivir have some cardiotoxic and proarrhythmic effects pronouncedly occur in patients with cardiovascular disorders. 10 Remdesivir is structurally like adenosine but with a longer half‐life. Because of its resemblance with ATP, remdesivir could inhibit atrioventricular node by binding to the A1 receptor on cardiac cells and induce sinus bradycardia. 26 Additionally, a decreased potassium level happens widely in Covid‐19 patients, which may lead to electrocardiographic changes such as QT prolongation and can increase the risk of bradycardia. 27 Our results support prior studies reporting some hepatic and renal reactions and QT prolongation are predominantly observed in male reports as AEs of remdesivir. 28
Many factors affect the occurrence of drug AE and some of these factors cannot be changed like age, presence of other diseases, or genetic factors. LMM analysis in our study showed significant effects of age and gender on remdesivir AE. It has been reported that older people are more than twice as susceptible to drug AEs as younger people. 29 Aging results in the decrease in the amount of water in the body that leads to the increase in the amount of fat tissue relative to water. Thus, drugs that dissolve in water reach higher concentrations and drugs that dissolve in fat accumulate more. Also, in aging people kidneys are less able to excrete drugs into the urine and the liver is less able to metabolize many drugs. 30 In addition, women have lower body weight and organ size, more body fat, different gastric motility, and lower GFR in comparison to men. These anatomical and physiological differences can affect the pharmacokinetics and pharmacodynamics of the drugs in the body including drug absorption, distribution, metabolism, and elimination. 31
The present study has some potential limitations. Our investigation was a single‐center study and the main limitations of our investigation were short‐term follow‐up and small sample size. Also, the study involved hospitalized Covid‐19 patients treated with remdesivir and the lack of Covid‐19 control patients who did not treat with remdesivir is another limitation. Thus, we cannot exactly conclude that AEs observed in the patients are due to remdesivir or SARS‐CoV‐2. Hence, more extensive studies with a control group are necessary to investigate remdesivir AEs and predict sex and age differences in remdesivir treatment outcomes.
5. CONCLUSION
We found that patients with mild Covid‐19 pneumonia may develop AE attributed to the remdesivir, despite having no liver and renal dysfunction. Moreover, remdesivir AE might be affected by gender and age of patients and clinicians should be more cautious about additive AE when other medicines are used in addition to remdesivir therapy.
AUTHOR CONTRIBUTIONS
Mohsen Sedighi contributed to conceptualization, project administration, methodology, and writing the original draft. Alireza Amanollahi contributed to data analysis, methodology, and writing the original draft. Nader Tavakoli, Omid M. Moghaddam, Hamed B. Ghafouri, and Seyede E. Hoseini contributed to the data collection and investigation. Nader Tavakoli contributed to funding acquisition, supervision, writing, and review, and editing.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary Information.
ACKNOWLEDGMENTS
The authors would like to thank the patients who participated in this study and our colleagues in the Trauma and Injury Research Center for their support of this study. This study was part of a research program in our center and financially supported by the Iran University of Medical Sciences (Grant number 99‐3‐69‐19919).
Sedighi M, Amanollahi A, Moradi Moghaddam O, Basir Ghafouri H, Hoseini SE, Tavakoli N. Linear mixed model analysis to evaluate correlations between remdesivir adverse effects with age and gender of patients with mild Covid‐19 pneumonia. J Med Virol. 2022;94:3783‐3790. 10.1002/jmv.27800
Contributor Information
Mohsen Sedighi, Email: sedighi.mo@iums.ac.ir.
Nader Tavakoli, Email: tavakoli.n@iums.ac.ir.
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bigdelian H, Sedighi M, Sabri MR, et al. Acute intracardiac thrombosis in children with coronavirus disease 2019 (COVID‐19). Front Pediatr. 2021;9:656720. 10.3389/fped.2021.656720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal G, Henry BM, Aggarwal S, Bangalore S. Cardiovascular safety of potential drugs for the treatment of coronavirus disease 2019. Am J Cardiol. 2020;128:147‐150. 10.1016/j.amjcard.2020.04.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11:222. 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilt TJ, Kaka AS, MacDonald R, Greer N, Obley A, Duan‐Porter W. Remdesivir for adults with COVID‐19: a living systematic review for American College of Physicians practice points. Ann Intern Med. 2021;174(2):209‐220. 10.7326/M20-5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Laar SA, de Boer MG, Gombert‐Handoko KB, Guchelaar HJ, Zwaveling J, LUMC‐Covid‐19 Research Group . Liver and kidney function in patients with Covid‐19 treated with remdesivir. Br J Clin Pharmacol. 2021;87(11):4450‐4454. 10.1111/bcp.14831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badgujar KC, Ram AH, Zanznay R, Kadam H, Badgujar VC. Remdesivir for COVID‐19: a review of pharmacology, mechanism of action, in‐vitro activity and clinical use based on available case studies. JDDT. 2020;10:264‐270. 10.22270/jddt.v10i4-s.4313 [DOI] [Google Scholar]
- 7. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 ‐ preliminary report. N Engl J Med. 2020;3:383(10):993‐994. 10.1056/NEJMc2022236 [DOI] [PubMed] [Google Scholar]
- 8. Estiverne C, Strohbehn IA, Mithani Z, et al. Remdesivir in patients with estimated GFR < 30 ml/min per 1.73 m2 or on renal replacement therapy. Kidney Int Rep. 2021;6(3):835‐838. 10.1016/j.ekir.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aleem A, Mahadevaiah G, Shariff N, Kothadia JP. Hepatic manifestations of COVID‐19 and effect of remdesivir on liver function in patients with COVID‐19 illness. Proc (Bayl Univ Med Cent). 2021;34(4):473‐477. 10.1080/08998280.2021.1885289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nabati M, Parsaee H. Potential cardiotoxic effects of remdesivir on cardiovascular system: a literature review. Cardiovasc Toxicol. 2021;13:1‐5. 10.1007/s12012-021-09703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ambrosino I, Barbagelata E, Corbi G, Ciarambino T, Politi C, Moretti AM. Gender differences in treatment of Coronavirus Disease‐2019. Monaldi Arch Chest Dis. 2020;3;90(4), 10.4081/monaldi.2020.1508 [DOI] [PubMed] [Google Scholar]
- 12. Aiswarya D, Arumugam V, Dineshkumar T, et al. Use of remdesivir in patients with COVID‐19 on hemodialysis: a study of safety and tolerance. Kidney Int Rep. 2021;6(3):586‐593. 10.1016/j.ekir.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H, Liu L, Zhang D, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;9;395(10235):1517‐1520. 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255‐2273. 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stoeckle K, Witting B, Kapadia S, An A, Marks K. Elevated inflammatory markers are associated with poor outcomes in COVID‐19 patients treated with remdesivir. J Med Virol. 2022;94(1):384‐387. 10.1002/jmv.27280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Groot NG, Bontrop RE. COVID‐19 pandemic: is a gender‐defined dosage effect responsible for the high mortality rate among males? Immunogenetics. 2020;72(5):275‐277. 10.1007/s00251-020-01165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu Z, Cai T, Fan L, et al. Clinical value of immune‐inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332‐339. 10.1016/j.ijid.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang T, Smith DA, Campbell C, et al. Longitudinal analysis of the utility of liver biochemistry as prognostic markers in hospitalized patients with corona virus disease 2019. Hepatol Commun. 2021;5(9):1586‐1604. 10.1002/hep4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu JY, Anand H, Frager SZ, Hou W, Duong TQ. Longitudinal progression of clinical variables associated with graded liver injury in COVID‐19 patients. Hepatol Int. 2021;15(4):1018‐1026. 10.1007/s12072-021-10228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nardo AD, Schneeweiss‐Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID‐19. Liver Int. 2021;41(1):20‐32. 10.1111/liv.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zampino R, Mele F, Florio LL, et al. Liver injury in remdesivir‐treated COVID‐19 patients. Hepatol Int. 2020;14(5):881‐883. 10.1007/s12072-020-10077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ackley TW, McManus D, Topal JE, Cicali B, Shah S. A valid warning or clinical Lore: an evaluation of safety outcomes of Remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother. 2020;20;65(2):e02290‐20. 10.1128/AAC.02290-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;16;395(10236):1569‐1578. 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldman JD, Lye D, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid‐19. N Engl J Med. 2020;5;383(19):1827‐1837. 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nadim MK, Forni LG, Mehta RL, et al. COVID‐19‐associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747‐764. 10.1038/s41581-020-00356-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barkas F, Styla C‐P, Bechlioulis A, Milionis H, Liberopoulos E. Sinus bradycardia associated with remdesivir treatment in COVID‐19: a case report and literature review. J Cardiovasc Dev Dis. 2021;12;8(2):18. 10.3390/jcdd8020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alfano G, Ferrari A, Fontana F, et al. Hypokalemia in patients with COVID‐19. Clin Exp Nephrol. 2021;25(4):401‐409. 10.1007/s10157-020-01996-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zekarias A, Watson S, Vidlin SH, Grundmark B. Sex differences in reported adverse drug reactions to COVID‐19 drugs in a global database of individual case safety reports. Drug Saf. 2020;43(12):1309‐1314. 10.1007/s40264-020-01000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hajjar ER, Hanlon JT, Artz MB, et al. Adverse drug reaction risk factors in older outpatients. Am J Geriatr Pharmacother. 2003;1(2):82‐89. 10.1016/S1543-5946(03)90004-3 [DOI] [PubMed] [Google Scholar]
- 30. Jimmy J, Padma GMR. Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospital. Pharmacol Res. 2006;54:226‐233. 10.1016/j.phrs.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 31. Alomar MJ. Factors affecting the development of adverse drug reactions (review article). Saudi Pharm J. 2014;22(2):83‐94. 10.1016/j.jsps.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


