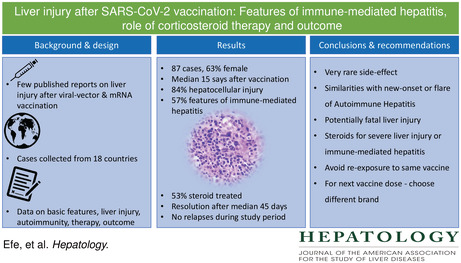
Abstract
Background and Aims
A few case reports of autoimmune hepatitis–like liver injury have been reported after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccination. We evaluated clinical features, treatment response and outcomes of liver injury following SARS‐CoV‐2 vaccination in a large case series.
Approach and Results
We collected data from cases in 18 countries. The type of liver injury was assessed with the R‐value. The study population was categorized according to features of immune‐mediated hepatitis (positive autoantibodies and elevated immunoglobulin G levels) and corticosteroid therapy for the liver injury. We identified 87 patients (63%, female), median age 48 (range: 18–79) years at presentation. Liver injury was diagnosed a median 15 (range: 3–65) days after vaccination. Fifty‐one cases (59%) were attributed to the Pfizer‐BioNTech (BNT162b2) vaccine, 20 (23%) cases to the Oxford‐AstraZeneca (ChAdOX1 nCoV‐19) vaccine and 16 (18%) cases to the Moderna (mRNA‐1273) vaccine. The liver injury was predominantly hepatocellular (84%) and 57% of patients showed features of immune‐mediated hepatitis. Corticosteroids were given to 46 (53%) patients, more often for grade 3–4 liver injury than for grade 1–2 liver injury (88.9% vs. 43.5%, p = 0.001) and more often for patients with than without immune‐mediated hepatitis (71.1% vs. 38.2%, p = 0.003). All patients showed resolution of liver injury except for one man (1.1%) who developed liver failure and underwent liver transplantation. Steroid therapy was withdrawn during the observation period in 12 (26%) patients after complete biochemical resolution. None had a relapse during follow‐up.
Conclusions
SARS‐CoV‐2 vaccination can be associated with liver injury. Corticosteroid therapy may be beneficial in those with immune‐mediated features or severe hepatitis. Outcome was generally favorable, but vaccine‐associated liver injury led to fulminant liver failure in one patient.
SARS‐COV‐2 vaccination and liver injury.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing coronavirus disease 2019 (Covid‐19) was first described in December 2019 in Wuhan, China.[ 1 ] SARS‐CoV‐2 is a highly transmissible and pathogenic virus that has spread worldwide and caused a pandemic. Although most Covid‐19 cases have mild symptoms, hospitalization and mortality rates are significant, especially in individuals with comorbid conditions.[ 2 , 3 ]
Vaccination is the most effective tool against Covid‐19. Due to the devastating global impact of Covid‐19, several vaccines were granted a fast‐track authorization. Currently, the most used vaccines are those produced by Pfizer‐BioNTech, Oxford‐AstraZeneca, and Moderna.[ 4 ] These vaccines have significantly reduced major Covid‐19 adverse outcomes such as hospitalization, intensive care unit admission and mortality.[ 4 , 5 ] Because massive vaccination program were started at the end of 2020, few serious adverse effects of SARS‐CoV‐2 vaccines have been reported.[ 6 ] Overall, SARS‐CoV‐2 vaccines appear safe and well tolerated.
SARS‐CoV‐2 vaccines trigger the interferon pathway as part of their mechanism of action, raising some concerns regarding the possibility of vaccine‐induced autoimmunity in susceptible individuals.[ 5 ] Several organ specific or systemic immune‐mediated diseases following SARS‐CoV‐2 vaccination have indeed been reported.[ 7 ] To the best of our knowledge, Bril et al.[ 8 ] reported the first case of liver injury after a first dose of Pfizer‐BioNTech vaccine. Additional cases have since been reported.[ 9 ] The clinical and histological findings of most patients resembled autoimmune hepatitis (AIH) and all reported patients showed spontaneous resolution or responded well to corticosteroid therapy.[ 9 , 10 ]
The aim of this international effort was to assemble a large case series in order to assess the clinical characteristics, efficacy of corticosteroid therapy and outcomes of patients who developed liver injury following SARS‐CoV‐2 vaccination.
PATIENTS AND METHODS
Patients
We retrospectively collected data from 18 countries on patients who developed liver injury after SARS‐CoV‐2 vaccination. All study collaborators independently identified cases and obtained predefined data from patient's medical records. Any treatment with immunosuppressive therapy was decided upon at the discretion of the local consulting physician. The Harran University Hospital at Şanlıurfa was the coordinating center (HRU/2021.17.29), and local ethical review boards of participating centers approved the study.
Liver injury
New‐onset liver injury was defined as elevations of alanine or aspartate aminotransferase (ALT or AST) ≥5× upper limit of normal (ULN) and/or alkaline phosphatase (ALP) ≥2× ULN or ALT/AST ≥3× UNL and bilirubin ≥2× ULN.[ 11 ] All patients were categorized for liver injury pattern by using the R‐value, which is defined as serum alanine aminotransferase (ALT)/upper limit of normal (ULN) divided by serum alkaline phosphatase (ALP)/ULN. Liver injury was categorized as hepatocellular if R ratio was >5, as mixed if 2–5 and as cholestatic if <2. The severity of the liver injury was categorized as (1) mild if serum enzyme elevations reached criteria for liver injury but bilirubin concentration was <2× ULN; (2) moderate if either bilirubin ≥2× ULN or symptomatic hepatitis; (3) severe if bilirubin concentration ≥2× ULN and signs of liver failure (INR ≥1.5, ascites and/or encephalopathy) or other organ failure considered to be due to liver injury; and (4) fatal if death from liver disease or the need for liver transplantation due to liver injury.[ 12 ]
Data collection
Case report forms included comprehensive laboratory and serologic data that included anti‐nuclear antibodies (ANA), anti‐smooth muscle antibodies (SMA), anti‐mitochondrial antibodies (AMA), anti‐liver/kidney microsome type 1 (LKM‐1), anti‐liver cytosol type 1 (LC‐1), anti‐soluble liver antigen/liver pancreas antigen (anti‐SLA/LP), serum immunoglobulin G (IgG) and ceruloplasmin levels, serological tests for hepatitis virus A‐C, Cytomegalovirus and Epstein–Barr virus. Autoimmune liver serology was evaluated according to local laboratory standards and a titer of 1:40 or higher was considered positive for ANA, SMA, LC‐1, and LKM‐1. Local pathologists in the participating centers evaluated liver biopsies; data from their reports were used in the study. Fibrosis was classified according to the METAVIR scoring system.[ 13 ] The simplified criteria were applied for the diagnosis of AIH.[ 14 ]
Statistical Analysis
Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL) version 26. The variables were investigated by using (Kolmogorov‐Smirnov/Shapiro‐Wilk's test) analytical methods and visual methods (histograms, probability plots) to determine whether or not they were normally distributed. Descriptive analyses were presented (by using tables of frequencies for the ordinal variables) median (min‐max) for the non‐normally distributed and ordinal variables and means and standard deviations for normally distributed variables. The Chi‐square test or Fisher's exact test where appropriate was performed to compare the proportions in different groups. The Kruskal‐Wallis tests were performed to compare the non‐normally distributed variables and ordinal variables among the groups and Mann–Whitney U test was performed to calculate the significance of pairwise differences by using Bonferroni correction to adjust for the multiple comparisons. A p value <0.05 was considered to represent statistical significance.
RESULTS
Characteristics of the study population
The general characteristics, clinical features, and outcomes are presented in Table 1. The preliminary data of eight cases were reported previously.[ 9 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ] The median age at the time of diagnosis was 48 (range 18–79) years and 55 (63%) were female. The median body mass index (kg/m2) was 25 (range 17–33). Twenty‐four (28%) patients had been diagnosed with other autoimmune disorders before liver injury onset. Autoimmune thyroid diseases were present in 12 (14%) patients, inflammatory bowel diseases in three (3%), sarcoidosis in three (3%), systemic lupus erythematosus in two (2%), celiac disease in two (2%), rheumatoid arthritis, pemphigus vulgaris, lichen planus, and multiple sclerosis in each one patient.
TABLE 1.
General characteristics of the study population
| Overall (n = 87) | Corticosteroid therapy (n = 46) | No corticosteroids (n = 41) | p value | |
|---|---|---|---|---|
| Age (years), median (min, max) | 48 (18–79) | 47 (20–69) | 49 (18–79) | 0.529 |
| Sex, female, n (%) | 55 (63.2) | 29 (63) | 26 (63.4) | 0.971 |
| Pre‐existing liver disease, n (%) | 12 (13.8) | 3 (6.5) | 9 (22) | 0.037 |
| Symptoms at liver injury onset, n (%) | ||||
| Jaundice | 34 (39.1) | 22 (47.8) | 12 (29.3) | 0.077 |
| Nausea | 55 (63.2) | 30 (65.2) | 25 (61) | 0.682 |
| Fatigue | 65 (74.7) | 36 (78.3) | 29 (70.7) | 0.420 |
| Fever | 10 (11.5) | 4 (8.7) | 6 (14.6) | 0.386 |
| Abdominal pain | 21 (24.1) | 9 (19.6) | 12 (29.3) | 0.291 |
| Rash | 7 (8) | 3 (6.5) | 4 (9.8) | 0.580 |
| Itching | 10 (11.5) | 4 (8.7) | 6 (14.6) | 0.386 |
| Peak ALT × ULN | 16.7 (3.1–203.7) | 21.9 (5–203.7) | 9.2 (3.1–81.8) | <0.001 |
| Peak AST × ULN | 15.4 (1.8–250) | 23.4 (2.7–250) | 9.2 (1.8–70.1) | <0.001 |
| Peak ALP × ULN | 1.3 (0.4–7.1) | 1.3 (0.4–7.1) | 1.4 (0.4–6.5) | 0.595 |
| Peak total bilirubin × ULN | 2.6 (0.3–23.2) | 5.3 (0.6–23.2) | 1.1 (0.3–19.5) | 0.003 |
| INR | 1.1 (0.6–3.8) | 1.3 (0.7–3.8) | 1 (0.6–2.4) | <0.001 |
| Pattern of injury, n (%) | ||||
| Hepatocellular | 73 (83.9) | 44 (95.7) | 29 (70.7) | 0.020 |
| Mixed | 9 (10.3) | 2 (4.3) | 7 (17.1) | 0.052 |
| Cholestatic | 5 (5.7) | ‐ | 5 (12.2) | 0.015 |
| ANA positivity, n (%) | 56 (67.5) | 33 (73.3) | 23 (60.5) | 0.215 |
| SMA positivity, n (%) | 15 (18.1) | 11(24.4) | 4 (10.5) | 0.101 |
| AMA positivity, n (%) | 5 (6) | 4 (8.9) | 1 (2.6) | 0.233 |
| IgG × UNL | 1.09 (0.41–2.71) | 1.19 (0.71–2.71) | 1.02 (0.41–1.97) | 0.001 |
| IgG > UNL, n (%) | 53 (67.1) | 35 (77.8) | 18 (52.9) | 0.020 |
| Fibrosis stage 0‐2, n (%) | 41 (93.2%) | 34 (91.9) | 7 (100) | 0.435 |
| Severity score, n (% | ||||
| Grade 1 | 38 (43.7) | 14 (30.4) | 24 (58.5) | 0.008 |
| Grade 2 | 31 (35.6) | 16 (34.8) | 15 (36.6) | 0.861 |
| Grade 3 | 17 (19.5) | 15 (32.6) | 2 (4.9) | 0.001 |
| Grade 4 | 1 (1.1) | 1 (2.2) | – | 0.342 |
| Liver transplant, n (%) | 1 (1.1) | 1 (2.2) | – | 0.342 |
| Days from peak ALT to normalization | 46 (15–185) | 60 (15–185) | 39 (18–114) | 0.085 |
Note: Values reported as median (min‐max) range.
Abbreviations: AMA, anti‐mitochondrial antibody, ANA, anti‐nuclear antibody and SMA, smooth muscle antibody were studied in 83 patients; IgG, immunoglobulin G, was measured in 79 patients; ULN, upper limit of normal; liver histology was performed in 44 patients.
Liver injury was identified after vaccination with the Pfizer‐BioNTech (BNT162b2) vaccine in 51 (59%) of the cases, the Oxford‐AstraZeneca (ChAdOX1 nCoV‐19) vaccine in 20 (23%) and the Moderna (mRNA‐1273) vaccine in 16 (18%). Forty (46%) developed liver injury after the first vaccine dose and 47 (54%) were diagnosed after the second dose. The median time from vaccination to the onset of liver injury was 15 (range: 3–65) days. Three patients had mild hepatitis after the first dose of vaccine but a more severe liver injury after a second dose of the same vaccine.
Most patients (92%) were symptomatic at presentation; fatigue 65 (75%), nausea 55 (63%) and jaundice 34 (39%) were the most reported symptoms. The pattern of liver injury was hepatocellular in 73 (84%), mixed in 9 (10%) and cholestatic in 5 (6%).
Autoantibodies were studied in 83 (95%) patients. Among these, ANA was positive in 56 (67%), SMA was positive in 15 (18%) and five (6%) cases showed seropositivity for AMA. Both anti‐SLA and LC‐1 were detected in one patient. The IgG level was measured in 79 (91%) patients and was high in 53 (67%) of these.
Immune‐mediated features
Serum autoantibody profile and IgG level were assessed in 79 cases, of whom 45 (57%) showed an immune‐mediated phenotype (positive autoantibodies and elevated IgG levels). Forty‐four patients also underwent a liver biopsy. General characteristics, biochemical findings, patterns of liver injury, frequency of severe liver injury were similar in patients with and without features of immune‐mediated hepatitis (Table 2). Among the 44 with liver histology, 34 (77%) were ascribed as probable/definite AIH according to the simplified criteria. Among 35 cases who did not have a liver biopsy, 12 (34%) still reached a score of probable AIH. Corticosteroid therapy was more often given to patients who had immune‐mediated hepatitis than to those without (71.1% vs. 38.2%, p = 0.003), but median time from liver injury to biochemical resolution was similar. Follow‐up duration after resolution of liver injury was longer in patients with immune‐mediated hepatitis than in those without (120 vs. 80 days, p = 0.036).
TABLE 2.
Characteristics of cases according to features of immune‐mediated hepatitis
| With immune‐mediated hepatitis (n = 45) | Without immune‐mediated hepatitis (n = 34) | p value | |
|---|---|---|---|
| Age, median years (range) | 49 (30–76) | 48 (18–79) | 0.556 |
| Sex (female), n (%) | 29 (64.4) | 19 (55.9) | 0.440 |
| Pre‐existing liver disease, n (%) | 4 (8.9) | 4 (11.8) | 0.675 |
| Autoimmune disease, n (%) | 15 (33.3) | 6 (17.6) | 0.118 |
| Peak ALT × ULN | 18.8 (3.1–81.8) | 10.7 (3.3–203.7) | 0.305 |
| Peak AST × ULN | 18.1 (2.6–250) | 13.1 (1.8–174.3) | 0.078 |
| Peak ALP × ULN | 1.3 (0.4–7.1) | 1.4 (0.6–6.5) | 0.770 |
| Peak total bilirubin × ULN | 5.8 (0.6–23.2) | 2.2 (0.3–17.9) | 0.079 |
| INR | 1.2 (0.6–3.8) | 1.1 (0.6–3.6) | 0.662 |
| Pattern of injury, n (%) | |||
| Hepatocellular | 40 (88.9) | 27 (79.4) | 0.245 |
| Mixed | 3 (6.7) | 4 (11.8) | 0.430 |
| Cholestatic | 2 (4.4) | 3 (8.8) | 0.429 |
| Fibrosis stage 0‐2, n (%) | 28 (90.3) | 13 (100) | 0.245 |
| Grade 3–4 liver injury, n (%) | 12 (26.7) | 5 (14.7) | 0.200 |
| Corticosteroid therapy, n (%) | 32 (71.1) | 13 (38.2) | 0.003 |
| Liver transplant, n (%) | ‐ | 1 (2.9) | 0.247 |
| Time from liver injury to normalization (days) | 54 (15–185) | 40 (15–120) | 0.137 |
| Follow‐up duration after normalization (days) | 120 (35–182) | 80 (45–192) | 0.036 |
Note: Eight patients (six without complete laboratory assessment and two with known AIH diagnosis) were not included in immune‐mediated phenotype analysis. Of these eight patients, one received corticosteroid therapy.
Concomitant medications
Twenty‐three (26%) patients were taking medication for concomitant diseases prior to the onset of liver injury. Thirteen had hypertension and/or diabetes mellitus. Therapy was not discontinued in any of these. Six patients were on statins since more than 2 years. Therapy was discontinued in three of these. No patient had relapse of liver injury following readministration of the same drug. Mesalazine was not withdrawn in three patients with inflammatory bowel diseases. One patient had active breast cancer and was under systemic chemotherapy (carboplatin, docetaxel and trastuzumab) when liver injury was observed. The chemotherapy protocol was not suspended and liver biochemistry spontaneously improved. One patient with AIH and other patient with pemphigus vulgaris were receiving azathioprine therapy, which was maintained during and after the period of liver injury. One patient was on weekly pegylated interferon alfa‐2a for polycythemia for two years. This therapy was not discontinued at the onset of liver injury. Three patients used both ibuprofen (400–1200 mg/day) and acetaminophen (1–3 g/day) for 3–4 days prior to the diagnosis of liver injury. No patient was taking an anti‐TNF‐α agent prior to the onset of liver injury.
Patients with pre‐existing liver diseases
Twelve (14%) patients who developed liver injury attributed to SARS‐CoV‐2 vaccination had pre‐existing liver disorders. Seven patients had NAFLD based on liver ultrasound and mildly elevated liver tests. Their ALT levels were 0.8–1.6× UNL in the last follow‐up before vaccination and peak ALT reached 5.2–28.5× UNL following vaccination. Two patients with AIH were in complete biochemical remission for more than one year before developing liver injury after vaccination (peak ALT > 10× UNL). One had spontaneous resolution and in the other biochemical response was obtained by increasing the prednisolone dose. A patient with primary biliary cholangitis (PBC) whose ALT levels were normal at last follow‐up before vaccination, presented with liver injury (ALT > 5× UNL and IgG 1.41× UNL) 20 days after vaccination. Laboratory values returned to normal levels without specific therapy. One patient with cured for previous HCV and another patient who was many years earlier transplanted for primary sclerosing cholangitis developed liver injury following vaccination. Both patients had spontaneous resolution of laboratory values during follow‐up.
For any reasons, 54 (62%) patients had checked aminotransferase levels on a median of 120 (20–340) days prior to the onset of liver injury. Aminotransferase levels were normal in 48 patients and were >1–3× UNL in six of whom four had pre‐existing NAFLD. On the other hand, only four of patients showed clinical or histological findings of advanced fibrosis.
Case characteristics according to vaccine type
The general characteristics, laboratory values and outcomes of the patients according to SARS‐CoV‐2 vaccine type are presented in the Table 3. An immune‐mediated phenotype was observed in similar proportions of patients who developed liver injury following vaccination with the Pfizer‐BioNTech, Oxford‐AstraZeneca and Moderna vaccines (59.6%, 55.6%, and 50%, p = 0.810). The rates of patients treated with corticosteroids were similar (47.1%, 60%, and 62.5%, p = 0.429). Overall, treatment response and outcomes were also similar among the three vaccine groups.
TABLE 3.
General characteristics of the study population according to type of SARS‐CoV‐2 vaccine
| Pfizer‐Biontech (n = 51) | Oxford‐AstraZeneca (n = 20) | Moderna (n = 16) | p value | |
|---|---|---|---|---|
| Age, median years (range) | 46 (18–71) | 47 (20–76) | 57 (21–79) | 0.157 |
| Sex, female, n (%) | 32 (62.7) | 10 (50) | 13 (81.3) | 0.154 |
| Pre‐existing liver disease, n (%) | 7 (13.7) | 2 (10) | 3 (18.8) | 0.751 |
| Peak ALT × ULN | 16.7 (3.1–203) | 11.7 (3.2–63.8) | 21.7 (5–66.4) | 0.519 |
| Peak AST × ULN | 14.9 (1.8–250) | 14 (2.6–169) | 21.4 (3.4–55) | 0.608 |
| Peak ALP × ULN | 1.3 (0.6–6.5) | 1.2 (0.7–2.2) | 1.3 (0.4–5.6) | 0.970 |
| Peak total bilirubin × ULN | 2.6 (0.5–19.5) | 5.4 (0.5–22.1) | 1.1 (0.3–23.2) | 0.210 |
| INR | 1.1 (0.7–3.6) | 1.3 (0.6–3.8) | 1 (0.6–2.7) | 0.289 |
| Pattern of injury, n (%) | ||||
| Hepatocellular | 43 (84.3) | 17 (85) | 13 (81.3) | 0.948 |
| Mixed | 5 (9.8) | 2 (10) | 2 (12.5) | 0.952 |
| Cholestatic | 3 (5.9) | 1 (5) | 1 (6.3) | 0.985 |
| ANA positivity, n (%) | 31 (63.3) | 15 (75) | 10 (71.4) | 0.603 |
| SMA positivity, n (%) | 8 (16.3) | 5 (25) | 2 (14.2) | 0.643 |
| IgG × UNL | 1.12 (0.41–2.61) | 1.05 (0.5–2.71) | 1.08 (0.60–1.84) | 0.833 |
| Immune‐mediated phenotype, n (%) | 28 (59.6) | 10 (55.6) | 7 (50) | 0.810 |
| Grade 3‐4 liver injury, n (%) | 9 (17.6) | 6 (30) | 3 (18.8) | 0.501 |
| Liver transplant, n (%) | 1 (2) | ‐ | ‐ | 0.700 |
| Corticosteroid therapy, n (%) | 24 (47.1) | 12 (60) | 10 (62.5) | 0.429 |
Treatment and outcome
Corticosteroids (20–100 mg/day, prednisolone equivalent dose) were given to 46 patients of whom 11 also received a second immunosuppressive drug, including azathioprine (50–100 mg/day) in nine and mycophenolate mofetil (1000 mg/day) in two. Plasma exchange (3–6 cycles) was performed in nine patients and three of these also received corticosteroid therapy. One patient received intravenous immunoglobulins in addition to corticosteroids. Corticosteroids were more often given to patients with grade 3–4 liver injury than to those with grade 1–2 liver injury (88.9% vs. 43.5%, p = 0.001). The median time from the onset of liver injury to normalization of aminotransferases was 46 (15–185) days, not significantly different between corticosteroid‐treated and nontreated patients (60 vs. 39, p = 0.085).
One patient developed mild liver injury after a first dose of Pfizer‐BioNTech vaccine but presented with severe liver injury following a second dose of the same vaccine. Treatment with prednisolone (40 mg/day, intravenously) and plasma exchange did not improve the liver function. The patient progressed into liver failure with hepatic encephalopathy and underwent living donor liver transplantation. Review of the liver biopsy showed features of immune‐mediated liver injury (Figure S1).
Two patients who developed liver injury following Oxford‐AstraZeneca vaccination were switched to Pfizer‐BioNTech for the second vaccine dose. Neither of these two had a liver injury after the second vaccine dose. Immunosuppression was withdrawn in 26% (12/46) of the patients during the study period and none of these relapsed after 44–140 days of follow‐up. Similarly, 41 cases who showed spontaneous resolution of liver injury did not develop relapse during a median 69 days of (35–172) follow‐up.
DISCUSSION
The current study describes the clinical presentation, laboratory features and prognosis of 87 patients who developed liver injury associated with SARS‐CoV‐2 vaccination. Overall outcomes with or without steroid therapy were good except for one patient who progressed into fulminant liver failure and required liver transplantation.
The liver injury was predominantly hepatocellular and showed features of immune‐mediated hepatitis. Among 44 patients with complete laboratory and histological work‐up, 34 (77%) had probable or definite AIH according to the simplified AIH criteria. Previously reported cases of post‐SARS‐CoV‐2 vaccine‐induced liver injury also mostly had features of AIH.[ 8 , 9 , 10 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ] The exact mechanism of SARS‐CoV‐2 vaccine‐induced liver injury is not known. The two vaccine formulations (mRNA and viral vector) encode the SARS‐CoV‐2 spike (S) protein. The entry of S protein in the human body elicits a strong stimulus to innate immunity, which results in cellular activation leading to proinflammatory cytokine and chemokine production.[ 5 ] Due to molecular similarity between S protein and liver specific proteins, an activated immune system may lead to destruction of liver proteins. A recent study showed that anti‐SARS‐CoV‐2 S protein antibodies reacted to human tissue antigens, which resulted in significant elevations of immune‐mediated markers, such as ANA, anti‐actin and AMA.[ 22 ]
Previous reports suggest corticosteroid treatment in patients with immune‐mediated liver injury.[ 23 ] A high proportion of individuals with immune‐mediated hepatitis received corticosteroid therapy in the present study. Corticosteroids were well tolerated by 46 treated patients and no patient experienced severe side effects. Corticosteroid therapy was also more often used in patients with laboratory features of severe hepatitis. This may explain why the time from peak aminotransferase to normalization was longer in corticosteroid‐treated patients.
One patient progressed into liver failure. To our knowledge, this is the first reported case to be transplanted due to liver failure attributed to SARS‐CoV‐2 vaccination.[ 24 ] Another 42‐year‐old woman had laboratory findings of severe hepatitis and developed low grade hepatic encephalopathy. This patient was listed for liver transplantation but fortunately improved after plasma exchange and corticosteroid therapy. A few cases of SARS‐CoV‐2 vaccine‐induced liver injury have been reported and all had good response to therapy. These reports provide limited information about severity of liver injury, but some had features of severe liver injury.[ 9 , 10 ]
Distinguishing immune‐mediated liver injury following vaccination from new‐onset or flare of a pre‐existing AIH is challenging.[ 25 , 26 ] In our study population, 48 patients had normal aminotransferase levels in the year prior to vaccination. Also, 41 patients who showed spontaneous resolution of liver injury did not develop relapse during follow‐up. Although immune‐mediated liver injury can show histological features resembling AIH, the presence of advanced fibrosis is highly suggestive of AIH.[ 27 ] Among the 44 biopsied patients, liver histology showed advanced fibrosis in only three (7%), and one had laboratory, imaging, and endoscopic features of cirrhosis. Another patient was seropositive for both anti‐SLA and anti‐LC‐1, which are both highly suggestive of AIH and rarely detected in other liver disorders.[ 28 ] The distinction between immune‐mediated liver injury and AIH is also facilitated by steroid response and outcome. In previous studies, patients with drug induced‐AIH were successfully withdrawn from corticosteroid therapy, whereas relapse is common in AIH.[ 26 , 29 ] Corticosteroid therapy was discontinued in 12 patients and none of these had relapse during follow‐up. These results suggest that most of our patients had new‐onset immune‐mediated liver injury rather than flare or unmasking of an underlying classical AIH.
Determining causality can be challenging also in the context of pre‐existing conditions and concomitant medication. Two AIH patients who had normal aminotransferases at last follow‐up developed liver injury following vaccination. Both were strictly adherent to immunosuppressive therapy. A spontaneous flare of AIH was therefore unlikely. Baseline aminotransferases were normal also in a woman with PBC who presented with features of hepatitis after vaccination. Importantly, all three patients developed symptoms within a few days after vaccination, which also suggests causality.
Statin therapy which in rare instances can lead to liver injury,[ 30 ] was discontinued in three of six patients. Reintroduction of the same drug did not result in relapse of liver injury. Interferon therapy is another known cause of immune‐mediated liver injury.[ 31 ] One patient was under pegylated interferon therapy which was not discontinued. Some patients used acetaminophen and/or ibuprofen for postvaccine symptoms. Acetaminophen doses were used in therapeutic doses in these patients and those who used ibuprofen also had an immune‐mediated hepatitis, which is not typical in ibuprofen‐associated induced liver injury.[ 32 ] These medications were therefore very unlikely to be causative.
A recent report described rapid onset liver injury after a first Moderna mRNA vaccine dose in a patient who developed severe AIH after a second dose.[ 33 ] Three of the patients in the current study experienced mild liver injury after the first vaccination but more severe liver injury following the second dose of the same vaccine (one underwent transplantation). Two cases did not experience liver injury when another vaccine type was chosen for the second dose. This data suggests that re‐exposure to the same vaccine type should be avoided after SARS‐CoV‐2 vaccine‐induced liver injury. A recent study demonstrated that heterogeneous vaccination (Oxford‐AstraZeneca followed by Moderna or Pfizer‐BioNTech) had significantly greater efficacy than twice homologous Oxford‐AstraZeneca vaccination.[ 34 ]
Data in the present study was derived from experienced hepatologists in 18 countries across Europe and the Americas. The strength of the study is the large number of cases with liver injury following SARS‐CoV‐2 vaccination. The retrospective nature of our study is one of the limitations. Due to the design of the study, the frequency of SARS‐CoV‐2 vaccine‐induced liver injury could not be estimated. It is also difficult to predict the long‐term impact of liver injury in these patients. Thirty‐four (39%) of patients were still on immunosuppression at the last follow‐up, which is rather short. A more recent study showed that corticosteroid‐treated patients with infliximab induced liver injury did not have a relapse after stopping immunosuppression.[ 35 ] It thus seems prudent to try to discontinue immunosuppression in patients with vaccine‐induced liver injury. If no relapse occurs, this would support that vaccination was indeed the etiology.
We do not discourage SARS‐CoV‐2 vaccination. The overall benefits of vaccination during the pandemic clearly outweigh the risks of liver injury and other rare side effects. The number of patients described here is minuscule compared to the population vaccinated worldwide. The current study rather aimed to increase awareness about this rare side effect, to promote early recognition and to provide guidance for adequate management. New‐onset or flares of immune‐mediated conditions are known phenomena after different vaccinations,[ 36 ] such as AIH following hepatitis A vaccination.[ 37 ] That SARS‐CoV‐2 vaccination can trigger immune‐mediated hepatitis is therefore not unexpected. We did not compare safety profiles and our data do not suggest a difference between vaccine types. That about half of our cases with liver injury were attributed to the Pfizer‐BioNTech vaccine rather reflects differences in vaccination strategies and vaccine availability between countries.
In conclusion, this large international case series provides evidence for the hepatotoxicity potential of SARS‐CoV‐2 vaccines (Pfizer‐BioNTech, Moderna and Oxford‐AstraZeneca). The clinical phenotype is mostly hepatocellular and can show features immune‐mediated hepatitis. Spontaneous resolution was common, steroid response was good and prognosis favorable. One patient however progressed into liver failure and underwent liver transplantation.
AUTHOR CONTRIBUTIONS
Cumali Efe, Thomas D. Schiano, Staffan Wahlin, and Ezequiel Ridruejo conceptualized the study. Cumali Efe, Anand V. Kulkarni, Bendetta Terziroli Beretta‐Piccoli, Tugrul Purnak, Bianca Magro, Mustafa Cengiz, Staffan Wahlin, and Ezequiel Ridruejo collected and analyzed data. Mustafa Cengiz performed statistical analysis. Cumali Efe, Anand V. Kulkarni, Bendetta Terziroli Beretta‐Piccoli, Bianca Magro, Albert Friedrich Stättermayer, Mustafa Cengiz, Daniel Clayton‐Chubb, Craig Lammert, Christine Bernsmeier, Özlem Gül, Fatima Higuera‐de la Tijera, Margarita Anders, Ellina Lytvyak, Mete Akın, Rodrigo Liberal, Mirta Peralta, Berat Ebik, Serkan Duman, Nurhan Demir, Yasemin Balaban, Álvaro Urzua, Fernando Contreras, Maria Grazia Venturelli, Yılmaz Bilgiç, Adriana Medina, Marcos Girala, Fulya Günşar, Maria‐Carlota Londoño, Theodoros Androutsakos, Ayelen Kisch, Alper Yurci, Fatih Güzelbulut, Yasir Furkan Çağın, Enver Avcı, Murat Akyıldız, Emine Kübra Dindar‐Demiray, Murat Harputluoğlu, Rahul Kumar, Sanjaya K. Satapathy, Manuel Mendizibal, Marcelo Silva, Stefano Fagiuoli, Stuart K. Roberts, Neşe Karadağ Soylu, Ramazan Idilman, Eric M. Yoshida, Aldo J. Montano‐Loza, George N. Dalekos, Ezequiel Ridruejo, Thomas D. Schiano, and Staffan Wahlin contributed data and approved the final manuscript. Cumali Efe, Thomas D. Schiano, and Staffan Wahlin interpreted data and prepared the manuscript for the final submission.
CONFLICTS OF INTEREST
Eric M. Yoshida received grants and honoraria from Intercept. He received grants from Gilead, Merck, AbbVie, Intercept, Genfit, Madrigal, Allergan, Celgene, Pfizer, Paladin Laboratories, and Novodisk. He received honoraria from Lupin. Stefano Fagiuoli advises and is on the speaker's bureau for AbbVie, Gilead, Novartis, and Kedrion. He is on the speakers' bureau for Intercept.
ETHICS STATEMENT
We confirm that the requirement for informed consent was waived by the institutional review committee. No donor organs were obtained from executed prisoners or other institutionalized persons.
Supporting information
Appendix S1 xxx
ACKNOWLEDGMENTS
Turkish Association for the Study of Liver (TASL) organized and supported data collection of Turkish patients.
Efe C, Kulkarni AV, Terziroli Beretta‐Piccoli B, Magro B, Stättermayer A, Cengiz M, et al. Liver injury after SARS‐CoV‐2 vaccination: features of immune‐mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022;00:1–11. 10.1002/hep.32572
Staffan Wahlin and Thomas D. Schiano share senior authorship.
[Correction added August 22, 2022 after first online publication: author Albert Friedrich Stättermayer's name was updated to "Albert Stättermayer"
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42. [DOI] [PubMed] [Google Scholar]
- 3. Efe C, Dhanasekaran R, Lammert C, Ebik B, Higuera‐de la Tijera F, Aloman C, et al. Outcome of COVID‐19 in patients with autoimmune hepatitis: an international multicenter study. Hepatology. 2021;73:2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khandker SS, Godman B, Jawad MI, Meghla BA, Tisha TA, Khondoker MU, et al. A systematic review on COVID‐19 vaccine strategies, their effectiveness, and issues. Vaccines (Basel). 2021;9:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teijaro JR, Farber DL. COVID‐19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID‐19 vaccines: a systematic review and meta‐analysis. Front Immunol. 2021;12:714170. 10.3389/fimmu.2021.714170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New‐onset autoimmune phenomena post COVID‐19 vaccination. Immunology. 2021;165:386–401. 10.1111/imm.13443 [DOI] [PubMed] [Google Scholar]
- 8. Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID‐19) vaccine: causality or casualty? J Hepatol. 2021;75:222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS‐CoV‐2 vaccination: a multicenter case series. J Hepatol. 2022;76:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Letter to the editor: exacerbation of autoimmune hepatitis after COVID‐19 vaccination. Hepatology. 2022;75:757–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayashi PH, Lucena MI, Fontana RJ, Bjornsson ES, Aithal GP, Barnhart H, et al. A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology. 2022. 10.1002/hep.32327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug‐induced liver injury. Clin Pharmacol Ther. 2011;89:806–15. [DOI] [PubMed] [Google Scholar]
- 13. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. [DOI] [PubMed] [Google Scholar]
- 14. Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76. [DOI] [PubMed] [Google Scholar]
- 15. Garrido I, Lopes S, Simões MS, Liberal R, Lopes J, Carneiro F, et al. Autoimmune hepatitis after COVID‐19 vaccine ‐ more than a coincidence. J Autoimmun. 2021;125:102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avci E, Abasiyanik F. Autoimmune hepatitis after SARS‐CoV‐2 vaccine: new‐onset or flare‐up? J Autoimmun. 2021;125:102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Londoño MC, Gratacós‐Ginès J, Sáez‐Peñataro J. Another case of autoimmune hepatitis after SARS‐CoV‐2 vaccination – still casualty? J Hepatol. 2021;75:1248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clayton‐Chubb D, Schneider D, Freeman E, Kemp W, Roberts SK. Autoimmune hepatitis developing after the ChAdOx1 nCoV‐19 (Oxford‐AstraZeneca) vaccine. J Hepatol. 2021;75:1249–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan CK, Wong YJ, Wang LM, Ang TL, Kumar R. Autoimmune hepatitis following COVID‐19 vaccination: true causality or mere association? J Hepatol. 2021;75:1250–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palla P, Vergadis C, Sakellariou S, Androutsakos T. Letter to the editor: autoimmune hepatitis after COVID‐19 vaccination: a rare adverse effect? Hepatology. 2022;75:489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghielmetti M, Schaufelberger HD, Mieli‐Vergani G, Cerny A, Dayer E, Vergani D, et al. Acute autoimmune‐like hepatitis with atypical anti‐mitochondrial antibody after mRNA COVID‐19 vaccination: a novel clinical entity? J Autoimmun. 2021;123:102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiler‐Normann C, Schramm C. Drug induced liver injury and its relationship to autoimmune hepatitis. J Hepatol. 2011;55:747–9. [DOI] [PubMed] [Google Scholar]
- 24. Efe C, Harputluoğlu M, Soylu NK, Yilmaz S. Letter to the editor: Liver transplantation following severe acute respiratory syndrome‐coronavirus‐2 vaccination‐induced liver failure. Hepatology. 2022;75:1669–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki Y, Kakisaka K, Takikawa Y. Letter to the editor: autoimmune hepatitis after COVID‐19 vaccination: need for population‐based epidemiological study. Hepatology. 2022;75:759–60. [DOI] [PubMed] [Google Scholar]
- 26. Björnsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Drug‐induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. 2017;15:1635–6. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug‐induced liver injury. Hepatology. 2011;54:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Efe C, Ozaslan E, Wahlin S, Purnak T, Muratori L, Quarneti C, et al. Antibodies to soluble liver antigen in patients with various liver diseases: a multicentre study. Liver Int. 2013;33:190–6. [DOI] [PubMed] [Google Scholar]
- 29. Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug‐induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–8. [DOI] [PubMed] [Google Scholar]
- 30. Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak‐Ublick GA, Watkins PB, et al. Drug‐induced liver injury. Nat Rev Dis Primers. 2019;5:58. [DOI] [PubMed] [Google Scholar]
- 31. Efe C, Heurgué‐Berlot A, Ozaslan E, Purnak T, Thiéfin G, Simsek H, et al. Late autoimmune hepatitis after hepatitis C therapy. Eur J Gastroenterol Hepatol. 2013;25:1308–11. [DOI] [PubMed] [Google Scholar]
- 32. Zoubek ME, Lucena MI, Andrade RJ, Stephens C. Systematic review: ibuprofen‐induced liver injury. Aliment Pharmacol Ther. 2020;51:603–11. [DOI] [PubMed] [Google Scholar]
- 33. Zin Tun GS, Gleeson D, Al‐Joudeh A, Dube A. Immune‐mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV‐19 and mRNA prime‐boost vaccination against symptomatic Covid‐19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Björnsson HK, Gudbjornsson B, Björnsson ES. Infliximab‐induced liver injury: clinical phenotypes, autoimmunity and the role of corticosteroid treatment. J Hepatol. 2022;76:86–92. [DOI] [PubMed] [Google Scholar]
- 36. Watad A, Bragazzi NL, McGonagle D, Adawi M, Bridgewood C, Damiani G, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: insights from an analysis of 500 cases. Clin Immunol. 2019;203:1–8. [DOI] [PubMed] [Google Scholar]
- 37. Van Gemeren MAJ, van Wijngaarden P, Doukas M, de Man RA. Vaccine‐related autoimmune hepatitis: the same disease as idiopathic autoimmune hepatitis? Two clinical reports and review. Scand J Gastroenterol. 2017;52:18–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 xxx
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


