Dear Editor,
As the number of infections with the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) rise beyond 430 million documented cases, 1 the postinfection sequelae and long term symptoms of coronavirus disease 2019 (COVID‐19) among the survivors has become a growing concern. 2 The postacute sequelae of COVID‐19 also called long COVID is a number of conditions and symptoms involving several organ systems including the immune system, hematological system, pulmonary and cardiovascular system, gastrointestinal system, and the nervous system, as well as general and constitutional symptoms such as fatigue, fever, and muscle weakness. While a number of studies have suggested the role of the autoimmune factors and persistence of viral fragments in the development of long‐term COVID and failure to return to baseline health, the role of other latent host viruses has not been ruled out. Incidents of coinfection with COVID‐19 and Epstein–Barr virus (EBV) in patients with aforementioned complications propose the hypothesis of EBV being one of the possible causative agents.
The EBV is a double‐stranded DNA virus of the herpes family best known as the agent responsible for infectious mononucleosis. Referred to as one of the most common viruses in humans, it has a ubiquitous distribution of 90% among the world population. As an oncogenic virus, it is associated with a variety of lymphoproliferative disorders such as Burkitt's lymphoma, Hodgkin's lymphoma, T and natural killer (NK) cell lymphomas, and nasopharyngeal carcinoma. It is characterized by lifelong latency in B cells and intermittent recrudescence of lytic infection caused by stressors. 3 , 4 The virus has the ability to switch from latent to lytic phase. The conversion process can be triggered by a variety of stimuli, including psychological stress. When the virus is reactivated, the patient may experience symptoms such as brain fog, fatigue, arthralgia, and skin rashes, among others. Recent studies suggest the possible interaction between SARS‐CoV‐2 and EBV. There have been a few hypothesizes regarding the mechanism of this interaction. One possible mechanism involves a decrease in CD8+ cells which are the primary cells responsible for immunity against EBV infection. 5 Chen et al. 6 found CD8+ count to be significantly lower in patients with SARS‐CoV‐2/EBV coinfection and proposed the idea of reactivation due to the decrease in CD8+ cells. A correlation between reduced CD8+ T cells and NK counts, EBV DNA levels, and COVID‐19 severity was observed in Paolucci et al.'s 7 study. Based on our systematic search of the MEDLINE database, we identified 11 studies reporting incidence of EBV reactivation during SARS‐COV‐2 pathogenicity. A meta‐analysis was conducted to assess EBV reactivation incidence based on the available evidence. The analysis was carried out using R (version 4.1.3; R Core Team, 2020) and the metafor package (version 3.0.2). 8 Our results included 993 COVID‐19 patients, most of whom were in a severe or critical state. The pooled results demonstrate that the incidence of EBV reactivation is about 0.48 (95% confidence interval [CI]: 0.30–0.67; I 2 = 97.02%). Our subgroup analysis based on patients' disease condition revealed an incidence of 0.66 (95% CI: 0.51–0.80; I 2 = 90.97%) among critically ill patients and an incidence of 0.20 (95% CI: 0.13–0.28; I 2 = 77.76%) among outpatients or patients without additional information with regard to disease severity (Figure 1). Further research is needed to determine the precise role of COVID‐19 in the reactivation of latent EBV and the consequences of this reactivation.
Figure 1.
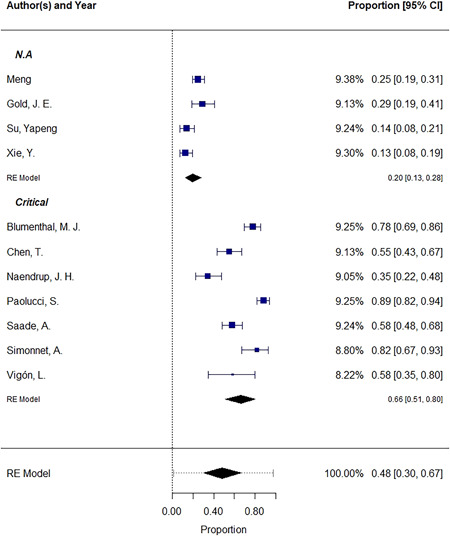
The pooled incidence of EBV reactivation. All measures are proportions of EBV reactivated cases among COVID‐19 patients. CI, confidence interval; COVID‐19, coronavirus disease 2019; EBV, Epstein–Barr virus; RE model, random‐effects model.
Recently, a longitudinal study on 309 COVID‐19 patients showed that in addition to SARS‐CoV‐2 viremia, EBV viremia within 1–2 weeks of onset of COVID‐19 is also associated with memory‐related long COVID symptoms. EBV viremia in the same study has been solely attributed to fatigue and sputum as long COVID symptoms. 9 The mechanisms through which EBV may influence the occurrence of long COVID could be attributed to the synergistic subversion and disruption of cellular and mitochondrial pathways. EBV encoded BZLF1 protein induces the degradation of p53 that normally governs DNA damage repair and apoptosis. Similarly, SARS‐CoV‐2 was also shown to degrade p53 in an Mdm2‐independent manner through NSP3 expression and RCHY1 activation. 10 , 11 Furthermore, altered metabolic profile and mitochondrial biogenesis in both viruses 10 can result in a sustained inflammatory response in SARS‐CoV‐2 and may mount EBV‐induced fatigue in an already compromised individual. 12 These would allow resurfacing of an otherwise latent EBV infection that may reside asymptomatically in its host.
The potential role of EBV and its reactivation in the context of SARS‐CoV‐2 infection severity and long COVID is evident in the literature. Gold et al. 3 published one of the first investigations on this topic. The authors suggested that the reactivation of the virus is observed soon after or within the initial phase of COVID‐19 as demonstrated by positive titers of acute EBV infection antibodies in the screened individuals. The findings of this study reveal that 30% of COVID‐19 patients showing symptoms of long‐term COVID and SARS‐CoV‐2 may trigger other viruses that contribute to these symptoms. They reported the most frequent symptoms to be fatigue, insomnia, headaches, myalgia, and confusion, and found a direct relationship between the number of reported long COVID symptoms and early antigen‐diffuse immunoglobulin G antibody titers. 3 Drugs used in the treatment of COVID‐19 may also play a major role in altering immune responses by means of regulating intracellular signaling pathways, thus prompting the reactivation process of EBV. The use of high‐dose corticosteroids has been stated as a risk factor for herpes virus reactivation. 13 Furthermore, lytic reactivation of EBV and Kaposi's sarcoma‐associated herpesvirus (human herpes virus‐8) and upregulation of viral lytic genes was seen in latently infected cells upon treatment with remdesivir in a dose‐dependent manner. 14
The results of the studies on the role of EBV and SARS‐CoV‐2 on disease severity has been conflicting. For instance, a previous study did not show increased disease severity in individuals with coinfection of EBV and SARS‐CoV‐2 and the results demonstrated no differences in terms of viral load and disease outcome. 15 On the other hand, EBV coinfection was associated with increased incidence of fever, higher aspartate aminotransferase and C‐reactive protein values, and corticosteroid use compared to SARS‐CoV‐2 infection alone in another study by Chen et al. 6 EBV reactivation was also associated with a longer duration of Intensive care unit stay. 16 However, stringent care is advised in evaluating the evidence of the causal association of EBV with COVID‐19 severity or long COVID symptoms, considering the potential confounding effects of the high prevalence of EBV in the general population and the reactivation of other latent viruses in patients, such as herpes simplex virus, cytomegalovirus, Varicella zoster virus (VZV), human herpes virus‐6 (HHV‐6), and human herpes virus‐7 (HHV‐7). 16 , 17 Indeed, there have been reports regarding higher incidence of diseases associated with VZV and HHV‐6 including Herpes zoster, Kawasaki disease, and pityriasis rosea during the pandemic period and/or along with coinciding SARS‐CoV‐2 infection. 18 All of these supports that SARS‐COV‐2 infection may be a possible inducer of latent virus reactivation. 17
Despite several studies have been published regarding EBV and COVID‐19, the findings are not strong enough to establish a definite conclusion. Efforts need to be maintained to elucidate the impact of COVID‐19 in the reactivation of latent EBV and the possible development of long COVID. In this context, prioritizing of COVID‐19 vaccination must be addressed, and a history of COVID‐19, even after complete recovery, should be recognized as a possible risk factor for EBV‐associated post‐COVID complications in the future management and monitoring of the patients.
AUTHOR CONTRIBUTIONS
Sepehr Aghajanian, Mohammad M. T. Athar, Omid K. Gargari: Writing – original draft. Arman Shafiee: Conceptualization, investigation, project administration, Supervision, writing – review and editing.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han Q, Zheng B, Daines L, Sheikh A. Long‐term sequelae of COVID‐19: a systematic review and meta‐analysis of one‐year follow‐up studies on post‐COVID symptoms. Pathogens. 2022;11(2):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to Epstein–Barr virus reactivation. Pathogens. 2021;10(6):763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shafiee A, Shamsi S, Kohandel Gargari O, et al. EBV associated T‐and NK‐cell lymphoproliferative diseases: A comprehensive overview of clinical manifestations and novel therapeutic insights. Rev Med Virol. 2022. :e2328. [DOI] [PubMed] [Google Scholar]
- 5. Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB. Immediate early and early lytic cycle proteins are frequent targets of the Epstein–Barr virus‐induced cytotoxic T cell response. J Exp Med. 1997;185(9):1605‐1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen T, Song J, Liu H, Zheng H, Chen C. Positive Epstein–Barr virus detection in coronavirus disease 2019 (COVID‐19) patients. Sci Rep. 2021;11(1):10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paolucci S, Cassaniti I, Novazzi F, et al. EBV DNA increase in COVID‐19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis. 2021;104:315‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viechtbauer W. Conducting meta‐analyses in R with the meta for package. Journal of Statistical Software, 2010;36(3):1‐48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 9. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post‐acute COVID‐19 sequelae. Cell. 2022;185(5):881‐895.e20. 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cardozo CM, Hainaut P. Viral strategies for circumventing p53: the case of severe acute respiratory syndrome coronavirus. Curr Opin Oncol. 2021;33(2):149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Viechtbauer W.. Conducting meta‐analyses in R with the meta for package. Journal of Statistical Software. 2010;36(3):1‐48. 10.1016/j.cell.2022.01.014 [DOI] [Google Scholar]
- 12. Nunn AVW, Guy GW, Botchway SW, Bell JD. SARS‐CoV‐2 and EBV; the cost of a second mitochondrial “whammy”? Immun Ageing. 2021;18(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naendrup JH, Garcia Borrega J, Eichenauer DA, Shimabukuro‐Vornhagen A, Kochanek M, Böll B. Reactivation of EBV and CMV in severe COVID‐19‐epiphenomena or trigger of hyperinflammation in need of treatment? A large case series of critically ill patients. J Intensiv Care Med. 2021; 1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen J, Dai L, Kendrick S, Post SR, Qin Z. The anti‐COVID‐19 drug remdesivir promotes oncogenic herpesviruses reactivation through regulation of intracellular signaling pathways. Antimicrob Agents Chemother. 2022;66:e0239521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blumenthal MJ, Lambarey H, Chetram A, Riou C, Wilkinson RJ, Schäfer G. Kaposi's sarcoma‐associated herpesvirus, but not Epstein–Barr virus, co‐infection associates with coronavirus disease 2019 severity and outcome in South African patients. Front Microbiol. 2021;12:795555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simonnet A, Engelmann I, Moreau AS, et al. High incidence of Epstein–Barr virus, cytomegalovirus, and human‐herpes virus‐6 reactivations in critically ill patients with COVID‐19. Infect Dis Now. 2021;51(3):296‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ciccarese G, Parodi A, Drago F. SARS‐CoV‐2 as possible inducer of viral reactivations. Dermatol Ther. 2020;31:942‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drago F, Ciccarese G, Rebora A, Parodi A. Human herpesvirus 6, 7 and Epstein–Barr virus reactivation in pityriasis rosea during COVID‐19. J Med Virol. 2021;93:1850‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


