Abstract
The coronavirus disease 2019 (COVID‐19) antiviral nirmatrelvir/ritonavir (Paxlovid) has been granted authorization or approval in several countries for the treatment of patients with mild to moderate COVID‐19 at high risk of progression to severe disease and with no requirement for supplemental oxygen. Nirmatrelvir/ritonavir will be primarily administered outside the hospital setting as a 5‐day course oral treatment. The ritonavir component boosts plasma concentrations of nirmatrelvir through the potent and rapid inhibition of the key drug‐metabolizing enzyme cytochrome P450 (CYP) 3A4. Thus nirmatrelvir/ritonavir, even given as a short treatment course, has a high potential to cause harm from drug–drug interactions (DDIs) with other drugs metabolized through this pathway. Options for mitigating risk from DDIs with nirmatrelvir/ritonavir are limited due to the clinical illness, the short window for intervention, and the related difficulty of implementing clinical monitoring or dosage adjustment of the comedication. Pragmatic options are largely confined to preemptive or symptom‐driven pausing of the comedication or managing any additional risk through counseling. This review summarizes the effects of ritonavir on drug disposition (i.e., metabolizing enzymes and transporters) and discusses factors determining the likelihood of having a clinically significant DDI. Furthermore, it provides a comprehensive list of comedications likely to be used in COVID‐19 patients which are categorized according to their potential DDI risk with nirmatrelvir/ritonavir. It also discusses recommendations for the management of DDIs which balance the risk of harm from DDIs with a short course of ritonavir, against unnecessary denial of nirmatrelvir/ritonavir treatment.
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) protease inhibitor nirmatrelvir/ritonavir (Paxlovid) has been granted authorization or approval in several countries for the treatment of patients with mild to moderate COVID‐19 at high risk of progression to severe disease and with no requirement for supplemental oxygen. Nirmatrelvir/ritonavir at an oral dose of 300/100 mg twice daily for 5 days reduced the risk of COVID‐19 related hospitalization and death within 28 days by 89% when compared with a placebo group when treatment was started within 3 days of COVID‐19 symptoms onset. 1 Due to the use of ritonavir, nirmatrelvir/ritonavir has a high potential to cause clinically important drug–drug interactions (DDIs) with other concurrent medications.
Ritonavir was originally approved in 1996 for HIV treatment at a dose of 600 mg twice daily but due to poor tolerability was discontinued as a therapeutic agent. However, as a potent inhibitor of the cytochrome P450 (CYP) 3A4 isoenzyme, low doses of ritonavir (i.e., mostly 100 mg administered once or twice daily) have been used for more than two decades to “boost” HIV protease inhibitors. 2 Similarly, ritonavir is used as a pharmacokinetic booster to increase the exposure of the COVID‐19 protease inhibitor nirmatrelvir, a CYP3A4 substrate. 3 , 4
Aside from affordability and availability, DDIs are the most important restriction to the widespread use of nirmatrelvir/ritonavir. In many settings, nirmatrelvir/ritonavir will be primarily prescribed by practitioners who may lack in‐depth knowledge of managing these complex DDIs associated with ritonavir, leading to unintended denial of therapy or the occurrence of serious adverse events due to inadvertent DDIs. Moreover, the short treatment course may allow some comedications to continue despite the presence of a DDI. For safe management, it is essential that prescribers are aware of drugs that are an absolute contraindication for use with nirmatrelvir/ritonavir but also know which drugs can be safely coadministered to prevent unnecessary denial of nirmatrelvir/ritonavir treatment or inappropriate withholding of long‐term comedications.
In this review, we summarize the effects of ritonavir on drug disposition (i.e., metabolizing enzymes and transporters) and discuss factors determining the likelihood of having a clinically significant DDI. Furthermore, using a standardized approach, we predicted potential DDIs with nirmatrelvir/ritonavir for a comprehensive list of comedications likely to be prescribed in COVID‐19 patients. Finally, we discussed the management of selected DDIs of interest.
PHARMACOLOGY OF RITONAVIR
Effect of ritonavir on drug‐metabolizing enzymes and drug transporters
Ritonavir is a potent CYP3A4 inhibitor reaching maximal inhibition effect at a dose of 100 mg and therefore can substantially increase the plasma concentrations of concurrent drugs predominantly metabolized by CYP3A4. 5 However, ritonavir is only a weak inhibitor of CYP2D6 at a boosting dose. 6 Ritonavir is also an inducer of CYP1A2, CYP2B6, CYP2C9, CYP2C19, and uridine diphosphate (UDP)‐glucuronyltransferase (UGT), and consequently can reduce the exposure (i.e., area under the curve (AUC)) of drugs metabolized by these enzymes. 7 In addition, ritonavir inhibits the transporters P‐glycoprotein (P‐gp) and breast cancer resistance protein (BCRP), expressed notably in the intestine, leading to an increased intestinal absorption of some drugs (e.g., direct‐acting oral anticoagulants). Ritonavir also inhibits the hepatic uptake transporters organic anion transporting polypeptides (OATP) 1B1 and 1B3, resulting in increased plasma concentrations of drugs such as statins. 7 , 8
Onset of ritonavir inhibitory and inducing effects
The onset of ritonavir inhibitory effect is rapid; thus even short courses of nirmatrelvir/ritonavir treatment can cause clinically relevant DDIs. Maximal inhibition of CYP3A4 was achieved 48 hours after initiating ritonavir in individuals treated with the CYP3A4 substrate midazolam. 9 In contrast, induction develops more slowly with maximal induction observed typically after 5–7 days of dosing for CYP3A4, although the time course for induction of non‐CYP3A4 enzymes and transporters has not been fully elucidated. 10 Thus when ritonavir is used for 5 days in the context of COVID‐19 treatment, its induction properties are less likely to be clinically relevant in comparison with chronic administration for HIV treatment.
Duration of CYP3A4 inhibitory effect after stopping ritonavir
Ritonavir irreversibly inhibits CYP3A4 leading to the loss of the enzyme. As a consequence, inhibition takes several days to reverse as it requires de novo enzyme synthesis to restore baseline metabolic activity. 11 , 12 Modeling suggests that over 80% of CYP3A4 inhibition will have resolved 3 days after stopping ritonavir in young and elderly adults (Figure 1 ), 11 although significant interindividual variability should be noted. 9 , 11 The time window for pausing drugs should also factor in the critical indication of some drugs. Real‐life data for the management of the nirmatrelvir/ritonavir interaction with the narrow therapeutic index drug tacrolimus showed that pausing tacrolimus for 8 days (i.e., 5 days during nirmatrelvir/ritonavir treatment + 3 days waiting period for the disappearance of CYP3A4 inhibition) resulted in tacrolimus levels within therapeutic range in most patients. A few individuals had supratherapeutic tacrolimus levels after re‐initiating tacrolimus at day 8 due to the slower CYP3A4 inhibition disappearance. 13 Based on these real‐life data, most comedications which are paused during nirmatrelvir/ritonavir therapy can be restarted 3 days after the last dose of nirmatrelvir/ritonavir. For narrow therapeutic index drugs, it is recommended to wait at least 3 days and, if possible, up to 5 days after completing nirmatrelvir/ritonavir treatment due to the large interindividual variability in the disappearance of CYP3A4 inhibition. The same timeline applies for comedications whose dosage has been reduced during nirmatrelvir/ritonavir treatment.
Figure 1.

Disappearance of CYP3A4 inhibition 1, 2 3, 4, and 5 days after stopping ritonavir for different age groups. The data are presented as mean value with the 95% confidence interval (adapted from ref. 11). CYP, cytochrome P450.
Effect of comedications on nirmatrelvir/ritonavir concentrations
Both nirmatrelvir and ritonavir are substrates of CYP3A4. 3 , 4 Strong CYP3A4 inhibitors will not increase nirmatrelvir/ritonavir concentrations to a clinically significant extent given that ritonavir per se inhibits maximally CYP3A4. However, the concentrations of nirmatrelvir/ritonavir can be significantly reduced by strong inducers which may potentially compromise its efficacy. The coadministration of nirmatrelvir/ritonavir and the strong inducer carbamazepine (300 mg twice daily) decreased nirmatrelvir AUC by 55%. 3 , 4 Thus, the coadministration with strong inducers is contraindicated in the product labels. 3 , 4 It is critical that prescribers are aware of the persisting inducing effect upon discontinuation of an inducer. 14 Thus, DDIs with strong inducers cannot be prevented and require the use of an alternative COVID‐19 therapy. Moderate inducers (e.g., efavirenz) are not anticipated to significantly alter nirmatrelvir/ritonavir concentrations as indicated by DDI studies with HIV protease inhibitors showing that twice daily ritonavir boosting was able to mostly counteract an efavirenz inducing effect. 15
FACTORS DETERMINING THE RISK OF HAVING A CLINICALLY RELEVANT DDI
A good understanding of the key factors that can increase the risk of having a clinically relevant DDI is critical to avoid an overestimation of the DDI risk, which could lead to unnecessary denial of nirmatrelvir/ritonavir treatment. Some key factors to consider when evaluating the DDI risk include the following:
Contribution of CYP3A4 metabolism to a comedication
The contribution of CYP3A4 to the overall metabolism/elimination of a comedication is critical to predict the magnitude of the DDI. Drugs predominantly metabolized by CYP3A4 (CYP3A4‐sensitive substrates) are expected to be profoundly impacted by ritonavir as the major metabolic pathway is inhibited. Conversely, DDIs tend to be mitigated for a drug eliminated by multiple CYPs or pathways as drugs can still be cleared through the unaffected pathways. For instance, ritonavir is predicted to increase simvastatin (metabolized 100% by CYP3A4) concentrations by 100‐fold, thereby exposing an individual to the risk of a deleterious adverse event. 16 Indeed, a patient treated with the strong CYP3A4 pharmacokinetic booster cobicistat developed a severe rhabdomyolysis‐induced kidney failure 10 days after initiation of simvastatin. 17 Given the high risk of harm, simvastatin is contraindicated with nirmatrelvir/ritonavir. Thus, simvastatin has to be paused to enable treatment with nirmatrelvir/ritonavir. On the other hand, ritonavir minimally increases the exposure of escitalopram, an antidepressant metabolized equally by CYP3A4, CYP2C19, and CYP2D6. 18 Additionally, the risk of QT interval prolongation with escitalopram is considered to be unlikely in the presence of nirmatrelvir/ritonavir given the latter's low propensity to prolong the QT interval. 4
Therapeutic index of the comedication
The narrow therapeutic index of a drug is also a determinant of higher risk for a clinically relevant DDI. For instance, the elimination half‐life and the plasma concentrations of the immunosuppressant drug tacrolimus were shown to be profoundly increased, reaching toxic levels only 3 days after initiating ritonavir in a patient on long‐term tacrolimus treatment. 19 Thus, the management of the DDI between tacrolimus and nirmatrelvir/ritonavir would require a substantial reduction in tacrolimus dose and close therapeutic drug monitoring (TDM) during and after completion of nirmatrelvir/ritonavir treatment to take into account the mechanism‐based inhibition of ritonavir. 13 , 20
POTENTIAL DDIs BETWEEN NIRMATRELVIR/RITONAVIR AND COMEDICATIONS LIKELY TO BE USED IN COVID‐19 PATIENTS
For safe prescribing, it is essential that clinicians are aware of drugs that are contraindicated for use with nirmatrelvir/ritonavir but also recognize which drugs can be safely coadministered. Thus, we predicted potential DDIs between nirmatrelvir/ritonavir and 371 comedications selected on the basis of either being prescribed at a high frequency in COVID‐19 patients or carrying potentially significant consequences for harm resulting from the DDI (Table 1 ) using a standardized approach as described below. In the following sections, we will discuss DDIs of interest within therapeutic classes compiled in the table.
Table 1.
Potential drug–drug interactions (DDIs) between nirmatrelvir/ritonavir and comedications classified by therapeutic classes. DDIs are categorized as: red flag for drugs that should not be coadministered due to the risk of deleterious adverse outcomes; amber flag for potential clinical DDIs manageable by monitoring or dosage adjustment; yellow flag for DDIs of weak clinical relevance with no need for monitoring or dosage adjustment; and green flag for no DDI. Symbols are used to distinguish red flag DDIs which cannot be prevented by stopping a comedication. This is notably the case for drugs characterized by a very long elimination half‐life or for strong inducers due to their prolonged induction upon discontinuation of the drug. Use of such medications would require an alternative COVID‐19 treatment. A symbol is also used for amber DDIs that may require a complex/impractical monitoring that may not be feasible outside a clinical setting. If such comedications cannot be stopped or replaced, the risk–benefit of prescribing nirmatrelvir/ritonavir should be evaluated. Amber DDIs without a symbol are manageable by counseling patients about potential DDIs and advising them to temporarily pause the comedication if feeling unwell, thus enabling treatment with nirmatrelvir/ritonavir. The list of drugs is incomplete, detailed information and additional DDIs are available at https://www.covid19‐druginteractions.org. 23
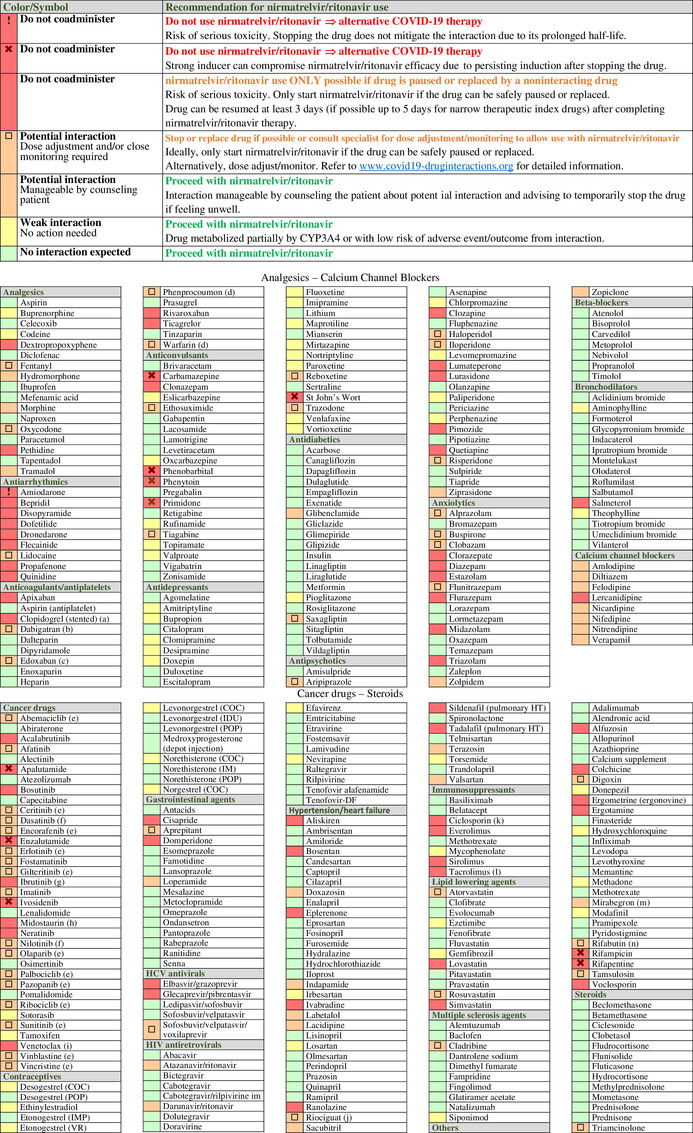
|
(a) Ritonavir reduces the conversion to clopidogrel's active metabolite, leading to insufficient inhibition of platelet aggregation. 35 Thus, it is recommended to avoid nirmatrelvir/ritonavir in patients at very high risk of thrombosis (e.g., early period post coronary stenting), 37 unless clopidogrel can be switched to the noninteracting drug prasugrel. However, nirmatrelvir/ritonavir treatment is possible in other clinical situations for which a transient loss in clopidogrel efficacy is acceptable (e.g., alternative to aspirin in intolerant patients).
(b) Reduce to 110 mg twice daily in individuals with normal renal function and to 75 mg twice daily in individuals with moderate renal impairment.
(c) Reduce edoxaban to 30 mg.
(d) Monitor international normalized ratio as clinically indicated.
(e) Decision to hold or dose adjust the cancer drug should be made in conjunction with the patient's oncologist. Consult www.covid19‐druginteractions.org 23 for details related to dosage adjustment.
(f) Accelerated or blast phase chronic myelogenous leukemia: do not coadminister; use alternative COVID‐19 therapy. In the indication of chronic phase chronic myelogenous leukemia, the decision to hold or dose adjust the cancer drug should be made in conjunction with the patient's oncologist. If it is decided to hold treatment, restart the cancer drug at least 3 days after completing nirmatrelvir/ritonavir. Alternatively dose adjust; consult www.covid19‐druginteractions.org 23 for details.
(g) The decision to hold ibrutinib treatment should be made in conjunction with the patient's oncologist. It may be dangerous to interrupt therapy in patients with high‐volume chronic lymphocytic leukemia or mantle cell lymphoma due to disease flare and/or cytokine release. Consider an alternative COVID‐19 therapy.
(h) Strong CYP3A4 inhibitors can substantially increase midostaurin exposure. Consider an alternative COVID‐19 treatment.
(i) Coadministration with nirmatrelvir/ritonavir is contraindicated at initiation and during the dose‐titration phase to minimize the risk of tumor lysis syndrome. Use an alternative COVID19 therapy.
(j) The European product label for riociguat does not recommend its use in the presence of strong inhibitors; the US product label recommends to start riociguat at a dose of 0.5 mg three times daily and to monitor for signs and symptoms of hypotension.
(k) The management of this interaction is challenging and would require dosage adjustment and therapeutic drug monitoring (TDM) of ciclosporin, which may not be possible given the short duration of nirmatrelvir/ritonavir treatment. An alternative COVID treatment should be considered. However, if TDM is available, an empiric dose reduction of ciclosporin has been suggested (reduce total daily dose by 80% and administer once daily) during treatment with nirmatrelvir/ritonavir (Days 1–5). Ciclosporin concentrations should be assessed on Day 6 or 7 and repeated every 2–4 days. 20
(l) The management of this interaction is challenging and would require a substantial reduction in tacrolimus dosage. Considering the complex management of this interaction, an alternative COVID treatment will need to be considered. However, if TDM for tacrolimus is available, it has been suggested to withhold all tacrolimus doses during treatment with nirmatrelvir/ritonavir (Days 1–5). It is advised to measure tacrolimus concentrations on Day 3 to assess the need for a one‐time tacrolimus dose during nirmatrelvir/ritonavir treatment. Tacrolimus concentrations should be assessed on Day 6 or 7 (and every 2–4 days thereafter) and concentrations used to guide the continued withholding or gradual reintroduction of tacrolimus. 20
(m) No dose reduction or monitoring in patients with normal renal function.
(n) Rifabutin is dosed at 150 mg once daily with nirmatrelvir/ritonavir.
COC, combined oral contraceptive; CYP, cytochrome P450; IM, intramuscular depot injection; IMP, implant; IUD, intrauterine device; POP, progestogen‐only pill; VR, vaginal ring.
Approach to predict potential DDIs with nirmatrelvir/ritonavir
The identification of the DDI risk was evaluated as described previously. 21 , 22 Briefly, data on the clinical pharmacology of nirmatrelvir/ritonavir and comedications were extracted from product labels and, where available, published studies on drug disposition. Since only three clinical DDI studies were conducted for nirmatrelvir/ritonavir (with itraconazole, with dabigatran and with carbamazepine), 3 , 4 clinical DDI studies or case reports involving HIV or hepatitis C virus (HCV) drugs boosted with ritonavir were also considered for the extrapolation of the DDI magnitude/risk. The propensity for a DDI was based on the potential of ritonavir to modulate (inhibit or occasionally induce) drug‐metabolizing enzymes and transporters contributing to the absorption, metabolism, and elimination of the coadministered drug. If there was evidence that a DDI may occur, then the clinical relevance was evaluated by taking into account factors such as the magnitude of the pharmacokinetic change and the therapeutic index. When assessing the clinical relevance of a DDI, we also took into account (i) the potential of nirmatrelvir/ritonavir to prevent severe disease in COVID‐19 patients at risk; (ii) the short treatment course; (iii) the possibility to monitor the drug effect or to adjust the dosage outside a clinical setting; and (iv) the option to safely pause the comedication during nirmatrelvir/ritonavir treatment. DDIs were classified into four levels: red flags for contraindicated drug associations that may have deleterious consequences; amber for potential clinically relevant interactions manageable by close monitoring or dosage adjustment (note: for some amber DDIs, coadministration with nirmatrelvir/ritonavir may or may not be feasible depending on the clinical setting/clinical condition of the patient); yellow for interactions of weak intensity with no need for additional monitoring or dosage adjustment; and green for no risk of DDI. Detailed information on the management of DDIs can be found in the University of Liverpool COVID‐19 interactions resource (www.covid19‐druginteractions.org), 23 which provides freely accessible up‐to‐date information on DDIs with nirmatrelvir/ritonavir and other COVID‐19 therapies. Furthermore, readers are directed to our previous publication for strategies on how to manage potential DDIs. 24 As a general rule, potentially interacting comedications should be paused temporarily if it is clinically appropriate to do so.
DDIs of interest within selected therapeutic classes
Analgesics
Potentially clinically relevant DDIs with nirmatrelvir/ritonavir can occur with opioid analgesics, notably those extensively metabolized by CYP3A4, like the widely used opioid oxycodone. The AUC of oxycodone (10 mg single dose) was shown to increase by threefold when coadministered with ritonavir (300 twice daily) or lopinavir/ritonavir (400/100 mg twice daily), 25 which could lead to adverse events such as respiratory depression. Thus, a reduction in oxycodone dose and monitoring for signs of opioid toxicity are advised in case of coadministration with nirmatrelvir/ritonavir. Conversely, no dosage adjustment is a priori needed for opioids undergoing UGT metabolism (e.g., morphine, hydromorphone) as the inducing effect of ritonavir on UGT does not reach maximal levels during the short nirmatrelvir/ritonavir treatment course.
Antiarrhythmics
Antiarrhythmics are narrow therapeutic index drugs which are not recommended with nirmatrelvir/ritonavir as an increase in their exposure can expose patients to the risk of arrhythmias or other serious adverse reactions. It should be highlighted that the antiarrhythmic amiodarone has a very long elimination half‐life (i.e., 55 days) 26 so that the DDI with nirmatrelvir/ritonavir cannot be mitigated even if pausing the drug; in this case an alternative COVID‐19 treatment is recommended.
Anticoagulants/antiplatelets
Anticoagulants include heparin/low molecular weight heparin, vitamin K antagonists, and the direct‐acting oral anticoagulants (DOACs). Heparins are not eliminated by CYPs and therefore can be coadministered with nirmatrelvir/ritonavir. Conversely, vitamin K antagonists are metabolized by multiple CYPs which can be inhibited or induced by ritonavir. For instance, warfarin is a mixture of enantiomers with its S‐enantiomer (more potent) largely metabolized by CYP2C9 while its R‐enantiomer is metabolized by CYP3A4 and CYP1A2. 27 Ritonavir inhibits CYP3A4 but induces CYP2C9 and CYP1A2. 7 Although reduction in warfarin exposure has been reported with the chronic use of ritonavir, 28 , 29 an increase in warfarin exposure is anticipated with the short nirmatrelvir/ritonavir treatment course as the onset of inhibition is more rapid than induction. For the management of this DDI, monitoring of international normalized ratio is recommended as clinically indicated. Significant DDIs are expected with DOACs as they are substrates of CYP3A4 and/or P‐gp. 30 Ritonavir was shown to enhance rivaroxaban AUC by 153%, 31 which may increase the risk of bleeding; thus coadministration with nirmatrelvir/ritonavir is not recommended. The management of this DDI would require pausing rivaroxaban and using alternative options for the anticoagulation based on the indication (i.e., edoxaban 30 mg once daily when used for atrial fibrillation; low molecular weight heparin for the prevention of thromboembolism in patients at high risk of clot). 23 The usual rivaroxaban treatment should be resumed at least 3 days (if possible up to 5 days) after the last nirmatrelvir/ritonavir dose. The decision to pause rivaroxaban and use an alternative treatment should be made on a case‐by‐case basis, in consultation with a cardiologist/hematologist. 23 Similarly, the use of apixaban is not recommended with nirmatrelvir/ritonavir, although the US label gives the possibility to administer apixaban at a reduced dose (2.5 mg twice daily) when combined with strong inhibitors of CYP3A4 and P‐gp. 23 Of interest, no adverse outcomes were reported in HIV‐infected patients treated with a reduced dose of apixaban while on ritonavir‐boosted regimens, 32 , 33 thus supporting the option to use a reduced dose of apixaban during nirmatrelvir/ritonavir treatment and for at least 3 days (if possible up to 5 days) after the last nirmatrelvir/ritonavir dose. Finally, the interaction between nirmatrelvir/ritonavir (300/100 mg twice daily for 5 days) and dabigatran was shown to increase dabigatran AUC by twofold. 4 In the case of coadministration, it is recommended to reduce dabigatran to 110 mg twice daily in individuals with normal renal function and to 75 mg twice daily in individuals with moderate renal impairment. 23 These dosing recommendations are based on the interaction between dabigatran and darunavir/cobicistat as this is of a similar magnitude to ritonavir given for a short duration (i.e., only an inhibitory effect on P‐gp with short duration ritonavir administration vs. a mixed inhibitory/inducing effect on P‐gp with long‐term ritonavir use). 34
Another DDI of interest is with the antiplaletet agent clopidogrel, a prodrug which is converted to its active metabolite by CYP3A4, CYP2B6, CYP2C19, and CYP1A2. 35 Coadministration with ritonavir may reduce the conversion to the active metabolite, leading to insufficient inhibition of platelet aggregation, whereas prasugrel's effect has been shown to be unaltered by ritonavir. 35 There is a report of a patient on ritonavir‐boosted HIV therapy who presented stent thrombosis episodes while concurrently treated with dual antiplatelet therapy comprising aspirin and clopidogrel. Replacement of clopidogrel by prasugrel prevented novel stent thrombosis episodes. 36 Thus, it is recommended to avoid nirmatrelvir/ritonavir in patients prescribed clopidogrel at very high risk of thrombosis (e.g., early period post coronary stenting) 37 and to substitute prasugrel if possible during nirmatrelvir/ritonavir treatment and for at least 3 days (up to 5 days) after nirmatrelvir/ritonavir treatment. It is also important to emphasize that nirmatrelvir/ritonavir treatment could be considered in other clinical situations for which a transient loss in clopidogrel efficacy is acceptable (i.e., clopidogrel used as an alternative to aspirin in intolerant patients or when used outside the critical period post coronary stenting associated with a higher risk of coronary artery stent thrombosis).
Anticonvulsants
Some anticonvulsants have inducing properties on drug metabolizing enzymes. Since induction persists upon discontinuation of an inducer, DDIs cannot be prevented; therefore an alternative COVID‐19 treatment has to be used in patients receiving anticonvulsants with strong inducing properties such as carbamazepine, phenobarbital, phenytoin, or primidone. However, twice daily ritonavir is able to counteract moderate or weak induction, allowing nirmatrelvir/ritonavir treatment with eslicarbazepine, oxcarbazepine, or rufinamide. 23
Antidepressants
Antidepressants are generally metabolized by multiple CYPs, including CYP2D6, which is weakly inhibited by ritonavir, 6 leading to DDIs of modest magnitude. 38 Nirmatrelvir/ritonavir can be used with most antidepressants with no need for dosage adjustment except for antidepressants primarily metabolized by CYP3A4 (e.g., trazodone) for which a dose adjustment may be needed. 23 Finally, the herbal St John's Wort is contraindicated with nirmatrelvir/ritonavir due to its significant inducing properties, particularly if the daily dose of hyperforin (constituent responsible for CYP and P‐gp induction) exceeds 1 mg. 39
Antidiabetics
Most antidiabetics can be used with nirmatrelvir/ritonavir as they are either eliminated renally (e.g., metformin) or undergo main metabolism via UGT (e.g., canagliflozin) or CYP2C9 (e.g., gliclazide), which are not fully induced during the short treatment course of nirmatrelvir/ritonavir. On the other hand, saxagliptin is primarily metabolized by CYP3A4 and its AUC was shown to be increased by 145% following the coadministration with the strong CYP3A4 inhibitor ketoconazole, which could increase the risk of hypoglycemia. 40 Therefore, it is recommended that saxagliptin dose does not exceed 2.5 mg with nirmatrelvir/ritonavir.
Antipsychotics
The dosage of antipsychotics is generally titrated based on the clinical response. The management of DDIs with nirmatrelvir/ritonavir can be challenging for antipsychotics metabolized by CYP3A4 (e.g., quetiapine, for which ritonavir is expected to increase AUC by eightfold and consequently the related risk of QT interval prolongation). 16 Their dosage would have to be reduced and increased again within a short period of time which could potentially destabilize the patient. The decision to modify the dosage or to pause the antipsychotic should be done in consultation with a specialist in mental health medicine with a careful evaluation of the benefit–risk of nirmatrelvir/ritonavir treatment.
Anxiolytics
Anxiolytics highly dependent on CYP3A4 metabolism (e.g., midazolam) and with a long elimination half‐life (e.g., diazepam) are contraindicated with nirmatrelvir/ritonavir due to the risk of extreme sedation and respiratory depression. 3 , 4 It is recommended to pause the anxiolytic during and for at least 3 days (if possible up to 5 days) after nirmatrelvir/ritonavir treatment completion to account for the CYP3A4 mechanism‐based inhibition by ritonavir.
Antihypertensives
Hypertension can be treated with calcium channel inhibitors (e.g., amlodipine), angiotensin‐converting enzyme (ACE) inhibitors (i.e., enalapril), beta‐blockers (e.g., metoprolol), angiotensin receptor blockers (e.g., olmesartan), or diuretics (e.g., furosemide). Nirmatrelvir/ritonavir does not significantly interact with agents of these therapeutic classes with the exception of calcium channel inhibitors as they are mainly metabolized by CYP3A4. For example, amlodipine AUC is increased by twofold in the presence of ritonavir, leading to the recommendation to reduce amlodipine dose by 50%. 41 However, given the short time treatment course of nirmatrelvir/ritonavir, dose adjustment can be optional in the case of amlodipine and most other calcium channel inhibitors as patients can be advised to temporarily pause the antihypertensive drug if feeling unwell. 23
Bronchodilators
Bronchodilators can be coadministered with nirmatrelvir/ritonavir with the exception of the CYP3A4 substrate salmeterol, which is not recommended as increased concentrations expose patients to the risk of serious and/or life‐threatening cardiovascular adverse events. 3 , 4
Cancer drugs
Several cancer drugs, notably the kinase inhibitors, which play an increasingly prominent role in the treatment of cancer, undergo CYP3A4 metabolism and/or are substrates of drug transporters. 42 Given their narrow therapeutic index, significant DDIs are expected with the concurrent use of nirmatrelvir/ritonavir, which may lead to important toxicities for some cancer drugs, thereby requiring an evaluation of the risk–benefit of prescribing nirmatrelvir/ritonavir. It should be mentioned that it is common practice to hold temporarily some tyrosine kinase inhibitors, cyclin‐dependent kinase inhibitors, and poly (ADP‐ribose) polymerase inhibitors during acute infections such as COVID‐19. 43 The decision to hold or dose adjust the cancer drug should be made in conjunction with the patient's oncologist. It may be safe to temporarily hold cancer drugs in patients who achieved disease control; however it could be potentially dangerous to pause treatment, for instance, in patients with high‐volume chronic lymphocytic leukemia or mantle cell lymphoma due to the risk or disease flare and/or cytokine release. 44 In such situations, as well as in patients receiving cancer drugs with strong inducing properties (e.g., apalutamide, enzalutamide), an alternative COVID‐19 therapy should be considered.
Contraceptives
Nirmatrelvir/ritonavir is unlikely to alter the effectiveness of contraceptives, including combined oral contraceptives. 45 Exposure of ethinylestradiol is not expected to be significantly reduced via UGT induction given the short treatment course of nirmatrelvir/ritonavir. In addition, the progestin component (responsible for the contraceptive efficacy) is generally increased by ritonavir due to inhibition of CYP3A4. 46
Gastrointestinal agents
Within this therapeutic class, potentially clinically significant DDIs can occur with the gastroprokinetic agents cisapride or domperidone, as inhibition of CYP3A4 by nirmatrelvir/ritonavir is expected to increase their exposures, which may result in serious and/or life threatening reactions, including cardiac arrhythmias. 3 , 4 It is recommended to hold these drugs during and for at least 3 days (if possible up to 5 days) after completing nirmatrelvir/ritonavir treatment.
HCV antivirals
Several first‐line HCV antivirals (i.e., elbasvir, glecaprevir, grazoprevir, velpatasvir, and voxilaprevir) are metabolized by CYP3A4 and are substrates of drug transporters inhibited by ritonavir (i.e., P‐gp and/or BCRP and/or OATP1B1) and therefore can be substantially impacted by nirmatrelvir/ritonavir. 47 For instance, the coadministration of the HIV drug darunavir/ritonavir increased grazoprevir AUC by 7.5 fold 48 and glecaprevir AUC by 4.8 fold, 49 which may lead to elevation of alanine aminotransferase. Coadministration with nirmatrelvir/ritonavir is not recommended and consideration must be given to holding elbasvir/grazoprevir and glecaprevir/pibrentasvir during and for at least for 3 days after the completion of nirmatrelvir/ritonavir treatment. However, the risk–benefit of prescribing nirmatrelvir/ritonavir should be carefully evaluated in patients with suboptimal adherence to their HCV therapy as further treatment interruption could potentially result in HCV treatment failure.
HIV antiretrovirals
Nirmatrelvir/ritonavir can be coadministered with all HIV drugs with no dose adjustment. Patients on ritonavir or cobicistat containing HIV regimens can continue their treatment as the additional ritonavir in Paxlovid for 5 days is unlikely to be problematic in terms of intolerance and it is probably simplest to continue the patient's current regimen. 23
Immunosuppressants
Based on DDIs studies with the strong inhibitor ketoconazole or ritonavir‐boosted HIV protease inhibitors, nirmatrelvir/ritonavir is expected to profoundly increase the exposure of the CYP3A4 and P‐gp substrates everolimus, 50 sirolimus, 51 ciclosporin, 52 and tacrolimus. 19 The management of these interactions are challenging and would require holding or substantially reducing the immunosuppressant dose with close TDM. However, TDM may not be feasible during the short nirmatrelvir/ritonavir treatment course and therefore an alternative COVID‐19 treatment should be considered. If TDM is available, early experience in four SARS‐CoV‐2–infected kidney transplant recipients on tacrolimus supports holding the immunosuppressant on the day of nirmatrelvir/ritonavir initiation and to monitor closely drug levels to guide the reintroduction of tacrolimus. 13 , 53
Lipid‐lowering agents
Potential clinically relevant DDIs between nirmatrelvir/ritonavir and drugs for dyslipidemia occur with statins. There are differences in the magnitude of DDIs depending on the involvement of CYP3A4 in the metabolism. Simvastatin and lovastatin are sensitive CYP3A4 substrates resulting in profound DDIs, 16 leading to use with nirmatrelvir/ritonavir being contraindicated due to the risk of rhabdomyolysis. The US prescribing information recommends to discontinue simvastatin or lovastatin at least 12 hours prior to initiation of nirmatrelvir/ritonavir. 4 These statins should be held for at least 3 days but preferably 5 days after completing nirmatrelvir/ritonavir treatment. It should be mentioned that even though other statins are less impacted by ritonavir, the pragmatic approach of temporarily pausing any statins during nirmatrelvir/ritonavir is acceptable as it will not negatively affect their therapeutic effect but can minimize the risk for adverse events related to a DDI.
Multiple sclerosis agents
Nirmatrelvir/ritonavir can be coadministered with most treatments for multiple sclerosis. It should be noted that the exposure of cladribine can be increased due to BCRP inhibition by ritonavir. 54 However, since cladribine is administered as two annual courses, each including 2 weeks’ treatment given 1 month apart, 54 cladribine can be mostly delayed to allow nirmatrelvir/ritonavir treatment.
Steroids
Most corticosteroids are substrates of CYP3A4; therefore their exposure is expected to be increased by nirmatrelvir/ritonavir. However, the risk of a Cushing's syndrome is considered to be low due to the short course of nirmatrelvir/ritonavir. A review of individuals developing a Cushing's syndrome indicates that this adverse effect mostly occurs after several weeks of concurrent administration of corticosteroids with ritonavir‐containing HIV regimens. 55 Monitoring for signs of systemic corticosteroid side effects is nevertheless recommended with triamcinolone due to its long half‐life and related higher risk of adverse effects.
Others
No significant DDIs are expected between nirmatrelvir/ritonavir and various treatments for rheumatoid arthritis (e.g., nonsteroidal and anti‐inflammatory drugs, steroids, hydroxychloroquine, methotrexate, or monoclonal antibodies).
SUMMARY
Nirmatrelvir/ritonavir, even given as a short treatment course, can cause significant DDIs, particularly for drugs that are predominantly metabolized by CYP3A4 and/or have a narrow therapeutic index or are substrates of P‐gp. DDI recommendations for nirmatrelvir/ritonavir are challenging as they need to balance the benefit of nirmatrelvir/ritonavir to prevent severe disease against the risk of having a potentially clinically relevant DDI, keeping in mind that the risk can be further increased for some drugs due to limited monitoring options outside the clinical setting. Another difficulty is that much advice is based on predicted DDIs as opposed to evidence generated by DDI studies, resulting in judgments based on “expert opinion.” However, even if the DDI magnitude can be extrapolated from previous studies with ritonavir‐boosted HIV drugs, there is nevertheless a lack of clinical experience with a short ritonavir treatment course, making the clinical relevance of certain DDIs with nirmatrelvir/ritonavir unclear. Thus, there will be often a need to balance the risk of “theoretical” DDIs against the benefit of nirmatrelvir/ritonavir. This may lead to differences in the evaluation of the risk–benefit of nirmatrelvir/ritonavir treatment among experts, particularly in patients with critical conditions (i.e., cancer or solid organ transplant recipients) for which pausing a drug or suboptimal therapy could have serious consequences. For example, due to the complex management of the tacrolimus – nirmatrelvir/ritonavir DDI, expert HIV pharmacists/pharmacologists recommend an alternative COVID‐19 treatment, 23 whereas organ transplant specialists would rather opt for nirmatrelvir/ritonavir treatment with management of the DDI by withholding tacrolimus and adjusting dosage with frequent TDM. 20
Even though some DDIs are complex, it should be emphasized that a potential risk of DDIs should not necessarily preclude nirmatrelvir/ritonavir use since many DDIs are manageable. Safe management of DDIs can only be carried out when prescribers are aware of their presence, underlining the importance of a full medicines reconciliation. Importantly, potentially serious DDIs can also occur with nonprescription drugs, including herbals (e.g., St John's Wort) or illicit substances (notably opioids); therefore the establishment of the list with all current drugs should also include these substances. If a potentially significant DDI is identified, the risk–benefit of prescribing nirmatrelvir/ritonavir should be evaluated, taking into account the potential severity of the DDI, strategies for the management of the DDI, and their applicability outside a clinical setting. Screening of DDIs using specialized resources, or prescription review by pharmacists/pharmacologists is advised to ensure safe prescribing. Extra caution is advised when reviewing prescriptions of individuals with significant polypharmacy or with renal/hepatic impairment.
FUNDING
No funding was received for this work. Open access funding provided by Universitat Basel.
CONFLICT OF INTEREST
C.M. received a research grant from Gilead and lecture honoraria from MSD and ViiV; D.R.K. has received consulting fees from Atea, Gilead, GSK, Janssen, Merck, Novartis, and ViiV, and research support from Atea, Gilead, Merck, and ViiV; F.M. has received educational grants and/or consulting fees from ViiV, Gilead, and Merck; A.B. has received educational grants and lecture honoraria from Gilead and Abbvie. S.G. has received no direct grants/research support but is an employee of the University of Liverpool and is funded by external research income sources awarded to the Liverpool Drug Interaction Group from BHIVA, EACS, Glasgow HIV Drug Therapy, NIHR, UKRI, AbbVie, Gilead, MSD, Novartis, Sobi, Thera Technologies, and ViiV; C.F. reports serving as a paid consultant for Gilead Sciences, Janssen, Merck, and ViiV Healthcare; A.P. has received research grants and/or consulting fees from ViiV, Gilead, MSD, Theratech, and Janssen; M.B. has received research grants and/or consulting fees from ViiV, Gilead, MSD, GSK, Novavax, Valneva, Cipla, Mylan, Janssen, and Roche; L.W. has received research grants and/or consulting fees from ViiV, Gilead, MSD, Theratech, Cipla, Mylan, and Janssen; D.B. is working at Radboud University Medical Center, which has received research grants from ViiV, Merck, and Gilead; D.J.B. has received educational grants from AbbVie, Novartis, Merck, Gilead, and Sobi and lecture/consultancy fees from AbbVie, Gilead, Merck, and ViiV; S.K. has received educational grants for the Liverpool drug interaction website (www.covid‐druginteractions.org) from AbbVie, Gilead, MSD, Novartis, and Sobi. S.K. has also received speakers' honoraria from ViiV Healthcare, Gilead Sciences, and AbbVie; consultancy fees from ViiV Healthcare and Merck; and research funding from Gilead Sciences and ViiV Healthcare.
References
- 1. Hammond, J. et al. Oral nirmatrelvir for high‐risk, nonhospitalized adults with COVID‐19. N. Engl. J. Med. 386, 1397–1408 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boffito, M. , Back, D. & Gatell, J.M. Twenty years of boosting antiretroviral agents: where are we today? AIDS 29, 2229–2233 (2015). [DOI] [PubMed] [Google Scholar]
- 3. Paxlovid Summary of Product Information <Paxlovid150mg/100mgfilm‐coatedtablets‐SummaryofProductCharacteristics(SmPC)‐(emc)(medicines.org.uk)> Accessed February 1, 2022.
- 4. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization for Paxlovid <FACTSHEETFORHEALTHCAREPROVIDERS:EMERGENCYUSEAUTHORIZATIONFORPAXLOVID(fda.gov)> Accessed May 5, 2022.
- 5. Eichbaum, C. , Cortese, M. , Blank, A. , Burhenne, J. & Mikus, G. Concentration effect relationship of CYP3A inhibition by ritonavir in humans. Eur. J. Clin. Pharmacol. 69, 1795–1800 (2013). [DOI] [PubMed] [Google Scholar]
- 6. Aarnoutse, R.E. et al. Effect of low‐dose ritonavir (100 mg twice daily) on the activity of cytochrome P450 2D6 in healthy volunteers. Clin. Pharmacol. Ther. 78, 664–674 (2005). [DOI] [PubMed] [Google Scholar]
- 7. Marzolini, C. , Gibbons, S. , Khoo, S. & Back, D. Cobicistat versus ritonavir boosting and differences in the drug‐drug interaction profiles with co‐medications. J. Antimicrob. Chemother. 71, 1755–1758 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Moss, D.M. , Siccardi, M. & Marzolini, C. Mechanisms of drug interactions II: transport proteins. In Drug Interactions in Infectious Diseases: Mechanisms and Models of Drug Interactions (eds. Pai, M. , Kiser, J.J. , Gubbins, P.O. & Rodvold, K.A. ) 49–85 (Humana Press, New York, 2018). [Google Scholar]
- 9. Katzenmaier, S. , Markert, C. , Riedel, K.D. , Burhenne, J. , Haefeli, W.E. & Mikus, G. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin. Pharmacol. Ther. 90, 666–673 (2011). [DOI] [PubMed] [Google Scholar]
- 10. Ramsden, D. et al. Perspectives from the innovation and quality consortium induction working group on factors impacting clinical drug‐drug interactions resulting from induction: focus on cytochrome 3A substrates. Drug Metab. Dispos. 47, 1206–1221 (2019). [DOI] [PubMed] [Google Scholar]
- 11. Stader, F. et al. Stopping lopinavir/ritonavir in COVID‐19 patients: duration of the drug interacting effect. J. Antimicrob. Chemother. 75, 3084–3086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Washington, C.B. et al. Effect of simultaneous versus staggered dosing on pharmacokinetic interactions of protease inhibitors. Clin. Pharmacol. Ther. 73, 406–416 (2003). [DOI] [PubMed] [Google Scholar]
- 13. Salerno, D.M. et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID‐19 in solid organ transplant recipients. Am. J. Transplant (2022). 10.1111/ajt.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kinvig H. et al. High dose rifampicin for the treatment of leprosy in HIV patients taking dolutegravir. Virtual Conference on Retroviruses And Opportunistic Infections, Boston, Massachusetts, March 8–11, 2020. Abstract 450.
- 15. Sekar, V.J. , De Pauw, M. , Marien, K. , Peeters, M. , Lefebvre, E. & Hoetelmans, R.M.W. Pharmacokinetic interaction between TMC114/r and efavirenz in healthy volunteers. Antivir. Ther. 12, 509–514 (2007). [PubMed] [Google Scholar]
- 16. Stader, F. et al. Analysis of clinical drug‐drug interaction data to predict magnitudes of uncharacterized interactions between antiretroviral drugs and comedications. Antimicrob. Agents Chemother 62, e00717–e00718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Godinho, R. , Bugnon, S. , Gracin, T. & Tataw, J. Severe rhabdomyolysis‐induced acute kidney injury following concomitant use of Genvoya (EVG/COBI/FTC/TAF) and simvastatin; a case report. BMC Nephrol 20, 69(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutierrez, M.M. , Rosenberg, J. & Abramowitz, W. An evaluation of the potential for pharmacokinetic interaction between escitalopram and the cytochrome P450 3A4 inhibitor ritonavir. Clin. Ther. 25, 1200–1210 (2003). [DOI] [PubMed] [Google Scholar]
- 19. Mertz, D. , Battegay, M. , Marzolini, C. & Mayr, M. Drug‐drug interaction in a kidney transplant recipient receiving HIV salvage therapy and tacrolimus. Am. J. Kidney Dis. 54, e1–e4 (2009). [DOI] [PubMed] [Google Scholar]
- 20. Lange, N.W. et al. Nirmatrelvir/ritonavir use: managing clinically significant drug‐drug interactions with transplant immunosuppressants. Am. J. Transplant (2022). 10.1111/ajt.16955. [DOI] [PubMed] [Google Scholar]
- 21. Seden, K. et al. Development of an evidence evaluation and synthesis system for drug‐drug interactions and its application to a systemic review of HIV and malaria co‐infection. PLoS One 12, e0173509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodge, C. et al. Drug interactions: a review of the unseen danger of experimental COVID‐19 therapies. J. Antimicrob. Chemother. 75, 3417–3424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. University of Liverpool COVID‐19 Drug interaction database <https://www.covid19‐druginteractions.org>. Accessed March 24, 2022.
- 24. Marzolini, C. et al. Prescribing nirmatrelvir‐ritonavir: how to recognize and manage drug‐drug interactions. Ann. Intern. Med. 175, 744–746 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nieminen, T.H. et al. Oxycodone concentrations are greatly increased by the concomitant use of ritonavir or lopinavir/ritonavir. Eur. J. Clin. Pharmacol. 66, 977–985 (2010). [DOI] [PubMed] [Google Scholar]
- 26. Pollak, P.T. , Bouillon, T. & Shafer, S.L. Population pharmacokinetics of long‐term oral amiodarone. Clin. Pharmacol. Ther. 67, 642–652 (2000). [DOI] [PubMed] [Google Scholar]
- 27. Kaminsky, L.S. & Zhang, Z.Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 73, 67–74 (1997). [DOI] [PubMed] [Google Scholar]
- 28. Bonora, S. et al. Drug interactions between warfarin and efavirenz or lopinavir/ritonavir in clinical treatment. Clin. Infect. Dis. 46, 146–147 (2008). [DOI] [PubMed] [Google Scholar]
- 29. Liedtke, M.D. , Vanguri, A. & Rathbun, R.C. A probable interaction between warfarin and the antiretroviral TRIO study regimen. Ann. Pharmacother. 46, e34 (2012). [DOI] [PubMed] [Google Scholar]
- 30. Egan, G. , Hughes, C.A. & Ackman, M.L. Drug interactions between antiplatelet or novel oral anticoagulant medications and antiretroviral medications. Ann. Pharmacother. 48, 734–740 (2014). [DOI] [PubMed] [Google Scholar]
- 31. Mueck, W. , Kubitza, D. & Becka, M. Co‐administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br. J. Clin. Pharmacol. 76, 455–466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nisly, S.A. & Stevens, B.N. Ritonavir or cobicistat‐boosted antiretroviral therapy and direct oral anticoagulants: a case for apixaban. Int. J. STD AIDS 30, 718–722 (2019). [DOI] [PubMed] [Google Scholar]
- 33. Lomakina, V. , Sozio, S.J. & Tekle, J. Use of apixaban in atrial fibrillation with ritonavir‐boosted antiretroviral therapy: a case report. J. Pharm. Pract (2022). 10.1177/08971900221074938. [DOI] [PubMed] [Google Scholar]
- 34. Lingineni, K. et al. Quantitative benefit‐risk assessment of P‐gp mediated drug‐drug interactions (DDIs) of dabigatran co‐administered with pharmacokinetic enhancers in patients with renal impairment. Clin. Pharmacol. Ther. 109, 193–200 (2021). [DOI] [PubMed] [Google Scholar]
- 35. Marsousi, N. et al. Impact of boosted antiretroviral therapy on the pharmacokinetics and efficacy of clopidogrel and prasugrel active metabolites. Clin. Pharmacokinet. 57, 1347–1354 (2018). [DOI] [PubMed] [Google Scholar]
- 36. Bravo, I. , Alvarez, H. , Marino, A. , Clotet, B. & Molto, J. Recurrent coronary disease in HIV‐infected patients: role of drug‐drug interactions. Br. J. Clin. Pharmacol. 84, 1617–1619 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260 (2018). [DOI] [PubMed] [Google Scholar]
- 38. Siccardi, M. et al. Prediction of drug‐drug interactions between various antidepressants and efavirenz or boosted protease inhibitors using a physiologically based pharmacokinetic modelling approach. Clin. Pharmacokinet 52, 583–592 (2013). [DOI] [PubMed] [Google Scholar]
- 39. Zahner, C. et al. No clinically relevant interactions of St John's Wort extract Ze 117 low in hyperforin with cytochrome P450 enzymes and P‐glycoprotein. Clin. Pharmacol. Ther 106, 432–440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel, C.G. , Li, L. , Girgis, S. , Kornhauser, D.M. , Frevert, E.U. & Boulton, D.W. Two‐way pharmacokinetic interaction studies between saxagliptin and cytochrome P450 substrates or inhibitors: simvastatin, diltiazem extended‐release, and ketoconazole. Clin. Pharmacol. 3, 13–25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Courlet, P. et al. Population pharmacokinetic modelling to quantify the magnitude of drug‐drug interactions between amlodipine and antiretroviral drugs. Eur. J. Clin. Pharmacol. 77, 979–987 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hussaarts, K.G.A.M. , Veerman, G.D.M. , Jansman, F.G.A. , van Gelder, T. , Mathijssen, R.H.J. & van Leeuwen, R.W.F. Clinically relevant drug interactions with multikinase inhibitors: a review. Ther. Adv. Med. Oncol. 11, 1758835918818347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kingston Health Sciences Centre . Management of nirmatrelvir/ritonavir (Paxlovid) drug‐drug interactions in oncology <PaxlovidDrug‐DrugInteractionsinOncology(antimicrobialstewardship.com)>. Accessed May 25, 2022.
- 44. Ontario COVID‐19 drugs and biologics clinical practice guidelines Working Group on behalf of the Ontario COVID‐19 science advisory table and University of Waterloo School of Pharmacy <Nirmatrelvir/Ritonavir(Paxlovid):WhatPrescribersandPharmacistsNeedtoKnow‐OntarioCOVID‐19ScienceAdvisoryTable(covid19‐sciencetable.ca)>. Accessed March 24, 2022.
- 45. National Institutes of Health . COVID‐19 NIH treatment guidelines panel's statement on potential drug‐drug interactions between ritonavir‐boosted nirmatrelvir (Paxlovid) and concomitant medications <Ritonavir‐BoostedNirmatrelvir(Paxlovid)|COVID‐19TreatmentGuidelines(nih.gov)>. Accessed March 24, 2022.
- 46. Scarsi, K.K. , Darin, K.M. , Chappell, C.A. , Nitz, S.M. & Lamorde, M. Drug‐drug interactions, effectiveness, and safety of hormonal contraceptives in women living with HIV. Drug Saf. 39, 1053–1072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smolders, E.J. , Jansen, A.M.E. , Ter Horst, P.G.J. , Rockstroh, J. , Back, D.J. & Burger, D.M. Viral hepatitis C therapy: pharmacokinetic and pharmacodynamics considerations: a 2019 update. Clin. Pharmacokinet. 58, 1237–1263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feng, H.P. et al. Pharmacokinetic interactions between the hepatitis C virus inhibitors elbasvir and grazoprevir and HIV protease inhibitors ritonavir, atazanavir, lopinavir, and darunavir in healthy volunteers. Antimicrob. Agents Chemother 63, e02142–e02118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kosloski, M.P. et al. Drug‐drug interactions of glecaprevir and pibrentasvir coamdinistered with human immunodeficiency virus antiretrovirals. J. Infect. Dis. 76, 73–84 (2004). [DOI] [PubMed] [Google Scholar]
- 50. Kovarik, J.M. , Beyer, D. , Bizot, M.N. , Jiang, Q. , Shenouda, M. & Schmouder, R.L. Blood concentrations of everolimus are markedly increased by ketoconazole. J. Clin. Pharmacol. 45, 514–518 (2005). [DOI] [PubMed] [Google Scholar]
- 51. Thomas, P.P. , Manivannan, J. , John, G.T. & Jacob, C.K. Sirolimus and ketoconazole co‐prescription in renal transplant recipients. Transplantation 77, 474–475 (2004). [DOI] [PubMed] [Google Scholar]
- 52. Vogel, M. et al. Management of drug‐to‐drug interactions between cyclosporine A and the protease‐inhibitor lopinavir/ritonavir in liver‐transplanted HIV‐infected patients. Liver Transpl. 10, 939–944 (2004). [DOI] [PubMed] [Google Scholar]
- 53. Wang, A.X. , Koff, A. , Hao, D. , Tuznik, N.M. & Huang, Y. Effect of nirmatrelvir/ritonavir on calcineurin inhibitor levels: early experience in four SARS‐CoV‐2 infected kidney transplant recipients. Am. J. Transplant (2022). 10.1111/ajt.16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hermann, R. , Karlsson, M.O. , Novakovic, A.M. , Terranova, N. , Fluck, M. & Munafo, A. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin. Pharmacokinet. 58, 283–297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saberi, P. , Phengrasamy, T. & Nguyen, D.P. Inhaled corticosteroid use in HIV‐positive individuals taking protease inhibitors: a review of pharmacokinetics, case reports and clinical management. HIV Med. 14, 519–529 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


