Abstract
The cutaneous side effects of COVID‐19 vaccines are being studied and their immunogenicity is most likely linked to the pathophysiology of psoriasis. Although uncommon, several cases of exacerbation and new onset of psoriasis have been reported globally after vaccination. To contribute to the literature on this intriguing topic, we present three cases of de novo psoriasis in adult patients following COVID‐19 vaccination. Our observations and a literature review show that this occurrence is independent of the type and brand of vaccines.
Keywords: AZD1222 vaccine, BNT162b2 vaccine, COVID‐19 vaccination, mRNA‐1273 vaccine, new‐onset psoriasis
1. INTRODUCTION
Shortly after the initiation of the novel coronavirus 2019 (COVID‐19) mass vaccination programs, healthcare systems desire further information regarding the effect and safety of the vaccines outside of clinical trials. The cutaneous side effects and the course of psoriasis after COVID‐19 vaccination have been discussed in the literature. 1 , 2 Although there is little information on the effect and safety of COVID‐19 vaccines in patients with psoriasis, there are extensive reviews and guidelines on these topics. 1 While uncommon, several cases of exacerbation and new onset of psoriasis postvaccination have been reported worldwide. 3 To the best of our knowledge, the present report is the first case series of de novo psoriasis in adult non‐psoriasis patients following COVID‐19 vaccination in Vietnam. Our findings could make a significant contribution to the literature and potentially aid fine‐tuning of the vaccination programs by informing participants of possible dermatological risks.
1.1. Case report
1.1.1. Case 1
A 51‐year‐old man had a chief complaint of skin lesions, which he recently noticed. One week after the first dose of AZD1222 vaccine, more scaly red lesions appeared and spread to both lower legs. After the second dose of AZD1222 vaccine, more lesions appeared and spread to the lower legs, thighs, and scalp. At that time, he was diagnosed with atopic dermatitis and treated with antihistamine and topical corticosteroids. The patient's condition was partially resolved. One week after the third dose of AZD1222 vaccine, the patient developed more lesions. He had infrequently controlled hypertension, was a 35‐pack‐year smoker, and drank alcohol occasionally. Upon examination, blanchable erythematous demarcated papules and plaques with silvery‐white dry scaling were found on his scalp, legs, and hands. Some of his nails had onycholysis and subungual hyperkeratosis, but the joints were not involved (Figure 1A,B).
FIGURE 1.
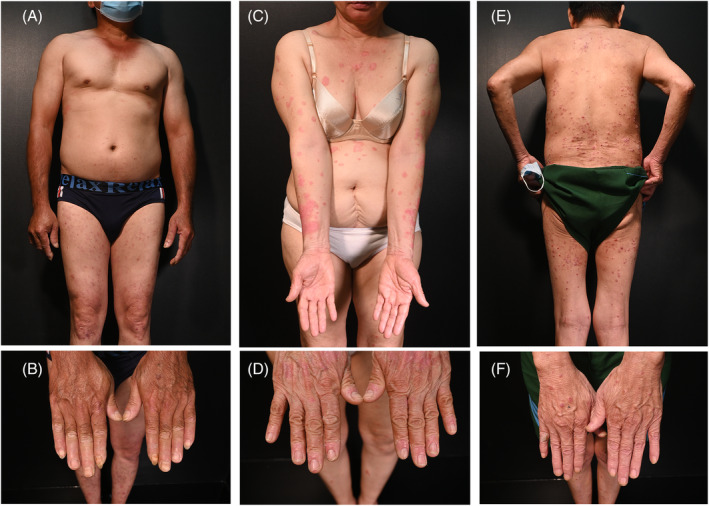
Patient 1: numerous small blanchable erythematous demarcated papules and plaques with silvery‐white dry scaling were found in both legs, and hands, with onycholysis and subungual hyperkeratosis in some nails (A, B). Patient 2: erythematous demarcated papules and plaques and scaling on trunk and extremities with mild onycholysis (C, D). Patient 3: well‐defined erythematous papules and plaques with white dry scaling over body without nail involvement (E, F).
The blood test results were normal. Skin biopsy revealed regular psoriasiform epidermal hyperplasia with loss of stratum granulosum and neutrophils in the upper layers of the stratum spinosum (spongiform pustule of Kogoj) (Figure S1). Based on these findings he was diagnosed with plaque psoriasis.
1.1.2. Case 2
A 68‐year‐old woman presented with a chief complaint of a skin eruption. She had hypertension, which was well controlled for 15 years but had no history of similar lesions previously. She received her first and second doses of the mRNA‐1273 vaccine, with only mild fever, pain, and swelling at the injection site. However, she had a mixed vaccine regimen, and a month after the third dose of the BNT162b2 vaccine, she noticed red and scaly lesions on her right wrist that rapidly spread to other sites. She reported no pruritus on the skin or pain in the joints (Figure 1C,D).
All blood test results were normal. Skin biopsy was consistent with psoriasis with dilated blood vessels, thinning of the suprapapillary layer, intermittent parakeratosis, and perivascular infiltration of lymphocytes (Figure S2 ).
1.1.3. Case 3
A 73‐year‐old man presented with the chief complaint of scaly red lesions on his skin. His medical history included COPD, hypertension, diabetes for more than 10 years, and SARS‐COV‐2 infection with no symptoms 4 months previously. One month after the first dose of the BNT162b2 vaccine, he noticed many itchy red and scaly lesions on his skin. On his scalp, trunk, and extremities, he had well‐defined blanchable erythematous papules and plaques with white dry scaling (Figure 1E,F). Joint pain was not noted. Blood tests revealed no abnormalities. Parakeratosis, orthokeratosis, and hyperkeratosis with elongated rete ridges surrounding dermal papillae were noted on histopathologic examination, along with dilated vessels in the dermal papillae and the collection of neutrophils in the stratum corneum (Munro's microabscess). Based on these findings he was diagnosed with guttate psoriasis (Figure S3).
2. DISCUSSION
Because psoriasis is an autoinflammatory skin disease, any change in the immune system might cause its manifestation in at‐risk people. However, the pathophysiology of new‐onset psoriasis following COVID‐19 vaccination remains unclear. The current understanding of this topic is based on vaccine immunogenicity against SAR‐CoV‐2 and the mechanism of de novo psoriasis development after other vaccinations. Regarding immunogenicity, mRNA and adenovector vaccines follow the same path of eliciting SARS‐CoV‐2 immunity after entry into dendritic cells in the lymph nodes. 4 Type I interferon and pro‐inflammatory cytokines and chemokines are then produced, causing naive T cells to differentiate into helper and cytotoxic T cells. T helper cells promote B‐cell differentiation into specific plasma cells that produce anti‐protein S antibodies. Furthermore, following a single dose of AZD1222, an increase in the production of TNF‐α and IFN‐γ, which are important cytokines in the pathophysiology of psoriasis, was observed on day 14. 5 After influenza, BCG, or tetanus‐diphtheria toxoid vaccination, production of IL‐6, a cytokine that upregulates Th17 cells, and development of Th17 cells, the key cells in psoriasis pathogenesis, increased. 6 Thus, the new onset of psoriasis after COVID‐19 vaccinations may be due to the immunogenicity of these vaccines, which upregulates cytokines IL‐6, TNF‐α, IFN‐γ, and Th17 cells.
Several cases of de novo guttate, plaque, erythrodermic, and pustular psoriasis have been observed globally following COVID‐19 immunization 3 , 7 , 8 , 9 , 10 (Table 1). In our report, guttate and plaque psoriasis was observed in one and two patient(s), respectively. Guttate psoriasis is often triggered by streptococcal or viral infections. New‐onset guttate psoriasis has been reported after vaccination with BNT162b2 and mRNA‐1273. 7 , 8 New‐onset plaque psoriasis has been reported after vaccination with mRNA vaccines and AZD1222. 3 , 9 Intriguingly, different vaccine regimens and brands were administered to our three patients. One patient received three doses of the AZD1222 vaccine, one had a BNT162b2 shot after two mRNA‐1273 doses, and the last patient had psoriasis onset after the first BNT162b2 vaccination.
TABLE 1.
Case studies of de novo psoriasis following COVID‐19 vaccination in literature
| Authors | Number of patient(s) a | Gender of patient(s) | Age of patient(s) | Vaccine regimen | Day(s) of onset | Psoriasis subtype(s) | Severity | History of COVID‐19 | Treatment(s) (response) |
|---|---|---|---|---|---|---|---|---|---|
| Wei et al. 3 | 1 | Male | 89 |
First dose: mRNA‐1273 Second dose: mRNA‐1273 |
24 after second dose | N/A | 60% BSA affected | No |
Ixekizumab acitretin 25 mg (resolved) |
| Lehmann et al. 7 | 1 | Female | 79 |
First dose: BNT162b2 |
10 after first dose | (mainly) guttate | N/A possibly mild | N/A |
calcipotriol/betamethasone ointment + UVB (N/A) |
| Song et al. 8 | 1 | Female | 23 | First dose: BNT162b2 | Two after first dose | Guttate | N/A; possibly mild | N/A | Topical calcipotriol/betamethasone (significantly improved) |
| Nagrani et al. 9 | 1 | Male | 65 |
First dose: AZD1222 b Second dose: AZD1222 |
10 after second dose | Plaque | 30% BSA | N/A | Apremilast, antihistamines and emollients (well‐responsed) |
| Elamin et al. 10 | 1 | Female | 66 | First dose: AZD1222 b | 21 after first dose | Pustular | Extensive, possibly moderate‐to‐severe | No |
Topical steroid, Acitretin 20 mg qd (resolved) |
| Our case reports | 3 |
Two males One female |
51, 68, and 73 | Three AZD1222 doses; one BNT162b2 dose; and mixed one dose of BNT162b2 after two doses of mRNA‐1273 |
7 days after the first AZD1222; 30 days after the first BNT162b2 dose; 30 days after the third BNT162b2 dose in a mixed regimen |
One guttate Two plaque |
Mild |
Yes: 1 No: 2 |
Topical calcipotriol/betamethasone antihistamines |
Included new‐onset psoriasis only.
Also known as ChAdOx1 nCoV‐19.
Various vaccine regimens have been observed worldwide prior to the onset of psoriasis. In most cases, new‐onset psoriasis appears approximately a month after the first or second dose of the vaccine. Lehmann et al. and Song et al. reported that psoriasis began 10 and 2 days after the first BNT162b2 dose, respectively. 7 , 8 Wei et al. and Nagrani et al. reported that psoriasis began 24 and 10 days after the second mRNA‐1273 and AZD1222 dose, respectively. 3 , 9 In two of our patients, the onset of psoriasis initiated 1 week and 1 month after receiving the first dose of AZD1222 and BNT162b2 vaccines, respectively. The onset in the other patient initiated 1 month after the third dose. We noted one patient who had been infected with SARS‐CoV‐2 long before the onset of psoriasis, which is similar to the case described by Wei et al. 3
Psoriasis can affect individuals of any age or sex. Men and women were included in this study. However, the reported age of onset of psoriasis postvaccination is high, ranging from 65 to 89 years, 3 , 7 , 9 , 10 except for one woman who was 23 when she developed psoriasis. 8 This is consistent with our findings of onset age between 51 and 73 years in all patients.
We performed skin biopsies to confirm the diagnosis of psoriasis. All patients in the reports were treated with a variety of anti‐psoriasis therapies, ranging from topical therapy alone to the combination of UVB, apremilast with antihistamine and emollients, and ixekizumab and acitretin. 3 , 7 , 8 , 9 , 10 Psoriasis resolved after a short period of treatment in most cases. For mild psoriasis, we used topical calcipotriol/betamethasone and antihistamines in all three patients. However, our study is limited by the lack of follow‐up.
In conclusion, based on our and worldwide cases that have been reported, we believe that new‐onset psoriasis after COVID‐19 immunization is a topic that should be further investigated. Psoriasis is a common dermatological condition that has a significant impact on the well‐being and quality of life of patients. More studies should investigate psoriasis and postvaccination skin reactions.
AUTHOR CONTRIBUTIONS
Tu Nguyen Anh Tran: data acquisition, manuscript drafting, and final approval of the version to be published.
Thuy Thi Phan Nguyen: data acquisition, manuscript drafting, and final approval of the version to be published.
Nguyen Nhat Pham: data acquisition, manuscript drafting, and final approval of the version to be published.
Nhi Thi Uyen Pham: data acquisition, manuscript drafting, and final approval of the version to be published.
Thao Thi Phuong Vu: data acquisition, manuscript drafting, and final approval of the version to be published.
Hao Trong Nguyen: manuscript drafting, critical revision for important intellectual content, and final approval of the version to be published.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICS STATEMENT
This study was approved by the Research Ethics Committee of the Ho Chi Minh City Hospital of Dermato‐Venereology. Informed consent was obtained from the patients for participation in the study and publication of the case report.
Supporting information
Figure S1 Parakeratosis (A), perivascular lymphocytic infiltration (B), neutrophils in the upper layers of the stratum spinosum (spongiform pustule of Kogoj) (C), and psoriasiform epidermal hyperplasia with loss of the stratum granulosum in overall (D) (patient 1).
Figure S2 Psoriasiform epidermal hyperplasia with elongated rete ridges (A), and features of other psoriasiform changes (parakeratosis, loss of the stratum granulosum, perivascular lymphocytic infiltration) (B‐D) (patient 2).
Figure S3 Parakeratosis, orthokeratosis and hyperkeratosis with elongated rete ridge surrounding dermal papillae (A), dilated vessels in dermal papillae and perivascular lymphocytic infiltration (B, C), and the collection of neutrophils in the stratum corneum (Munro's microabscess) (D) (patient 3).
ACKNOWLEDGMENTS
The authors thank Psoriasis Clinics, Ho Chi Minh City Hospital of Dermato‐Venereology, for their assistance in data collection. This article was not funded by any private, commercial, or not‐for‐profit entities.
Tran TNA, Nguyen TTP, Pham NN, Pham NTU, Vu TTP, Nguyen HT. New onset of psoriasis following COVID‐19 vaccination. Dermatologic Therapy. 2022;35(8):e15590. doi: 10.1111/dth.15590
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Niebel D, Novak N, Wilhelmi J, et al. Cutaneous adverse reactions to COVID‐19 vaccines: insights from an immuno‐dermatological perspective. Vaccine. 2021;9(9):944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun Q, Fathy R, McMahon DE, Freeman EE. COVID‐19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39(4):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei N, Kresch M, Elbogen E, Lebwohl M. New onset and exacerbation of psoriasis after COVID‐19 vaccination. JAAD Case Rep. 2022;19:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teijaro JR, Farber DL. COVID‐19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ewer KJ, Barrett JR, Belij‐Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV‐19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270. [DOI] [PubMed] [Google Scholar]
- 6. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lehmann M, Schorno P, Hunger RE, et al. New onset of mainly guttate psoriasis after COVID‐19 vaccination: a case report. J Eur Acad Dermatol Venereol. 2021;35(11):e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song WJ, Lim Y, Jo SJ. De novo guttate psoriasis following coronavirus disease 2019 vaccination. J Dermatol. 2022;49(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagrani P, Jindal R, Goyal D. Onset/flare of psoriasis following the ChAdOx1 nCoV‐19 Corona virus vaccine (Oxford‐AstraZeneca/Covishield): report of two cases. Dermatol Ther. 2021;34(5):e15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford‐AstraZeneca COVID‐19 vaccine. Clin Exp Dermatol. 2022. January;47(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Parakeratosis (A), perivascular lymphocytic infiltration (B), neutrophils in the upper layers of the stratum spinosum (spongiform pustule of Kogoj) (C), and psoriasiform epidermal hyperplasia with loss of the stratum granulosum in overall (D) (patient 1).
Figure S2 Psoriasiform epidermal hyperplasia with elongated rete ridges (A), and features of other psoriasiform changes (parakeratosis, loss of the stratum granulosum, perivascular lymphocytic infiltration) (B‐D) (patient 2).
Figure S3 Parakeratosis, orthokeratosis and hyperkeratosis with elongated rete ridge surrounding dermal papillae (A), dilated vessels in dermal papillae and perivascular lymphocytic infiltration (B, C), and the collection of neutrophils in the stratum corneum (Munro's microabscess) (D) (patient 3).
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


