ABSTRACT
Anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibodies have been found in breast milk following both natural SARS‐CoV‐2 infection and coronavirus disease 2019 (COVID‐19) vaccination. This was a prospective study to evaluate the temporal changes in amount and neutralization capacity of anti‐SARS‐CoV‐2 antibodies in breast milk stimulated by natural infection and by vaccination. Serial breast milk samples were collected from postnatal women who were recruited through convenience sampling. We found a rapid increase in neutralizing SARS‐CoV‐2‐specific antibodies in breast milk from both study groups. Amongst the infection group, the median immunoglobulin A (IgA) level was 16.99 (range, 0–86.56) ng/mL and median binding capacity was 33.65% (range, 0–67.65%), while in the vaccination group these were 30.80 (range, 0–77.40) ng/mL and 23.80% (range, 0–42.80%), respectively. In both groups, both binding capacity and IgA levels decreased progressively over time after peaking. Neutralizing activity had become undetectable by about 150 days after the first dose of the vaccine, but a vaccine booster dose restored secretion of neutralizing IgA, albeit with different levels of response in different individuals. This highlights the importance of the vaccine booster dose in sustaining neutralizing antibody levels in breast milk, which may potentially provide protection for very young children, who cannot receive the COVID‐19 vaccine. © 2022 International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: antibody, breast milk, COVID‐19, natural infection, SARS‐CoV‐2, vaccination
CASE SERIES
This study aimed to evaluate the temporal changes in amount and neutralizing activity of anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibodies in breast milk stimulated by natural infection and by vaccination. The study was prospective and included postnatal, lactating women who were recruited through convenience sampling and who had either laboratory‐confirmed SARS‐CoV‐2 infection during pregnancy or had had their first coronavirus disease 2019 (COVID‐19) vaccination. Participants provided informed consent (ethics approval ref. 2020.210; 2020.214). Breast milk samples were self‐collected into sterile containers. The concentration of immunoglobulin A (IgA) reactive to the receptor binding domain (RBD) of the SARS‐CoV‐2 spike protein in skimmed acellular milk was evaluated using quantitative enzyme‐linked immunosorbent assays (ELISA) (ImmunoDiagnostics Limited, Sha Tin, Hong Kong). Results were interpreted as negative when the optical density (OD) was < 0.2 and positive when OD was ≥ 0.2. Specific IgA concentrations (in ng/mL) were calculated for the positive samples. The SARS‐CoV‐2 S1RBD‐ACE2 binding assay is a competitive ELISA assay (ImmunoDiagnostics Limited) which imitates the virus‐binding process. The neutralizing activity of anti‐SARS‐CoV‐2 antibodies was determined as the percentage of inhibition of the binding of SARS‐CoV‐2 spike protein‐RBD (S1RBD) to angiotensin converting enzyme 2 (ACE2). This percentage of inhibition was defined as the binding capacity. Descriptive data are presented as median (range). Generalized estimating equations (GEE) were adjusted to estimate the change in binding capacity and the change in IgA level over time from infection or vaccination, and the relationship between binding capacity and IgA level. P‐values and slope estimate with 95% CI are reported. Analyses were performed using statistical software R version 4.0.5 1 and the geepack package was used for the GEE model 2 .
Amongst the infection group (n = 18; 75 observations), the median infection‐to‐sampling interval was 45 (range, 3–192) days. None of these women was vaccinated against SARS‐CoV‐2 before the study ended. Their median IgA level was 16.99 (range, 0–86.56) ng/mL and median binding capacity was 33.65% (range, 0–67.65%). Amongst the vaccination group (n = 8; 93 observations), the median first‐dose‐to‐sampling interval was 71 (range, 0–334) days. None of these women had been diagnosed previously with COVID‐19 or was diagnosed before the study ended. Their median IgA level was 30.80 (range, 0–77.40) ng/mL and median binding capacity was 23.80% (range, 0–42.80%). Overall, higher binding capacity was observed after infection than after vaccination (Figure 1). A statistically significant reduction in IgA levels and binding capacity with increased infection‐to‐sampling interval was observed in both groups (Figure 1). Four participants had a vaccine booster dose during the study period and their IgA levels and binding capacity were restored from zero to at least 21.55 ng/mL and 13.6%, respectively (Figure 2). There was a positive linear relationship between binding capacity and IgA level in both study groups (Figure 3).
Figure 1.
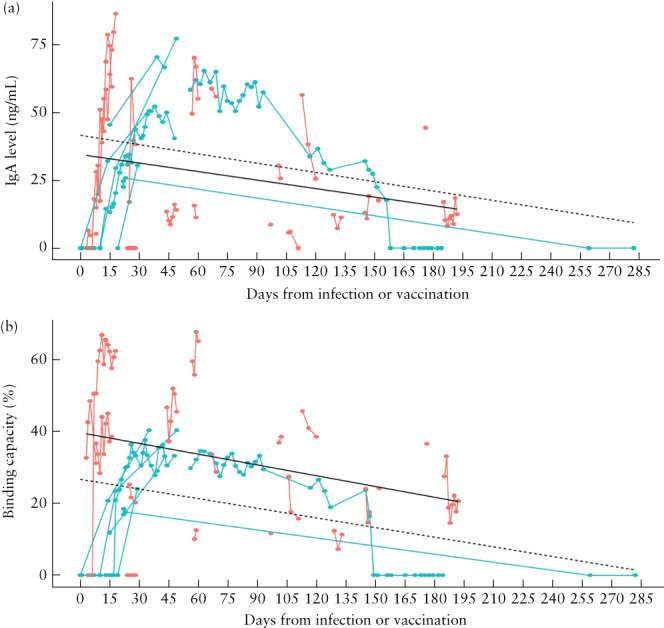
Immunoglobulin A (IgA) level in breast milk (a) and binding capacity (b) according to time from natural infection with severe acute respiratory syndrome coronavirus 2 ( ) or first vaccination for coronavirus disease 2019 (
) or first vaccination for coronavirus disease 2019 ( ). Circles are individual measurements and measurements of individuals are linked by lines. (a) Black lines represent the generalized estimating equations (GEE) model for the relationship between IgA level and days from infection (
). Circles are individual measurements and measurements of individuals are linked by lines. (a) Black lines represent the generalized estimating equations (GEE) model for the relationship between IgA level and days from infection ( ; slope, –0.11; 95% CI, –0.20 to –0.01; P = 0.03) or vaccination (
; slope, –0.11; 95% CI, –0.20 to –0.01; P = 0.03) or vaccination ( ; slope, –0.11; 95% CI, –0.26 to 0.03; P = 0.1). (b) Black lines represent the GEE model for the relationship between binding capacity and days from infection (
; slope, –0.11; 95% CI, –0.26 to 0.03; P = 0.1). (b) Black lines represent the GEE model for the relationship between binding capacity and days from infection ( ; slope, –0.10; 95% CI, –0.18 to –0.02; P = 0.009) or vaccination (
; slope, –0.10; 95% CI, –0.18 to –0.02; P = 0.009) or vaccination ( ; slope, –0.09; 95% CI, –0.16 to –0.01; P = 0.02).
; slope, –0.09; 95% CI, –0.16 to –0.01; P = 0.02).
Figure 2.
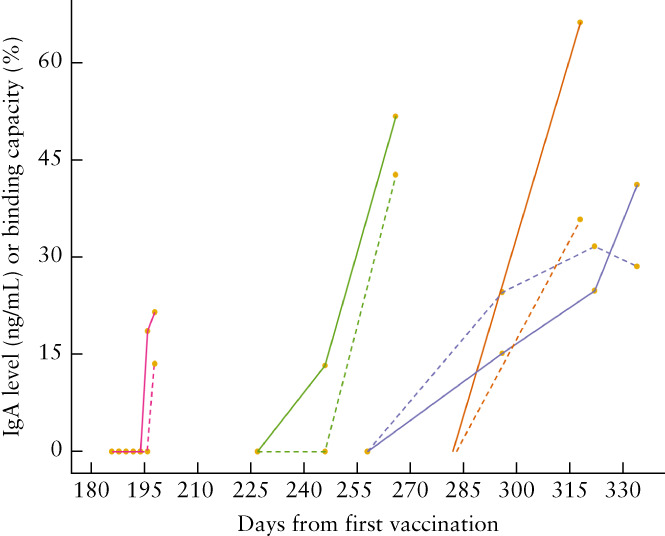
Immunoglobulin A (IgA) level in breast milk ( ) and binding capacity (
) and binding capacity ( ) according to time from first vaccination for coronavirus disease 2019, between days 180 and 330, in four patients who received a vaccine booster dose. The first measurement in each case was at the time of booster vaccination.
) according to time from first vaccination for coronavirus disease 2019, between days 180 and 330, in four patients who received a vaccine booster dose. The first measurement in each case was at the time of booster vaccination.
Figure 3.
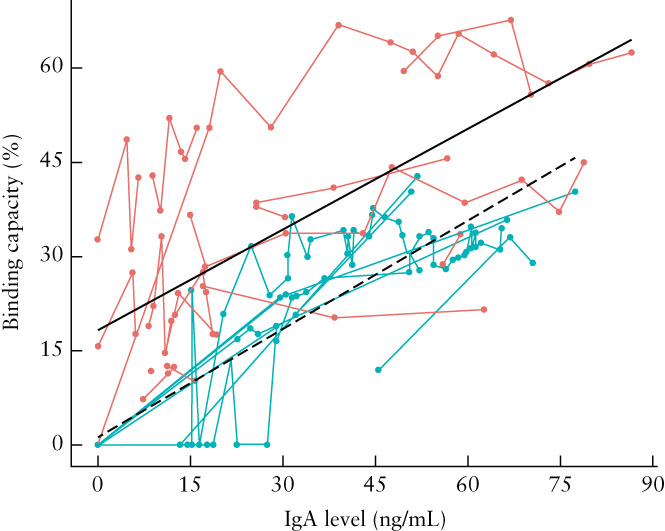
Relationship between binding capacity and immunoglobulin A (IgA) level in breast milk of women who had natural infection with severe acute respiratory syndrome coronavirus 2 ( ) or who had been vaccinated for coronavirus disease 2019 (
) or who had been vaccinated for coronavirus disease 2019 ( ). Circles are individual measurements and measurements of individuals are linked by lines. Black lines represent the generalized estimating equations model for their relationship in the infection group (
). Circles are individual measurements and measurements of individuals are linked by lines. Black lines represent the generalized estimating equations model for their relationship in the infection group ( ; slope, 0.58; 95% CI, 0.50–0.65; P < 0.001) and in the vaccination group (
; slope, 0.58; 95% CI, 0.50–0.65; P < 0.001) and in the vaccination group ( ; slope, 0.54; 95% CI, 0.28–0.80; P < 0.001).
; slope, 0.54; 95% CI, 0.28–0.80; P < 0.001).
DISCUSSION
SARS‐CoV‐2‐specific neutralizing antibodies have been found in breast milk from lactating women who had received the COVID‐19 vaccine 3 , 4 . In this study we demonstrated a rapid increase in anti‐SARS‐CoV‐2‐specific IgA levels in breast milk and in the binding capacity following both natural infection and COVID‐19 vaccination, and showed that both wane as time passes. The neutralizing activity had become undetectable by about 150 days after the first dose of the vaccine. These findings suggest that breast milk from lactating women who have contracted SARS‐CoV‐2 naturally or received the COVID‐19 vaccination may provide protection for very young children, who cannot receive the vaccine. However, the period of protection is not indefinite. We found that a vaccine booster dose is effective in retriggering the secretion of neutralizing IgA in breast milk after the level has become undetectable. This highlights the importance of the booster dose in order to sustain neutralizing antibody levels in breast milk. It is important to consider the observed variability of response of the neutralizing antibodies after a booster dose in different individuals (Figure 2). The binding capacity of antibodies in the breast milk from one participant remained undetectable 21 days after the booster dose, despite the IgA level being detectable, whereas another participant had both a detectable IgA level and neutralizing activity 13 days after the booster dose. More research is needed to evaluate specifically the effect of the booster dose on the antibodies in breast milk in order to determine the need for and timing of future COVID‐19 vaccine boosters.
ACKNOWLEDGMENT
For recruiting the pregnant SARS‐CoV‐2 patients during the study period, we wish to thank T. Ma and F. N. Yu, Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Hong Kong SAR, China; C. W. Kong, Department of Obstetrics and Gynaecology, United Christian Hospital, Hong Kong SAR, China; T. K. Lo, Department of Obstetrics and Gynaecology, Princess Margaret Hospital, Hong Kong SAR, China; P. L. So, Department of Obstetrics and Gynaecology, Tuen Mun Hospital, Hong Kong SAR, China; W. C. Leung, Department of Obstetrics and Gynaecology, Kwong Wah Hospital, Hong Kong SAR, China; W. Shu, Department of Obstetrics and Gynaecology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China; K. W. Cheung, Department of Obstetrics and Gynaecology, Queen Mary Hospital, Hong Kong SAR, China. None was compensated for his or her contributions. This work was supported by funding from Ferring Pharmaceuticals (Saint‐Prex, Switzerland). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2021. https://www.r‐project.org/.
- 2. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw 2006; 15: 1–11. [Google Scholar]
- 3. Low JM, Low YW, Zhong Y, Lee CYC, Chan M, Ng NBH, Amin Z, Ng YPM. Titres and neutralising capacity of SARS‐CoV‐2‐specific antibodies in human milk: a systematic review. Arch Dis Child Fetal Neonatal Ed 2022; 107: 174–180. [DOI] [PubMed] [Google Scholar]
- 4. Perl SH, Uzan‐Yulzari A, Klainer H, Asiskovich L, Youngster M, Rinott E, Youngster I. SARS‐CoV‐2–specific antibodies in breast milk after COVID‐19 vaccination of breastfeeding women. JAMA 2021; 325: 2013–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


