To the Editor:
Adolescent solid organ transplant recipients (SOTRs) have attenuated antibody responses to two-dose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination.1 , 2 Although adult SOTR studies suggest a third vaccine improves immunogenicity, their benefit in adolescents is unknown.3 , 4 We report the antibody response and safety of a third mRNA vaccine dose (D3) in adolescent SOTRs.
After approval by the Johns Hopkins Medicine Institutional Review Board, samples from SOTRs (12–18 years) in our multicenter, observational study who received D3 were analyzed for antibodies to SARS-CoV-2 spike protein receptor-binding domain (positive: ≥0.8, maximum: >2500 U/ml).1 , 5 Samples were collected at three time points: pre-D3 (1–9 months post-D2), 1 month post-D3, and 3 months post-D3. Fisher’s exact, Wilcoxon signed-rank, and McNemar’s tests were used as appropriate.
Forty-two participants received three BNT162b2 doses and one received three mRNA-1273 doses. Samples were available for 43 participants pre-D3, 43 1 month post-D3, and 31 3 months post-D3. Median (IQR) age was 15 (13–16) years; 44.2% were male, and 76.7% were White (Table S1). Participants were median (IQR) 10 (6–13) years from transplant, and heart transplant (41.9%) was most common. Four (9.3%) participants reported pre-D1 SARS-CoV-2 infections and four (9.3%) reported breakthrough infections (Table S2).
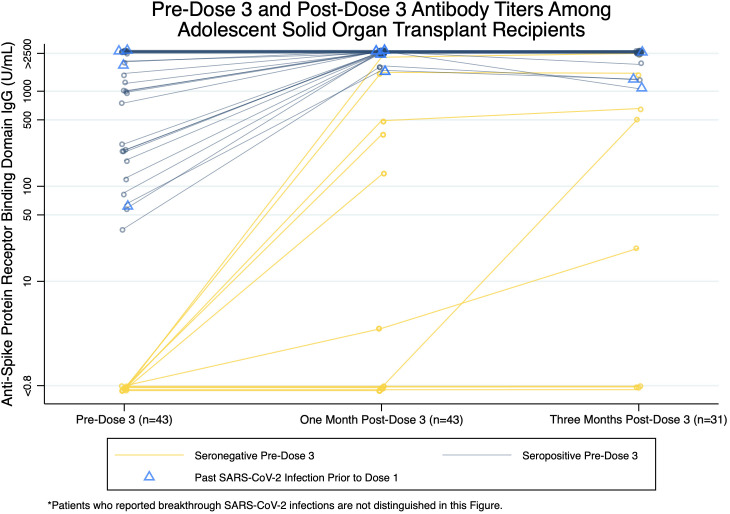
Antibody titers were positive in 32/43 (74.4%) participants pre-D3 and 38/43 (88.4%) 1 month post-D3 ( Figure 1). Of participants with positive pre-D3 titers, titers were lower pre-D3 compared to 1 month post-D3 (median [IQR]: 1769.5 [211.3–>2500], >2500 [2500–>2500] U/ml; p < .001), and the proportion with titers ≥1000 U/ml increased from 56.3% to 100% (p < .001). Of participants with negative pre-D3 titers, 6/11 (54.5%) seroconverted 1 month post-D3 (median [IQR]: 418.4 [132.3–1581] U/ml) and 5/11 (45.5%) remained seronegative (Table S3). Having received a transplant within 3 years was associated with negative 1 month post-D3 titer (p = .04) (Table S1).
FIGURE 1.
Pre-dose 3, 1 month post-dose 3, and 3 months post-dose 3 antibody titers among adolescent SOTRs. Samples were available for 43 participants pre-D3 (32 positive, 11 negative), 43 participants 1 month post-D3 (38 positive, 5 negative), and 31 participants 3 months post-D3 (28 positive, 3 negative). Dark blue circles and lines represent antibody titer trends of participants with positive pre-D3 titers (n = 32), and yellow circles and lines represent antibody titer trends of participants with negative pre-D3 titers (n = 11). Participants who reported past SARS-CoV-2 infection prior to receiving dose 1 (n = 4) are marked by light blue triangles. Jittered and darker shapes and lines represent multiple participants. Participants who reported breakthrough SARS-CoV-2 infections during the study period are not distinguished in this Figure. Participant samples were processed using the qualitative and semi-quantitative Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay that tests for total antibody against the receptor-binding domain of the SARS-CoV-2 spike protein. Per the manufacturer, a positive threshold of ≥0.8 U/mL was used. The minimum titer reported by the assay is <0.8 U/ml and the maximum titer is >2500 U/ml. 6/11 participants with negative pre-D3 titers seroconverted to have positive 1 month post-D3 titers, while 5/11 participants remained seronegative. 19/32 participants had positive pre-D3 titers lower than the assay’s maximum titer, of which 2/19 had their 1 month post-D3 titer increase by 1600–1800 U/ml and 17/19 had their 1 month post-D3 titer increase to the assay’s maximum. 13/32 participants with positive pre-D3 titers at the assay’s maximum titer continued to have 1 month post-D3 titers of >2500 U/ml. Among participants with 3 month post-D3 samples, 27/31 with positive 1 month post-D3 titers remained seropositive, 3/31 with negative 1 month post-D3 titers remained seronegative, and 1/31 with negative 1 month post-D3 titer and pre-D3 breakthrough SARS-CoV-2 infection seroconverted to have a positive 3 months post-D3 titer
From one to three months post-D3, 27/31 (87.1%) participants remained seropositive, 3/31 (9.7%) remained seronegative, and 1/31 (3.2%) with breakthrough infection seroconverted. There was no statistically significant difference between 1 and 3 months post-D3 titers (median [IQR]: >2500 [1631–>2500], >2500 [1300–>2500] U/ml; p = .79).
Thirty-seven (86.0%) and twenty-two (51.2%) participants completed surveys at 1 and 3 months post-D3, respectively. Main D3 side effects were local pain (73.0%) and fatigue (43.2%). No participants reported allergic reactions, myocarditis, or new neurological conditions. One heart recipient reported acute organ rejection unrelated to vaccination 3 months post-D3.
In this observational cohort, 88.4% of adolescent SOTRs had positive antibody responses 1 month post-D3, an increase from 63–73% post-D2.1 , 2 54.5% of participants with prior negative responses seroconverted and 100% with positive responses increased or remained at maximum titer. Additionally, titers remained stable 3 months post-D3. There were no vaccine-related adverse events and four breakthrough infections. With concerns over new variants and vaccine authorization, our results in this small convenience sample suggest the benefit of a third SARS-CoV-2 vaccine for antibody response in adolescent SOTRs. Future studies are needed to examine the association of spike antibodies with neutralizing antibodies and clinical protection as well as durability with longer follow-up.
ACKNOWLEDGMENTS
The authors thank the Johns Hopkins COVID-19 Transplant Vaccine Study pediatric participants and caregivers, without whom this research could not be possible. This research was also made possible with the generous support of the Ben-Dov family and Trokhan Patterson family. This work was supported by grant K24AI144954 (Dorry L. Segev) from the National Institute of Allergy and Infectious Diseases, grant K23DK115908 (Jacqueline M. Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases, grant K08HS026510-01A1 (Amy G. Feldman) from the Agency for Healthcare Research and Quality, and grant KL2TR003099 (Olga Charnaya) from the Johns Hopkins Institute for Clinical and Translational Research (ICTR).
DISCLOSURE
The authors of thismanuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Lara A. Danziger-Isakov MD MPH has the following disclosures: consulting and/or Data and Safety Monitoring Board member: Takeda, Merck; contracted clinical research agreements paid to her institution: Ansun Bio-Pharma, Astellas, Merck, Pfizer, Takeda, Viracor. Noelle H. Ebel MD has the following disclosures: consulting for Mirum. Amy G. Feldman MD has the following disclosures: consulting for Albireo. Evelyn K. Hsu MD has the following disclosures: contracted clinical research agreements paid to her institution: Gilead, Mirum, Albireo. Saeed Mohammad MD has the following disclosures: consulting for Albireo and Mirum. Emily R. Perito MD has the following disclosures: contracted clinical research agreements paid to her institution: Gilead, Albireo. Dorry L. Segev MD PhD has the following disclosures: consulting and/or speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific, Regeneron, and AstraZeneca. Douglas B. Mogul MD MPH PhD has the following disclosures: salary with Mirum. The remaining authors of this manuscript have no disclosures or conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Table S1-S3
REFERENCES
- 1.Qin CX, Auerbach SR, Charnaya O, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccination in pediatric solid organ transplant recipients. Am J Transplant. 2022;22(2):669–672. doi: 10.1111/ajt.16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haskin O, Ashkenazi-Hoffnung L, Ziv N, et al. Serological response to the BNT162b2 COVID-19 mRNA vaccine in adolescent and young adult kidney transplant recipients. Transplantation. 2021;105(11):e226–e233. doi: 10.1097/TP.0000000000003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karaba AH, Zhu X, Liang T, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22(4):1253–1260. doi: 10.1111/ajt.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roche Diagnostics. Elecsys® Anti-SARS-CoV-2 S. https://diagnostics.roche.com/us/en/products/params/elecsys-anti-sars-cov-2-s.html. Accessed August 11, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1-S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.