Abstract
The development of carbon-based nanomaterials has extensively facilitated new discoveries in various fields. Carbon nanotube-based nanocomposites (CNT-based nanocomposites) have lately recognized as promising biomaterials for a wide range of biomedical applications due to their unique electronic, mechanical, and biological properties. Nanocomposite materials such as silver nanoparticles (AgNPs), polymers, biomolecules, enzymes, and peptides have been reported in many studies, possess a broad range of antibacterial activity when incorporated with carbon nanotubes (CNTs). It is crucial to understand the mechanism which governs the antimicrobial activity of these CNT-based nanocomposite materials, including the decoupling individual and synergistic effects on the cells. In this review, the interaction behavior between microorganisms and different types of CNT-based nanocomposites is summarized to understand the respective antimicrobial performance in different conditions. Besides, the current development stage of CNT-based nanocomposite materials, the technical challenges faced, and the exceptional prospect of implementing potential antimicrobial CNT-based nanocomposite materials are also discussed.
Keywords: Carbon nanotubes, Functionalization, Pathogens, Antimicrobial mechanisms, Toxicity
Introduction
Carbon is one of the most readily available elements in nature. 1 Over the past decades, carbon-based nanomaterials have attracted great attention from researchers and scientists due to their remarkable physicochemical characteristics. 2,3 There are a wide range of carbon nanostructure (CNS) materials have been discovered for various application, such as carbon nanotubes (CNTs), nano-diamond, fullerenes, carbon nano-onions, nanofibers, and others carbon-based nanomaterials. 3,4 Among them, CNT is one of the most extensively used materials, especially in biomedical field. 5,6 CNTs are characterized as hollow and concentric cylindrical structures formed by rolled graphene sheets with a remarkable high aspect ratio. 7,8 CNTs can be metallic and semi-conductive properties based on the rolling angle. 9 The classification of CNTs depends on the number of graphene sheets that roll upon their surfaces, such as single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). 10 The latter comprises multiple single-walled nanotubes that are clustered with each other inside the tubes. Among different types of CNTs, the antimicrobial activity of SWCNTs is higher due to its greater physicochemical properties. 11-14 Kang et al demonstrated the first report on the antimicrobial activity of purified SWCNTs, that the purified form of SWCNTs and MWCNTs showed significant impact on the integrity of bacterial membranes upon direct contact. 15 In addition, the morphology and metabolic activities were also compromised. 16 In their work, the antimicrobial effect of SWCNTs seemed to be stronger than MWCNTs, probably due to their small size which provides a larger surface area to facilitate the membrane perturbation. Besides, the oxidative stress plays an additional role in the CNTs’ antimicrobial mechanisms. 17 Haung et al investigated the mechanical effects that influenced the antimicrobial properties of CNTs, such as low wear rates, low friction coefficients, favorable tribological characteristics, and high corrosion resistance. 18 Based on the studies conducted by Chen et al, the SWCNTs played a significant role as “nano-darts” which penetrated bacterial cell walls, reduced membrane potential, released intracellular constituents (DNA and RNA), and ultimately disrupted the bacterial membrane. 19 More studies have suggested that MWCNTs displayed no mutagenic impact in microbial assays with S. typhimurium and Escherichia coli. 20 However, inhibition of microbial growth by CNTs depends on the concentration and treatment time. 21 On the other hand, the distinguishing characteristic of sp 2 carbon-based nanomaterials (including CNTs) exhibits exceptional electronic structure that causes semi-conductivity and pseudo metallic conductivity. Vecitis et al investigated this aspect and found that the metallic CNTs were demonstrated higher antimicrobial activity as compared to semi-conducting nanotubes. 22 Thus, the electronic effects also play an important role in the antimicrobial activity of CNTs. However, the photosensitization process may also activate the CNTs which causes the formation of reactive oxygen species (ROS) in bacterial cells. 23
Determining the dispersibility of the fibrous colloids is particularly necessary for CNTs. Unmodified CNTs are amphiphobic in nature and insoluble in most of the solvents. Therefore, the dispersion of CNTs may cause aggregation to occur that describes the interfacial surface area with pathogens. 17 There were a few studies discriminated between aggregated and dispersed CNTs, indicating the diverging of microbial toxicity based on different dispersibility of CNTs. 22
CNTs have been extensively used in a wide range of medical and pharmaceutical fields. 24-27 A large variety of nanocomposites, such as silver, enzymes, antimicrobial peptides (AMPs), and polymers are adsorbed on the surface of CNTs to increase the antimicrobial activity of nanotubes. 26,28,29 Up to date, a promising new method has been developed to resolve the antimicrobial resistance by combining bioactive molecules or antimicrobial drugs with CNTs and then developing new antimicrobial therapy options. 5,28,30 In addition, toxicological assessments of CNTs should be taken into consideration especially in the presence of catalytically active iron and other possible byproducts that were embedded in the nanotubes. 31
The covalently functional groups or molecules which are adsorbed on the surface of nanotubes significantly alter the microbial reactions, 32 which will be further discussed in this review. Besides, the previous studies on the interaction behavior of CNT-based-nanocomposites with microorganism, antimicrobial activity, the toxicity/biosafety profile of modified CNTs, the current research trend, and the development potential of CNTs will also be reviewed in this work. 33,34
The main challenge of using CNT-based-nanocomposites in antimicrobial research is that the raw CNTs are insoluble in any solvent due to the strong van der Waals interactions among nanotubes. In conjunction with their hydrophobic characteristic, CNTs do not disperse in solution but to form bundles or aggregates, as mentioned earlier. Undoubtedly, such hydrophobic characteristic and the intermolecular attractions between tubes should initially be overcome by any means. 35 The lack of solubility or dispersibility of CNTs, especially in water, will vastly restrict their use in biological and biomedical applications. On the other hand, it precludes the notion of derivatization of CNTs, which provides the opportunity to conjugate various bioactive molecules, such as therapeutic drugs, targeting ligands and proteins. 36 Hence, some promising and devising strategies are required to prevail over these limitations to pave the way of utilizing these organic materials as drug delivery systems.
Antimicrobial performance of pristine CNTs
CNTs are one of the competitive nanomaterials which have been extensively used in the development of antimicrobial surfaces. The antimicrobial activity of CNTs depends on various factors including their composition and surfaces such as length, size, number of graphene layers, and physical disposition (dispersion or aggregation). 17,19,37 Table 1 lists several studies on the antimicrobial properties of SWCNTs and MWCNTs against different microbial pathogenic strains.
Table 1. The antimicrobial performance of pristine carbon nanotubes in different studies .
| Types of CNTs | Synthesis method | Concentration | Species | Main findings | References |
| SWNTs | CO disproportionation | 5 µg/mL | E. coli | Releasing intracellular content due to irrecoverable outer membrane damage. | 15 |
| SWNTs | CO disproportionation | 5 µg/mL | E. coli | Microbial cells lost their cellular integrity. | 16 |
| MWNTs | CVD method | 5 µg/mL | E. coli | Many of the bacterial cells remain intact and preserve their outer membrane. | 17 |
| SWNTs and MWNTs | CVD method | 20 µg/mL, 50 µg/mL, 100 µg/mL | L. acidophilus, E. coli, B. adolescentis, E. faecalis, and S. aureus | The antimicrobial mechanism is associated with length-dependent wrapping and diameter-dependent piercing upon microbial cell membrane damage and the release of intracellular contents. | 19 |
| MWNTs | Nanocycle productions | 1.5 mg/L-1 – 100 mg/L-1 | E. coli | The MIC values of MWNTs were high, indicating low microbial toxicity. | 38 |
| MWNTs | - | - | E. coli, B. subtilis, and P. aeruginosa | The viability results demonstrated that the toxicity of MWNTs (2-log cell density reduction) against selected pathogens. | 39 |
| DWNTs and MWNTs | NE scientific productions | 20 µg/mL – 100 µg/mL | Staphylococcus aureus, P. aeruginosa, K. pneumoniae, and C. albicans | MWNTs demonstrated higher antimicrobial activity than DWNTs against selected pathogens. | 21 |
| MWNTs | Nanotech Labs productions | 20 mg/20 mL | P. fluorescens | The percentage of inactivated bacteria by MWNTs was recorded at 44%. It was observed that MWNTs showed a significant effect on the inhibition of microbial adhesion due to the electrochemical potential. | 40 |
| SWNTs | - | 5 µg/mL | Escherichia coli, and Bacillus subtilis | No obvious physical destruction was observed below 10 nN of applied force. | 41 |
| SWNT, DWNT, and MWNT | Electric arc discharge, and CCVD | 100 µg/mL | Staphylococcus aureus, P. aeruginosa, and C. albicans | Microbial death induced by the aggregation of CNTs that were trapped on the microbial cell surface. | 42 |
| SWNTs, and MWNTs | - | 0.2 mg/mL | E. coli | Laser-activated CNTs had the potential to control the growth of bacteria. | 43 |
Up to date, various mechanisms have been suggested to quantify the CNTs toxicity and their biosafety. In 2007, Kang et al reported for the first time that single-walled CNTs were showed strong antimicrobial activity, which caused cell membrane destruction via direct contact and thus, reducing the cell viability by 80%. 15 In 2008, another study on the gene expression analysis showed that the impairment of cell membrane is the main mechanism of CNT-biocidal. The authors found that the pathogens exposed to CNTs are exhibited oxidative stress, accompanied by the destruction of cell membranes and release of intracellular contents. 17 Nagai and Toyokuni also reported the occurrence of the impairment of cell membrane by direct piercing of the pathogen surface. 44 On the other hand, it has been reported that the length of CNTs plays a crucial role in their interactions with the cellular membrane, where longer tubes demonstrate lower toxicity to the pathogen. 4,16 In addition, Aslan et al observed that the toxicity of shorter SWCNTs is greater due to the higher density of open tube ends. 26 This observation has been supported by another study, 45 where nanotubes with smaller diameter tend to cause the destruction of underlined cell membrane via cellular surface interaction. Besides, the microbial death can be induced by the aggregation of CNTs that are trapped on the microbial cell surface. 41,42 While on the other hand, Arias et al found that CNTs with a larger diameter of 15-30 nm were interacted with pathogens mainly via their sidewalls. 46
Similarly, many studies have shown that SWCNTs are highly toxicity to the pathogen than MWCNTs and convincingly causing the destruction of the cell membrane of pathogen. 17,19 In the study carried out by Kang et al, the majority of E. coli bacterial cells were flattened after incubated with SWCNTs for one hour but remained intact when incubated with MWCNTs. 17 In addition, they also observed that E. coli exhibited higher level of stress-related genes in the presence of SWCNTs, as compared to MWCNTs.
In contrary to the studies mentioned above, Young et al proposed that the toxicity of MWCNTs is greater for bacteria as compared to SWCNTs. 47 Saleemi et al have recently studied on the antimicrobial effects of CNTs (DWCNTs and MWCNTs) against different microbial strains, such as Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Candida albicans. 21 It was reported in their study that non-covalently dispersed MWCNTs exhibited higher antimicrobial activity than DWCNTs.
Despite the inconsistent reports, CNTs still remain competitive among other nanomaterials in fighting against a broad range of microorganisms in view of the fact that their antimicrobial activity has been traced and confirmed on various microbial strains, including S. aureus, E. coli, Enterococcus faecalis, Lactobacillus acidophilus, and Bifidobacterium adolescentis. 15,17,19 However, their broad-spectrum antimicrobial properties against various types of pathogens need to be further investigated.
Antimicrobial properties of functionalized SWCNTs-based nanocomposites
The significant antimicrobial properties of SWCNTs should be highlighted as an effective antimicrobial agent to inhibit the growth of microorganisms on different biomedical surfaces. Their interaction behavior with different types of microorganisms should be further addressed. Therefore, this section will focus on the effect of functionalized SWCNTs with different nanocomposites to increase their antimicrobial ability towards various microbial strains, as summarized in Table 2.
Table 2. Overview on the antimicrobial activity of functionalized SWCNTs-based nanocomposites in different studies .
| Material blend | Concentration | Species | Main findings | References |
| f-SWNTs with functional groups (-OH, -COOH, -NH2) | 50-200 µg/mL | S. aureus, B. Subtilis, and S. typhimurium | SWNTs functionalized with -OH and -COOH functional group showed more microbial inhibition rate (7-log reduction) against selected pathogens, while SWNTs with -NH2 displayed antimicrobial activity only at high concentrations. | 46 |
| Silver-SWNTs functionalized with peptides (TP226, TP359, TP557) | 5 µg/mL | S. aureus | The viability of bacteria increased by 4-log in non-treated skin model, whereas treated skin with functionalized silver-SWNTs showed antimicrobial activity only 1-log reduction. | 28 |
| Functionalized SWNTs with DNA and lysozyme (LSZ) | ~25 mg/L | S. aureus, and M. lysodeikticus | Layer by layer coating of DNA- and LSZ-SWNTs displayed high antimicrobial activity (with 84% microbial reduction). | 29 |
| SWNTs incorporated inside poly(lactic-co-glycolic acid) | < 2% by weight | E. coli, and S. epidermidis | The metabolic activity of bacteria was considerably decreased (98%) with SWNTs-PLGA, while 15-20% reduction rate observed with pure PLGA. | 26 |
| SWNTs-polyvinyl-N-carbazole (PVK) nanocomposite | 3 wt.% | E. coli, and B. subtilis | SWNTs-PVK nanocomposite induced a higher rate of bacterial inactivation (90% for B. subtilis and 94% for E. coli) in the planktonic cells and showed a significant reduction of biofilm formation. | 48 |
| SWNTs assembled with poly(L-glutamic acid) (PGA) and poly(L-lysin)(PLL) (layer-by-layer) | < 2% by weight | E. coli, and S. epidermidis | SWNTs/PGA/PLL showed a higher rate of antimicrobial activity (90%) against selected pathogens than non-treated samples of PGA/PLL (with 20% reduction rate). | 26 |
| Oxidized SWNTs with poly(vinyl alcohol) (PVOH) nanocomposite | 0-10% (w/w) | Pseudomonas aeruginosa | The viability of cell deposited on the surface of O-SWNTs-PVOH gradually decreased with increasing in nanotubes loading. | 49 |
| SWNTs/porphyrin composite | 0.04 mg/mL | S. aureus | In the presence of visible light, SWNTs/porphyrin induced damage to the cell membrane. | 50 |
| Functionalized-SWNTs/ poly(ethylene glycol) (PEG) and poly(ε caprolactone) composites | 0.5-1.0 wt.% | P. aeruginosa, and S. aureus | The proliferation of tested bacteria inhibited by f-SWNT/copolymer complex to a lower extent as compared to pure polymer complex. | 51 |
| SWNTs bound with polyamide membranes | 0.1-0.2 mg/mL | E. coli | The complex of nanocomposite inactivated the microbial cells by 60% after 1 h of contact time. | 50 |
Functionalized with carboxyl, hydroxyl and amine groups
As mentioned earlier, there are different functionalization methods for CNTs, such as covalent and non-covalent methods. Notably, CNTs can be functionalized/modified with acid/carboxyl moieties for the formation of CNTs-bacterial aggregates to increase their interaction with pathogens. 46 Previous studies reported that the functionalization of CNTs facilitated their binding with microbial cells. 24,52 In their work, the effects of various surface functional groups of SWCNTs were studied, including -NH2, -COOH, and -OH on their microbial inhibitory effects against S. aureus, B. subtilis, and Salmonella typhimurium. They found that functionalized SWCNTs with cationic – NH2 group inhibited bacterial growth only at high concentrations, while the SWCNTs with anionic – COOH and neutral – OH groups demonstrated strong inhibitory effects (7-log reduction) against selected pathogens. The strong inhibitory effects mean that some cells or all cells in the cell population were inactivated after treatment with SWCNTs-COOH and SWCNTs-OH. These surface groups such as – COOH and – OH were derived directly from the surface of SWCNTs, whereas – NH2 group was modified with a long chain CH3 (CH2)16CH2-NH2. They suggested that direct contact with the SWCNTs is the likely mechanism causing bacterial cell death, the long carbon chain may affect the interactions of SWCNTs – NH2 group with microbial cells in such a way that cylindrical shape of SWCNTs may not be in close direct contact with microbial cell walls that probably account for the reduced inhibitory effects of SWCNTs – NH2. In addition, functionalized SWCNTs tend to promote bacterial interaction with nanotubes irrespective of the surface functional group and their inhibitory effects are presented in a selective manner. 46
SWCNTs coated with silver nanoparticles
Many studies have demonstrated the antimicrobial activity of silver nanoparticles (AgNPs) and other metal oxides together with their inhibitory effects on the infections. 24,53 Chaudhari et al observed that the antimicrobial properties of silver coated SWCNTs can be modified with AMPs against S. aureus applied in a skin model. 28 In their study, the proliferation of bacteria was considerably inhibited by 105 CFU/g of silver coated functionalized CNT after skin treatment. 28 In general, AgNPs have the tendency to bind and penetrate the bacterial cell membrane and therefore causing cell death by altering the permeability of membrane. Besides, the ROS may also be produced in the process. 54 Moreover, it has been proven that AMPs show antimicrobial effect on various fungi, bacteria, and viruses. 53 Hence, the synergistic effects of AgNPs with AMPs increase the toxicity of nanotubes and the findings can be useful for the development of novel antimicrobial therapies. 28 Chaudhary et al attached SWCNTs to AgNPs and bio-conjugated this approach to AMPs TP359 to evaluate the antimicrobial activity of SWCNTs-adsorbed AgNPs against S. aureus, Streptococcus pyogenes, Salmonella enterica serovar Typhimurium, and E. coli. 55 They found that the conjugation showed a strong synergistic antibacterial effect of TP359 with SWCNTs-adsorbed AgNPs. Another study conducted by Kumar et al reported the antibacterial potency of decorated SWCNTs with AgNPs in cotton fabrics against S. aureus and E. coli. 56 They observed that the fabrics coated with SWCNTs-AgNPs showed excellent antibacterial properties against selected pathogens. In addition, AgNPs on silica-coated SWCNTs substrate showed significantly inactivated the bacterial growth (E. coli) as compared to AgNPs on plasma-treated SWCNTs substrates that lose their hydrophilicity during AgNPs deposition. 57 In another study, silver-based biohybrids demonstrated antioxidant and antimicrobial properties against S. aureus, E. coli, and E. faecalis. 58 The silver-based biohybrids consisting of phytonanosilver, CNTs, and cholesterol-containing liposomes showed higher reduction rates of microbial growth and antioxidant activity.
However, Chang et al used a facile and simple one-step approach for the synthesis of CNTs and graphene oxide with AgNPs against E. coli and S. aureus. 59 They observed that the synthesized nanomaterials showed antibacterial activity, but the graphene oxide AgNPs exhibited highest disinfection property. The lipid peroxidation assay and antioxidant enzyme activities proved that the nanomaterials were able to induce O2 - oxidative stress on bacteria, thus affected the cell membrane integrity and ultimately caused cell death. Another study showed the antimicrobial properties of SWCNTs coated with Ag-doped TiO2 nanoparticles against E. coli and S. aureus. 60 The results demonstrated that synthesized nanocomposites have a strong antibacterial activity against both types of bacteria, while S. aureus appeared to be less susceptible to the nanocomposite samples than E. coli under illumination by UV light. Park et al synthesized pegylated single walled carbon nanotubes (pSWCNTs) coated AgNPs with enhanced antibacterial properties and evaluated their effects on foodborne pathogenic bacteria. 61 They found a significant reduction in proteins associated with bacterial biofilm formation, quorum sensing and maintenance of cellular architecture, and cell motility in surviving foodborne pathogen. Moreover, Singh et al prepared a hybrid SWCNTs/Ag/PPy based nanocomposite by using a facile and cost-effective one-pot synthesis technique. 62 The prepared nanocomposites have the ability to inhibit the growth of selected bacterial strains such as S. aureus, P. aeruginosa, E. coli and B. cereus completely within 24 h. Zhu et al proposed a new antimicrobial nanoplatform of mesoporous silica-coated and AgNPs-coated SWCNTs developed by a N-[3-(trimethoxysilyl)propyl]ethylene diamine (TSD)-mediated approach (SWCNTs@mSiO2-TSD@Ag). 63 In this system, they compared commercial AgNPs and TSD modified mesoporous coated SWCNTs and found that the nanosystem of SWCNTs@mSiO2-TSD@Ag showed a strong antimicrobial activity against multi-drug resistant bacterial strains by damaging the cell membrane of bacteria and eventually a quick release of Ag ions. Yun et al revealed the antibacterial activity of CNTs-Ag and GO-Ag against both gram-positive and gram-negative pathogens. 64 They observed that antimicrobial activity of CNTs-Ag was higher as compared to GO-Ag nanocomposites that may be due to the excellent dispersion of AgNPs into the CNTs. Another study showed that carbon-Ag nanocomplex prevented the microbial growth against methicillin-resistant S. aureus, K. pneumoniae, Acinetobacter baumannii, and Burkholderia cepacian. 65 These nanocomposites can also be used to prevent the proliferation of bio-defense pathogen such as Yersinia pestis.
Immobilization of enzyme with SWCNTs
CNTs can also be modified with natural enzymes, such as lysozyme (LSZ) to enhance their toxicity towards different bacterial species including S. aureus, and Micrococcus lysodeikticus as investigated. 29 The antimicrobial activity of LSZ and its mechanisms comprising of cell wall lysis via hydrolyzing the beta 1, 4 linkages between N-acetylglucosamine and N-acetylmuramic acid on peptidoglycan have been also previously described. 24,66
SWCNTs associated with polymers
Biomaterials that could inactivate the microbial cells are needed to reduce the infections associated with medical devices. The chemical modifications of CNTs improve their dissolution properties and chemical compatibility, while functionalization with polymer enhances the dispersibility and solubility of CNTs as well as increases the interfacial interaction to polymeric matrices in their composites. For instance, SWCNTs in the form of deposited aggregates and membrane coatings have been demonstrated to be highly toxic to the microbial cells. Their ease of modification and chemical stability render SWCNTs attractive nanomaterials to the antimicrobial biomaterials. Moreover, SWCNTs in their pure form are expensive and only provide a limited range of material properties, thus they are unlikely to develop as ideal antimicrobial materials. However, SWCNTs modified with polymers could enhance the antimicrobial property and also provide a wide range of mechanical, degradation, and structural properties.
Aslan et al made SWCNTs nanocomposite with poly (lactic-co-glycolic acid) (PLGA) matrix and investigated their antimicrobial activity against Staphylococcus epidermidis and E. coli. 26 They reported that SWCNTs-PLGA had reduced the bacterial viability with a 98% cell reduction as compared to pure PLGA. The metabolic activity of the bacteria was significantly reduced as reported. 26 Moreover, the SWCNTs association with polyvinyl-N-carbazole caused a higher inactivation rate of planktonic cells (90% for B. subtilis and 94% for E.coli) and reduced their biofilm formation. 48 Similarly, nanocomposite prepared by SWCNTs associated with poly(L-glutamic acid) and poly(L-lysine) resulted in high inactivation rate of E. coli and S. epidermidis up to 90%. 67 Goodwin et al synthesized a nanocomposite namely SWCNTs-poly(vinyl alcohol) and investigated its antimicrobial activity against P. aeruginosa. 49 The bacterial viability gradually reduced with increasing concentrations of SWCNTs. 49 Sah et al reported that the formation of SWCNTs nanocomposite with photosensitive molecules (porphyrin) exhibited a sufficient antimicrobial effect against S. aureus. 50 The bacterial cells were treated with porphyrin appended SWCNTs in the presence of visible light using tungsten-halogen lamp. They observed that it formed the short lived first excited state (1PS) after absorption of light by porphyrin and the first excited singlet state encounters the intersystem crossing, resulting in a long-lived excited triple state which is primarily responsible for various chemical reactions. The photochemical reaction initiated by the transfer of electrons from the triple excited state (3PS) to the SWCNTs that inhibits the electron-hole recombination and ultimately transfer the electron to the ambient molecular oxygen to form ROS. The formation of ROS causes destruction of bacterial cell wall that leads to the bacterial cell death. 50 Furthermore, SWCNTs which were covalently bound with polyamide membranes had successfully inactivated 66% of bacteria and caused a delay in membrane biofouling. 68 In contrast, previous studies reported that non-ionic surfactant and hydrophilic polymers were suitable materials to adhere the CNTs surface which to be applied in various biomedical applications. For instance, poly(ethylene glycol) (PEG) is the most effective coating agents due to its high hydrophilicity. Cajero-Zul et al used a linear and branched PEGs attached to the surface of CNTs were assessed as effective nanosystems to be used as a new medium. 51 They observed that bacteria exposed to SWCNTs-copolymer of star-shaped poly (ethylene glycol) (PEG) and poly(ε caprolactone) (PCL) did not demonstrate antimicrobial activity, but the thermal and mechanical properties of nanocomposites were better than the ones of their polymeric matrix. The star-shaped PCL-PEG copolymer structure was assessed by using different techniques, such as FTIR, GPC, 1H-NMR, and 13C-NMR spectroscopies. Moreover, the molecular structure of star-shaped copolymer enables the poly(ethylene glycol) (PEG) chains be exposed to the bacterial action and prevented their growth. 51 In view of that, certain SWCNT-nanocomposites show significant antimicrobial activity, while some others display a combination of antimicrobial properties.
Antimicrobial properties of functionalized MWCNTs nanocomposites
MWCNTs have been widely studied and used in many sectors due to their unique physicochemical properties and antimicrobial potential. MWCNTs functionalized with various materials are extensively studied and implemented to produce effective antimicrobial surfaces. 69,70 Table 3 summarized the previous studies which were conducted with respect to MWCNTs biocidal effects and their interaction with a broad range of microorganisms.
Table 3. Overview on the antimicrobial activity of functionalized MWCNTs-based nanocomposites in different studies .
| Material blend | Concentration | Species | Main findings | References |
| 50-200 µg/mL | S. aureus, B. subtilis, and S. typhimurium | MWNTs functionalized with -OH and -COOH functional group did not significantly induce antimicrobial activity on selected pathogens. | 46 | |
| 25 µg/mL | E. coli, B. subtilis, and S. aureus | MWNTs-COOH inactivated the bacterial cells by 30% for B. subtilis, 40% for E. coli, and 50% for S. aureus, respectively. | 71 | |
| 20 μg/mL | S. aureus, E. coli, and P. aeruginosa | MWNTs-COOH inactivated the bacterial cells by 26.9% for P. aeruginosa, 34.1% for E. coli, and 22.8% for S. aureus, respectively. | 72 | |
| f-MWNTs with functional groups (-OH, -COOH, -NH2) | 20 mg/20 mL | S. aureus, E. coli, and P. aeruginosa | MWNTs-COOH inactivated the bacterial cells by 26.8 ± 1.1 for P. aeruginosa, 20 ± 0.8 for E. coli, and 14.7 ± 0.5 for S. aureus, respectively. | 73 |
| 20 µg/mL, 50 µg/mL, 100 µg/mL | E. coli, S. aureus, E. faecalis, L. acidophilus, and B. adolescentis | MWNTs-COOH and MWNTs-OH induced dose-dependent microbial inhibition against selected pathogens. | 21 | |
| 1000 µg/mL | V. parahaemolyticus | Antimicrobial activity of functionalized-MWNTs was time-dependent. Functionalized nanotubes that did not pierce into the cell membrane, rather wrapped around the surface of the pathogen. | 74 | |
| 0–100 mg/mL | Group A Streptococcus | Carboxylated-MWNTs functionalized with antibodies may have the potential to mitigate the bacterial infections of soft tissue. | 75 | |
| 20 µg/mL – 100 µg/mL | Staphylococcus aureus, P. aeruginosa, K. pneumoniae, and C. albicans | Microbial growth was inhibited by non-covalently dispersed CNTs and relied heavily on the treatment time and concentration. MWNTs demonstrated higher antimicrobial effect on selected pathogens. | 21 | |
| Surfactant- functionalized MWNTs with sodium dodecylbenzene sulfonate (SDBS), sodium cholate (SC), sodium dodecyl sulfate (SDS), triton X-100 (TX-100), dodecyltrimethylammonium bromide (DTAB), cetyltrimethylammonium bromide (CTAB), and polyvinylpyrrolidone (PVP) | 1.0, 0.5, 0.25 and 0.125 mg/mL | S. mutans | Functionalized-MWNTs caused cell membrane rupture via direct contact. | 76 |
| 0.1, 0.5, 1 mg/mL | E. coli | Functionalized-MWNTs penetrated the bacterial cell membrane due to electrostatic forces between bacterial membrane and surfactant. | 77 | |
| AgNPs-coated MWNTs | 2-30 wt% | E. coli | The cell membrane of bacteria damaged via direct contact. | 78 |
| f-MWNTs with lysine | 0.01875 to 0.6 mg/mL | S. aureus, E. coli, S. agalactiae, S. typhimurium, S. dysgalactiae, and K. pneumoniae | Electrostatic adsorption presented between the bacterial membrane and positive charges lysine groups on MWNTs. | 79 |
| MWNTs functionalized with amphiphilic dendrimer poly(propyleneimine) | 25 µg/mL | E. coli, B. subtilis, and S. aureus | MWNTs-nanocomposite inactivated the bacterial cells by 96.5% for S. aureus, 96.6% for B. subtilis, and 87% for E. coli, respectively. | 71 |
| MWNTs functionalized with aromatic dendrimer polyamide | 20 μg/mL | S. aureus, E. coli, and P. aeruginosa | MWNTs-nanocomposite inactivated the bacterial cells by 35.5% for S. aureus, 65.2% for P. aeruginosa, and 72.6% for E. coli, respectively. | 72 |
| Poly(amidoamine)-grafted MWNTs | 20 mg/20 mL | S. aureus, E. coli, and P. aeruginosa | MWNTs-nanocomposite complex inactivated the bacterial cells by 60 ± 1.8% for P. aeruginosa, 34.1 ± 1.2% for E. coli, and 22.8 ± 0.9% for S. aureus, respectively. | 73 |
| Oxidized MWNTs/poly(vinyl alcohol) nanocomposite | 0-10% (w/w) | P. aeruginosa | MWNTs-poly(vinyl alcohol) was able to reduce the viability of bacteria with increasing concentrations of nanotubes. | 49 |
| MWNTs-chitosan hydrogels | 25, 50, 100 mg/40 mL | S. aureus, E. coli, and C. tropicalis | MWNTs-chitosan hydrogels exhibited higher antimicrobial activity against S. aureus and C. tropicalis than E. coli. | 80 |
| 0.01%, 0.1% and 0.2% (w/w) | E. coli, S. pneumoniae, S. racemosum, C. albicans, P. aeruginosa, E. coli, G. candidum, and A. fumigatus | MWNTs nanocomposite showed strong microbial inhibition rate against Gram-positive bacteria than Gram-negative bacteria. | 81 |
MWCNTs attached with functional groups
The attachment of functional groups to the surface of CNTs is to prevent desorption processes and unwanted absorption of the molecules from the biological medium. 82-86 The efficacy of microbial growth inhibition depends on the adsorption rate of various functional groups on the surface of CNTs. Pasquini et al assessed the association between functional group attachment to the surface of CNTs and microbial cytotoxicity. 32 They further corelated the toxicity with physicochemical properties and functionalized SWCNTs agglomeration state, and reported that no direct correlation was identified between the bacterial cytotoxicity and thermal, physicochemical, and structural properties of f-SWCNTs. The aggregation of nanoparticle was superior to the individual chemical and physical properties of functional groups when evaluating the f-SWCNTs cytotoxicity. 32 Moreover, MWCNTs functionalized with surface functional group (-COOH) have significantly reduced the viability of bacteria by 30% for B. subtilis, 27% for P. aeruginosa, 20~40% for E. coli, and 15~50% for S. aureus. 71-73 Chen et al showed that MWCNTs with functional groups (-OH, -COOH) demonstrated a significant dose-dependent antimicrobial effect on pathogens, such as E. coli, S. aureus, E. faecalis, L. acidophilus, and B. adolescentis. 19 Ding et al were also observed the same effect on Vibrio parahaemolyticus. 87 Arias et al reported that MWCNTs functionalized with -COOH, -NH2, and -OH did not induce significant antimicrobial activity as compared to SWCNTs. 46 The antimicrobial activity of non-covalently dispersed CNTs (DWCNTs and MWCNTs) against S. aureus, K. pneumoniae, P. aeruginosa, and C. albicans has been extensively studied. 21 In their findings, the microbial growth which was prevented by non-covalently dispersed CNTs relied heavily on the treatment time and concentration. The functionalized MWCNTs with a compound named ethanolamine, were showed suppression of the microorganisms growth as compared to pristine MWCNTs. 88 Another study showed that MWCNTs modified with oxygen groups could increase the antimicrobial properties. 89
MWCNTs coated with silver nanoparticles
Like SWCNTs, silver-coated-MWCNTs displayed remarkable antimicrobial performance. The data showed that silver/MWCNTs complex inhibited the bacterial growth by 93.7~99% for S. epidermidis and E. coli, 56.7% for S. aureus, 100% for Sphingomonas spp. and Methylobacterium spp. and 69.7% for P. aeruginosa. 73,90,91 The amphiphilic dendrimers poly(propyleneimine) formed a complex with silver-coated-MWCNTs that inactivated the bacteria by percentage of > 90% for S. aureus, B. subtilis, and E. coli. 71 Similarly, polymer colloids immobilization with silver/MWCNTs complex exhibited strong antimicrobial effect on S. aureus and E. coli. 92 However, immobilization of silver sulfide (Ag2S) quantum dots with poly(amidoamine)-grafted MWCNTs has demonstrated microbial growth inhibition by 55.7% for S. aureus, 97.8% for E. coli, and 78.5% for P. aeruginosa. Besides, Ag2S-MWCNTs showed better antimicrobial activity as compared to cadmium sulfide quantum dots coated-MWCNTs. 73
MWCNTs blended with noble metals
For more promising results, MWCNTs were also blended with other noble metals, such as copper nanoparticles, to reduce the viability of bacteria by 75%. 93 Likewise, bacteria (E. coli) treated with zinc oxide-coated-MWCNTs showed strong antimicrobial activity. 94 A nanocomposite complex comprises of MWCNTs, titanium, and gold have demonstrated significant microbial growth inhibition against B. subtilis, K. pneumoniae, S. aureus, C. albicans, Streptococcus pneumoniae, Proteus vulgaris, and Shigella dysenteriae. 95 Besides, titanium alloy-coated-MWCNTs blended with rifampicin were able to inhibit the formation of biofilm for up to 5 days. 27
Immobilized enzyme onto MWCNTs
Some enzymes like chloroperoxidase (CPO) and laccase were immobilized on the surface of MWCNTs to reduce the viability of bacteria by 99% for S. aureus and E. coli. The laccase immobilized with MWCNTs inhibited the microbial growth and spore formation for B. cereus and B. anthracis by > 99%. 96 In order to understand their bactericidal mechanism, CPO catalyzed the oxidation of chloride into HOCl by H2O2 in the acidic conditions. The rapidly produced HOCl responds to H2O2 to provide singlet oxygen. Both singlet oxygen and HOCl are strong oxidants and have a wide range of antibacterial activity that may be exploited to inhibit, control, or reduce microbial growth. Moreover, the solution-phased CPO catalysis was very effective against E. coli and S. aureus. 96 In contrast, the main antimicrobial agent in the laccase + methyl syringate (MS) system is the hydroxyl radical, which may be produced in different ways. For example, one of the mechanisms is called Haber–Weiss reaction that produces hydroxyl radical from H2O2 and superoxide radical. Once superoxide radical is produced by the one-electron MS radical transfer to O2, it may efficiently undergo dismutation to H2O2 that causes the destruction of bacterial cells. 96
Polymers adsorbed onto MWCNTs
The antimicrobial activity has also been observed for MWCNTs combined with different polymers. Murugan et al studied the antimicrobial activity of MWCNTs modified with dendrimer, such as amphiphilic poly(propyleneimine) and found that the microbial growth was inhibited by 87% for E. coli, 96.6% for B. subtilis, and 96.5% for S. aureus. 71 The MWCNTs prepared by Neelgund et al with aromatic polyamide dendrimer exhibited a good antimicrobial effect on P. aeruginosa (65.2%) and E. coli (72.6%). 72 In contrary, MWCNTs functionalized with poly(amidoamine) were consistently inhibited all selected bacterial growth. 73 The antimicrobial activity of nanocomposites can be enhanced with increasing concentrations of MWCNTs. This is proven by Goodwin et al where MWCNT-poly (vinyl alcohol) has successfully reduced the viability of bacteria with increasing concentrations of nanotubes. 49
MWCNTs-based hydrogels nanocomposite
Currently, the antimicrobial activity of MWCNTs-based chitosan hydrogels has been extensively studied due to the physiological nature of the hydrogel-based materials. Interestingly, chitosan has also been previously used as an antimicrobial agent in many studies. For instance, a strong antimicrobial activity of MWCNTs-based chitosan hydrogels against S. aureus, E. coli, and Candida tropicalis was observed. 80 Mohammad et al also reported that MWCNTs-based chitosan hydrogel exhibited a wide range of antimicrobial activity. 81
The antimicrobial mechanisms of CNTs
Various antimicrobial mechanisms have been proposed in previous literature, with one of the examples shown in Figure 1: (1) attachment of CNTs on the microbial cell surface to promote the transmembrane electron transfer and induce cell wall and membrane damage; (2) protein dysfunction and DNA damage when CNTs penetrating bacterial cells; (3) formation of secondary products, such as ROS. 97
Figure 1.
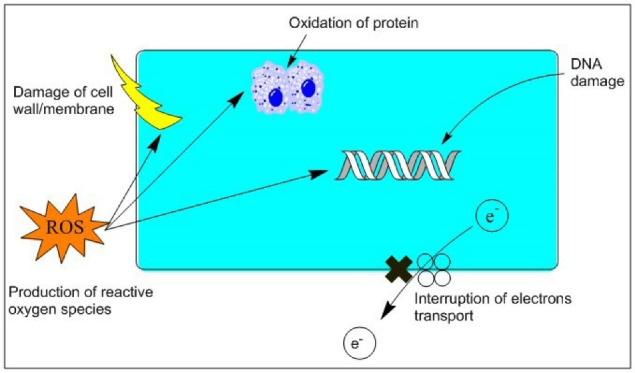
Antimicrobial mechanism of CNTs (Reprinted with permission from Li et al; Copyright, Elsevier).
Many studies reported that the destruction of pathogens cell membrane causes the leakage of intracellular contents and then followed by the death of microbial cell. For instance, Kang et al reported the first direct evidence that bacterial cell membrane damage occurred due to the direct contact between SWCNTs and pathogen, resulted in the leakage of intracellular contents such as DNA, RNA, and protein. 15 Few studies observed that 60 minutes contact time between bacteria and SWCNTs was sufficient to destroy the membrane, whereas others demonstrated that a longer time (up to days) was needed to obtain the same results. 15-17,46,98 Arias et al studied the physical contacts between microbial cells and aggregated SWCNTs using scanning electron microscopy (SEM) and reported the side walls of SWCNTs interacted with the Salmonella cells. 46 The mechanism that describes the death of bacterial cells often starts from the destruction of cell membranes and then followed by the discharge of intracellular materials. The adherence of CNTs alters the cellular structure, permeability, and proton motive force of the cellular membrane. Some studies have observed that CNTs tend to distort the cell morphology and the cellular membrane integrity when brought to contact with bacterial cells. Kang et al studied the loss of bacterial (E. coli) cellular integrity by SEM and confirmed the cytoplasmic contents efflux by measuring the concentration of RNA and DNA. 17 With the presence of SWCNTs, a two-fold increase of RNA and five-fold increase of plasmid DNA were found in the solutions, indicating the severe damage in cellular membrane integrity. 17 Similarly, Saleemi et al reported the antimicrobial mechanisms of double-walled and multi-walled CNTs against S. aureus, P. aeruginosa, K. pneumoniae, and C. albicans. 21 They found that both types of CNTs were wrapped around the surface of pathogens and caused severe damage to the cell wall/membrane of the selected pathogens. 21 Furthermore, Liu et al suggested that the dispersed SWCNTs were acted as “nano darts” in the solution, attacking both Gram-negative and Gram-positive bacteria, which convincingly increased the bacterial cell death. 99 Another similar result was reported after bacterial cells (Ralstonia solanacearum) were incubated with CNTs. 100
Generation of oxidative stress and ROS
Oxidative stress is considered as the main mechanism to induce the toxicity of CNTs in microbial cells. When CNTs penetrate the microbial cells, the ROS are generated, including hydrogen peroxide (H2O2), superoxide anion (O2•_), organic hydroperoxides, and hydroxyl radicals (OH•). The oxidative stress produces these free radicals and initiates the unsaturated phospholipids peroxidation in cellular membranes, which thereafter generating peroxyl radical intermediates that cause the severe destruction of nucleic acids and lipoproteins. The lipid peroxidation also induces membrane malfunction by changing the membrane fluidity. This has caused the conformational variations in membrane proteins, resulting in bacterial cell death. 101
The physicochemical properties of CNTs, such as electrophilic nature and surface area, are the factors to determine the amount of production of ROS in bacterial cells. Bacterial cell destruction occurs when the activities of the antioxidant enzyme are damaged by excessive ROS generated inside the cell. After bacterial cells were exposed to CNTs, the genes (part of oxyR and soxRS systems) that are associated with oxidative stress were expressed. 16,17 On the other hand, Vecitis et al investigated the toxicity mechanism of SWCNTs against E. coli using in vitro model of SWCNTs-mediated glutathione oxidation, a redox state mediator and non-enzymatic antioxidant that protects the pathogen from oxidative stress. 22 Their results indicate a rise in glutathione oxidation and the lipid peroxidation in the microbial membrane with an increasing fraction of metallic SWCNTs. The imbalance of both oxidant and antioxidant therefore causes oxidative stress to increase inside the bacteria. In addition, it has been proved that toxicity of C60 is primarily caused by the oxidative stress on microbial cells. DNA microarray report showed that variations in the expression of oxidative stress-related genes were observed after the cells were treated with CNTs. 16 However, some studies indicated that oxidative stress is not the only factor to cause microbial cell death. For instance, a study conducted by Liu et al assessed the oxidation-reduction capacity of SWCNTs and reported the loss of thiol groups (-SH) on the proteins both outside and inside the cell membranes of Bacillus subtilis and E. coli after treated with SWCNTs. 99 Under anoxic conditions, no thiol oxidation was observed following treatment with SWCNTs, indicating that SWCNTs remained outside the microbial cell and were unable to penetrate the cell membrane to oxidize the intracellular proteins. These results suggested that oxidative stress produced by SWCNTs may not play a significant role in the antimicrobial activity. 99
Destruction of DNA
Many studies have reported that the adhesion of CNTs to microbial cells is the main trigger of the antimicrobial effect. However, CNTs may attach to the surface of bacteria (S. mutans) through entanglement without bacterial cell membrane damage, as reported. 102 Simon-Deckers et al studied the adsorption of bacteria (Cupriavidusmetallidurans or E. coli) onto the CNTs (MWCNTs) by transmission electron microscopy and found that CNTs can induce protein dysfunction and DNA damage. 103 The direct contact between CNTs and DNA could cause the destruction of DNA dominated through the single-strand breaks. Moreover, CNTs may diminish the power of supercoiled DNA base stacking and make the conformational variation in DNA. In general, nanomaterials can induce antimicrobial effects by destroying the cell membrane of bacteria or by passing through the membranes and specifically targeting the intracellular components such as protein, RNA, and DNA, as shown in Figure 2. 104,105
Figure 2.
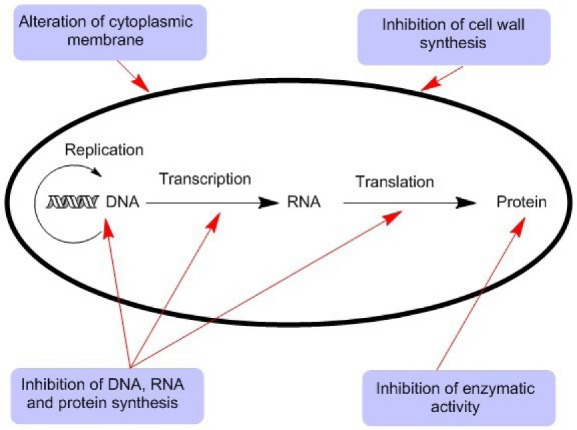
The different antimicrobial mechanisms of nanomaterials.
Nonetheless, CNTs can also obliquely contact with DNA without entering the cell. This can be accomplished by secondary effects (such as ROS), with the free radicals generated through the interaction of CNTs with the cellular environment. For instance, the ROS may interact with the DNA, causing changes in the structure of DNA and thereafter inhibiting the repairing mechanisms. 106 CNTs have the tendency to bind with the side chains of amino acid and SH groups of proteins to reduce their electrical properties. 107 Besides, CNTs contain metallic catalytic residues (e.g. nickel) during their synthesis process, where this transition metal (nickel) is involved in the Fenton reaction to generate hydroxyl radicals that react with the protein molecules.
Toxicity/biosafety profile of CNTs
CNTs have been extensively used in various biomedical applications. However, the detailed biosafety profile should be further investigated due to their toxicological effects on the human body. The high surface area to volume ratio of CNTs has increased their absorption rate, but this could possibly induce high toxicity and reactivity to the biological system and the environment, as reported. 108,109 The authors proposed that the interaction of CNTs with the biological systems can induce cytotoxic effects, such as ROS production, allergy, DNA damage, cytotoxicity to normal cells, and protein dysfunction. 110,111 The toxicity of CNTs depends on their size, shape, types of coating, aggregation, reactivity, mode of interaction with cells, and types of cell and tissue. 112-114 Even though the research data on the toxicity assessment of CNTs through in vivo and in vitro study models is still limited, the toxicity profile of CNTs must initially be evaluated before widely used as microbial growth inhibition agents.
Despite the significant number of achievements that has been made in the research of CNTs-polymer nanocomposites over the last decade, 115-117 there are some drawbacks to be taken into consideration. For example, weak interfacial bonding and poor dispersion remain a big problem for successfully integrating CNTs into the polymeric matrices. There are many challenges experienced when investigating CNTs as filler nanomaterials to be resolved. A homogeneous dispersion of CNTs in the polymeric matrix is difficult to accomplish and requires a strong interfacial interaction between polymeric matrix and nanotubes. 116,118 Previously, researchers have tried to efficiently reinforce CNTs with the polymeric matrix. 119,120 The homogeneous dispersion of CNTs is crucial to proficient reinforcement in the polymer nanocomposites. 121 Much efforts have been made to improve the CNTs dispersion, such as physical treatment, and chemical- and surfactants-based modifications of CNT’s surface. 120,121 Notably, a strong interfacial interaction is very important to take full benefit of the unique properties of CNTs. Therefore, functionalization of CNTs has been proposed to enhance the bonding at the interface and to significantly improve the dispersion of CNTs. 121,122
Application of CNTs in urinary tract devices
Due to the remarkable physicochemical properties of CNTs, there are numerous CNTs-based devices that have been extensively used in various biomedical application, such as urinary tract devices (e.g., the ureteral stents and urinary catheters) used in clinical practice even though it may cause urinary tract infections (UTIs) in some cases. Previous studies reported that UTIs were associated with 17% of hospital-acquired infections and had a prevalence rate of 27% and 36% in Europe and USA respectively. 123,124 The formation of biofilm by microorganisms is adhered to the inert or living surfaces, then surrounded by self-produced extracellular polymeric substances, and promotes bacterial growth within hours (see Figure 3). 104,125 Such process can cause significant harmful effect on human. 126 Hospital-acquired infections are the major cause of mortality in the United States and the infection rate is 65% due to microbial biofilms. 127 Most of the infections are initiated from medical instruments, such as bladder catheters (10~30%), fracture fixation and dental implantation devices (5~10%), and heart assistant instruments (25~50%). 128
Figure 3.
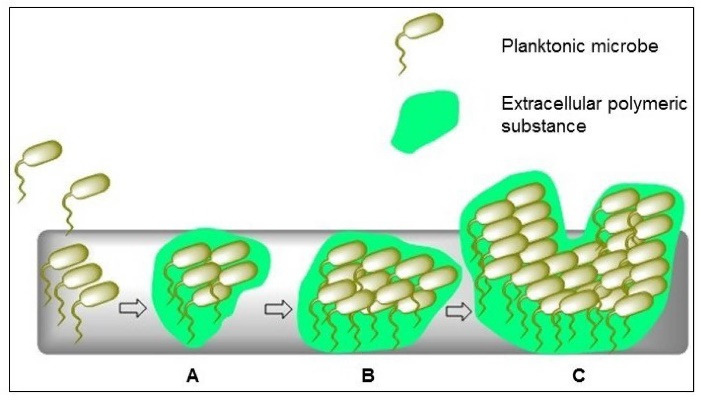
Schematic illustration of biofilm formation strategy. (A) Planktonic or free-floating bacteria attached to the surface. (B) Formation of bacterial self-produced extracellular polymeric substance (EPS) and colonized on the surface to produce a complex three-dimensional structure. (C) Bacterial communities produced within hours.
To overcome this drawback, application of various polymers has been implemented for biomedical instruments. 129 For instance, silicone polydimethylsiloxane (PDMS) has been applied in the urinary catheters and several implants primarily for the vesicoureteral reflux correction in the bladder. 130,131 The pathogen (E. coli) which is associated with 80% of UTIs may re-emerge and persist in the bladder after antibiotic treatment. 132-134 Moreover, the cost of replacing the infected implants during revision surgery can be twice the cost of primary implant operation. 125
On the other hand, the biofilm control approach has been suggested to reduce the infection, where the coating surfaces are used to discharge the antimicrobial agents over time. This approach comes with some limitations, including their toxicity to human cells, uncontrolled discharge of agent, and depletion of antimicrobial molecules, and the microbial resistance development. 26,135 In addition, more promising approaches have been applied to control the biofilm formation, such as reducing initial cellular adhesion via the physical methods which are unlikely causing bacterial resistance. 29,136 Thus, PDMS can be widely used in the membrane biofilm reactors for the treatment of wastewater due to its great cell adhesion properties, and to develop valuable compounds. 137-141
Recently, the authors have successfully incorporated MWCNTs into the PDMS in order to control the adhesion of bacteria (E. coli). 142 They found that small amounts of pristine MWCNTs (0.1%) caused a reduction by 20% on bacterial adhesion, while the oxidized form of MWCNTs (treated with nitric acid) also increased 20% of bacterial adhesion. These results matched by an earlier study conducted by Arias and Yang, where functionalized MWCNTs (with functional group (-OH)) did not show considerable antimicrobial activity against pathogens. 46 In contrary, Chen et al have demonstrated that functionalized MWCNTs (with functional group (-OH) showed significant dose-dependent microbial growth inhibition. 19 Therefore, both studies indicated that the surface performance may be affected by specific experimental conditions. Consequently, more studies need to be carried out to investigate further the behaviors of the MWCNTs/PDMS composites in microbial growth environment and inhibit the biofilm formation on the biomedical devices. 142
However, CNT-polymer nanocomposites have been widely applied in the medical and pharmaceutical fields, often displaying significant developments in the thermal, mechanical, optical, and electrical properties of the nanocomposites as compared to polymer alone. 143 In addition to the use in the fabrication of biosensors, CNTs have been applied in the development of drug delivery systems due to their immense potential in the biomedical field. 5,25,144 Previous studies showed that CNTs can be adhered to the cell membrane and used as coatings for medical implants to enhance the cell growth and attachment. 145-147 Similarly, incorporation of CNTs into polymers has demonstrated to increase the cell proliferation and attachment, with significant effects in tissue engineering scaffolds and cell culture substrate. 80,148-151 In case of implantable medical devices, bacterial adhesion on the surface of implant often causes implant failure and severe infections. Therefore, the antifouling properties of CNTs have rendered them as potential nanomaterial for a broad range of biomedical applications. 24 The antifouling coatings do not specifically kill the pathogen, but to prevent bacteria from being attached to the surfaces that allow biofilms to form. 152,153 In general, the antifouling mechanisms of nanomaterials involve exclusion steric repulsion, low surface energy, electrostatic repulsion, releasing of biocide, and killing of microbes upon direct contact with the coatings, which inhibit the biofilm formation by plankton bacteria, as shown in Figure 4. 104 The antibacterial activity of CNTs mainly depends on the number of graphene layers, aspect ratio, and physical disposition. 154 Previous studies demonstrated the effectiveness of MWCNTs nanocomposites in the removal of bacterial adhesion and biofilm. 40,80 For instance, PDMS is widely used in the production of implants and medical devices in the biomedical industry. 155 Specifically, in the manufacturing of UTI devices, PDMS is usually applied due to its great biocompatibility, excellent chemical stability, and mechanical resistance. 156,157 However, the use of PDMS in the biomedical sector carries some drawbacks, such as it is vulnerable to non-specific surface attachment of pathogens and protein. Currently, various studies reported the enhancement of antibiofouling characteristics of PDMS by the attachment of MWCNTs. 158,159
Figure 4.
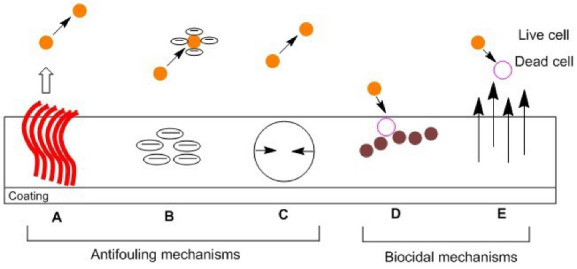
Antifouling and biocidal mechanisms of nanomaterials. (A) Steric repulsion: nanomaterials incorporated with the coating surfaces that provide physical berries to microbes, proteins, and cells. (B) Electrostatic repulsion: prevent the attachment of pathogens due to charges on the coating surfaces. (C) Low surface energy: reduction of external bacterial adhesion. (D) Releasing of biocide: start killing the microbes by the release of silver ions and nitric oxide. (E) Contact-active: kill multi-drug resistant (MDR) pathogens upon contact with the coatings.
With respect to PDMS/CNTs nanocomposites, various reports have been conducted on their thermal, electrical, and mechanical properties, suggesting that CNTs attachment may be beneficial. 160-163 Another best example of these composites’ application is the production of electrically conductive materials for biomedical implants with sensing ability. 164,165 Thus, a detailed analysis at the interface level could provide insights into the application of CNTs-based coatings in different medical implants.
Conclusion and outlook
CNTs are remarkable nanomaterials for various biomedical applications, specifically used in developing the antimicrobial surfaces. The antimicrobial properties of CNTs depend on multiple factors, which respectively affect the overall performance. For instance, the surface functionalization of CNTs plays a crucial role to improve their biocompatibility and hydrophilicity. There are different other materials, such as metals, polymers, and biomolecules, which could be blended with nanotubes for developing effective CNTs-based nanocomposites, that show high antimicrobial activity towards a wide range of microorganisms. Some studies have proposed that SWCNTs are more effective in microbial growth inhibition than MWCNTs, while there are some other studies supporting the use of MWCNTs for antimicrobial activities. This proves that the antimicrobial mechanisms of different types of CNTs are yet to be fully understood. Consequently, further research work is required for the development of CNTs-based nanocomposites to produce new antimicrobial surfaces. The toxicity or biosafety profile of CNTs-based nanocomposites should also be carefully studied before they can be widely used in biomedical applications.
Carbon nanostructures (CNSs) emerged about three decades ago, with significant development progress been reported over a short period of time. In recent times, most of the carbon nanomaterials are still under extensive research to discover their potential as an antimicrobial agent. While many carbon-based products are commercially available, other antimicrobial materials such as AgNPs and polymers are still preferred for three main reasons; (1) large-scale production of CNSs is challenging, (2) the production of CNSs is costly and time consuming, and (3) the cytotoxicity/biosafety profile of CNSs has not been fully interpreted. 166 Therefore, upcoming research should primarily focus on the large-scale production of non-toxic CNSs at minimal cost. Furthermore, the functionalization of CNTs seems to convincingly increase the overall efficiency in various biomedical applications, paving the way for broad integration in biomaterials. While safety profile of CNTs still needs to be carefully investigated, the production of new biomaterials for nanomedicine application will require their demand and superiority in the near future.
Acknowledgments
This project was supported by the Research Collaboration between Faculty of Health and Medical Sciences, and Faculty of Innovation and Technology, Taylor’s University Lakeside Campus, Malaysia under the Flagship Grant with project code: TUFR/2017/001/05.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Shah KA, Tali BA. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: a review on carbon sources, catalysts and substrates. Mater Sci Semicond Process. 2016;41:67–82. doi: 10.1016/j.mssp.2015.08.013. [DOI] [Google Scholar]
- 2.Zhu Z, Garcia-Gancedo L, Flewitt AJ, Xie H, Moussy F, Milne WI. A critical review of glucose biosensors based on carbon nanomaterials: carbon nanotubes and graphene. Sensors (Basel) 2012;12(5):5996–6022. doi: 10.3390/s120505996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maas M. Carbon nanomaterials as antibacterial colloids. Materials (Basel) 2016;9(8):617. doi: 10.3390/ma9080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Jumaili A, Alancherry S, Bazaka K, Jacob MV. Review on the antimicrobial properties of carbon nanostructures. Materials (Basel) 2017;10(9). 10.3390/ma10091066 [DOI] [PMC free article] [PubMed]
- 5.Saliev T. The advances in biomedical applications of carbon nanotubes. Carbon. 2019;5(2):29. doi: 10.3390/c5020029. [DOI] [Google Scholar]
- 6.Anzar N, Hasan R, Tyagi M, Yadav N, Narang J. Carbon nanotube-a review on synthesis, properties and plethora of applications in the field of biomedical science. Sens Int. 2020;1:100003. doi: 10.1016/j.sintl.2020.100003. [DOI] [Google Scholar]
- 7.Bera B. A review on polymer, graphene and carbon nanotube: properties, synthesis and applications. Imp J Interdiscip Res. 2017;3(10):61–70. [Google Scholar]
- 8.Rahman G, Najaf Z, Mehmood A, Bilal S, Shah AU, Mian SA, Ali G. An overview of the recent progress in the synthesis and applications of carbon nanotubes. C (Basel) 2019;5(1):3. doi: 10.3390/c5010003. [DOI] [Google Scholar]
- 9.Zhang X, Wen R, Huang Z, Tang C, Huang Y, Liu Y. et al. Enhancement of thermal conductivity by the introduction of carbon nanotubes as a filler in paraffin/expanded perlite form-stable phase-change materials. Energy Build. 2017;149:463–70. doi: 10.1016/j.enbuild.2017.05.037. [DOI] [Google Scholar]
- 10.Kobashi K, Ata S, Yamada T, Futaba DN, Okazaki T, Hata K. Classification of commercialized carbon nanotubes into three general categories as a guide for applications. ACS Appl Nano Mater. 2019;2(7):4043–7. doi: 10.1021/acsanm.9b00941. [DOI] [Google Scholar]
- 11.Oyelami AO, Semple KT. Impact of carbon nanomaterials on microbial activity in soil. Soil Biol Biochem. 2015;86:172–80. doi: 10.1016/j.soilbio.2015.03.029. [DOI] [Google Scholar]
- 12.Maleki Dizaj S, Mennati A, Jafari S, Khezri K, Adibkia K. Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bull. 2015;5(1):19–23. doi: 10.5681/apb.2015.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azizi-Lalabadi M, Hashemi H, Feng J, Jafari SM. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv Colloid Interface Sci. 2020;284:102250. doi: 10.1016/j.cis.2020.102250. [DOI] [PubMed] [Google Scholar]
- 14.Lee ES, Kim YO, Ha YM, Lim D, Hwang JY, Kim J. et al. Antimicrobial properties of lignin-decorated thin multi-walled carbon nanotubes in poly(vinyl alcohol) nanocomposites. Eur Polym J. 2018;105:79–84. doi: 10.1016/j.eurpolymj.2018.05.014. [DOI] [Google Scholar]
- 15.Kang S, Pinault M, Pfefferle LD, Elimelech M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007;23(17):8670–3. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- 16.Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24(13):6409–13. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- 17.Kang S, Mauter MS, Elimelech M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ Sci Technol. 2008;42(19):7528–34. doi: 10.1021/es8010173. [DOI] [PubMed] [Google Scholar]
- 18.Haung CF, Chan YH, Chen LK, Liu CM, Huang WC, Ou SF. et al. Preparation, characterization, and properties of anticoagulation and antibacterial films of carbon-based nanowires fabricated on surfaces of Ti implants. J Electrochem Soc. 2013;160(6):H392–H7. doi: 10.1149/2.109306jes. [DOI] [Google Scholar]
- 19.Chen H, Wang B, Gao D, Guan M, Zheng L, Ouyang H. et al. Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small. 2013;9(16):2735–46. doi: 10.1002/smll.201202792. [DOI] [PubMed] [Google Scholar]
- 20.Di Sotto A, Chiaretti M, Carru GA, Bellucci S, Mazzanti G. Multi-walled carbon nanotubes: lack of mutagenic activity in the bacterial reverse mutation assay. Toxicol Lett. 2009;184(3):192–7. doi: 10.1016/j.toxlet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Saleemi MA, Fouladi MH, Yong PVC, Wong EH. Elucidation of antimicrobial activity of non-covalently dispersed carbon nanotubes. Materials (Basel) 2020;13(7):1676. doi: 10.3390/ma13071676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vecitis CD, Zodrow KR, Kang S, Elimelech M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano. 2010;4(9):5471–9. doi: 10.1021/nn101558x. [DOI] [PubMed] [Google Scholar]
- 23.Chae SR, Watanabe Y, Wiesner MR. Comparative photochemical reactivity of spherical and tubular fullerene nanoparticles in water under ultraviolet (UV) irradiation. Water Res. 2011;45(1):308–14. doi: 10.1016/j.watres.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 24.Upadhyayula VK, Gadhamshetty V. Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: a review. Biotechnol Adv. 2010;28(6):802–16. doi: 10.1016/j.biotechadv.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 25.He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, Pham-Huy C. Carbon nanotubes: applications in pharmacy and medicine. Biomed Res Int. 2013;2013:578290. doi: 10.1155/2013/578290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslan S, Loebick CZ, Kang S, Elimelech M, Pfefferle LD, Van Tassel PR. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid) Nanoscale. 2010;2(9):1789–94. doi: 10.1039/c0nr00329h. [DOI] [PubMed] [Google Scholar]
- 27.Hirschfeld J, Akinoglu EM, Wirtz DC, Hoerauf A, Bekeredjian-Ding I, Jepsen S. et al. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomedicine. 2017;13(4):1587–93. doi: 10.1016/j.nano.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhari AA, Joshi S, Vig K, Sahu R, Dixit S, Baganizi R. et al. A three-dimensional human skin model to evaluate the inhibition of Staphylococcus aureus by antimicrobial peptide-functionalized silver carbon nanotubes. J Biomater Appl. 2019;33(7):924–34. doi: 10.1177/0885328218814984. [DOI] [PubMed] [Google Scholar]
- 29.Nepal D, Balasubramanian S, Simonian AL, Davis VA. Strong antimicrobial coatings: single-walled carbon nanotubes armored with biopolymers. Nano Lett. 2008;8(7):1896–901. doi: 10.1021/nl080522t. [DOI] [PubMed] [Google Scholar]
- 30.Mehra NK, Jain K, Jain NK. Pharmaceutical and biomedical applications of surface engineered carbon nanotubes. Drug Discov Today. 2015;20(6):750–9. doi: 10.1016/j.drudis.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN. et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006;165(1):88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Pasquini LM, Hashmi SM, Sommer TJ, Elimelech M, Zimmerman JB. Impact of surface functionalization on bacterial cytotoxicity of single-walled carbon nanotubes. Environ Sci Technol. 2012;46(11):6297–305. doi: 10.1021/es300514s. [DOI] [PubMed] [Google Scholar]
- 33.Saleemi MA, Yong PVC, Wong EH. Investigation of antimicrobial activity and cytotoxicity of synthesized surfactant-modified carbon nanotubes/polyurethane electrospun nanofibers. Nano Struct Nano Objects. 2020;24:100612. doi: 10.1016/j.nanoso.2020.100612. [DOI] [Google Scholar]
- 34.Saleemi MA, Hosseini Fouladi M, Yong PVC, Chinna K, Palanisamy NK, Wong EH. Toxicity of carbon nanotubes: molecular mechanisms, signaling cascades, and remedies in biomedical applications. Chem Res Toxicol. 2021;34(1):24–46. doi: 10.1021/acs.chemrestox.0c00172. [DOI] [PubMed] [Google Scholar]
- 35.Revathi SU, Vuyyuru MA, Dhanaraju MD. Carbon nanotube: a flexible approach for nanomedicine and drug delivery. Asian J Pharm Clin Res. 2015;8(1):25–31. [Google Scholar]
- 36.Battigelli A, Ménard-Moyon C, Da Ros T, Prato M, Bianco A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv Drug Deliv Rev. 2013;65(15):1899–920. doi: 10.1016/j.addr.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S. et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168(2):121–31. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Vassallo J, Besinis A, Boden R, Handy RD. The minimum inhibitory concentration (MIC) assay with Escherichia coli: an early tier in the environmental hazard assessment of nanomaterials? Ecotoxicol Environ Saf. 2018;162:633–46. doi: 10.1016/j.ecoenv.2018.06.085. [DOI] [PubMed] [Google Scholar]
- 39.Hartono MR, Kushmaro A, Chen X, Marks RS. Probing the toxicity mechanism of multiwalled carbon nanotubes on bacteria. Environ Sci Pollut Res Int. 2018;25(5):5003–12. doi: 10.1007/s11356-017-0782-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Nghiem J, Silverberg GJ, Vecitis CD. Semiquantitative performance and mechanism evaluation of carbon nanomaterials as cathode coatings for microbial fouling reduction. Appl Environ Microbiol. 2015;81(14):4744–55. doi: 10.1128/aem.00582-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Ng AK, Xu R, Wei J, Tan CM, Yang Y. et al. Antibacterial action of dispersed single-walled carbon nanotubes on Escherichia coli and Bacillus subtilis investigated by atomic force microscopy. Nanoscale. 2010;2(12):2744–50. doi: 10.1039/c0nr00441c. [DOI] [PubMed] [Google Scholar]
- 42.Olivi M, Zanni E, De Bellis G, Talora C, Sarto MS, Palleschi C. et al. Inhibition of microbial growth by carbon nanotube networks. Nanoscale. 2013;5(19):9023–9. doi: 10.1039/c3nr02091f. [DOI] [PubMed] [Google Scholar]
- 43.Kim JW, Shashkov EV, Galanzha EI, Kotagiri N, Zharov VP. Photothermal antimicrobial nanotherapy and nanodiagnostics with self-assembling carbon nanotube clusters. Lasers Surg Med. 2007;39(7):622–34. doi: 10.1002/lsm.20534. [DOI] [PubMed] [Google Scholar]
- 44.Nagai H, Toyokuni S. Differences and similarities between carbon nanotubes and asbestos fibers during mesothelial carcinogenesis: shedding light on fiber entry mechanism. Cancer Sci. 2012;103(8):1378–90. doi: 10.1111/j.1349-7006.2012.02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K. et al. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicology. 2010;4(2):207–46. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- 46.Arias LR, Yang L. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir. 2009;25(5):3003–12. doi: 10.1021/la802769m. [DOI] [PubMed] [Google Scholar]
- 47.Young YF, Lee HJ, Shen YS, Tseng SH, Lee CY, Tai NH. et al. Toxicity mechanism of carbon nanotubes on Escherichia coli. Mater Chem Phys. 2012;134(1):279–86. doi: 10.1016/j.matchemphys.2012.02.066. [DOI] [Google Scholar]
- 48.Ahmed F, Santos CM, Vergara RA, Tria MC, Advincula R, Rodrigues DF. Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ Sci Technol. 2012;46(3):1804–10. doi: 10.1021/es202374e. [DOI] [PubMed] [Google Scholar]
- 49.Goodwin DG Jr, Marsh KM, Sosa IB, Payne JB, Gorham JM, Bouwer EJ. et al. Interactions of microorganisms with polymer nanocomposite surfaces containing oxidized carbon nanotubes. Environ Sci Technol. 2015;49(9):5484–92. doi: 10.1021/acs.est.5b00084. [DOI] [PubMed] [Google Scholar]
- 50.Sah U, Sharma K, Chaudhri N, Sankar M, Gopinath P. Antimicrobial photodynamic therapy: single-walled carbon nanotube (SWCNT)-porphyrin conjugate for visible light mediated inactivation of Staphylococcus aureus. Colloids Surf B Biointerfaces. 2018;162:108–17. doi: 10.1016/j.colsurfb.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 51.Cajero-Zul LR, López-Dellamary FA, Gómez-Salazar S, Vázquez-Lepe M, Vera-Graziano R, Torres-Vitela MR. et al. Evaluation of the resistance to bacterial growth of star-shaped poly(ε-caprolactone)-co-poly(ethylene glycol) grafted onto functionalized carbon nanotubes nanocomposites. J Biomater Sci Polym Ed. 2019;30(3):163–89. doi: 10.1080/09205063.2018.1558487. [DOI] [PubMed] [Google Scholar]
- 52.Wang FY, Wang TY, Liao TY, Liu MY. The complete mitochondrial genome sequence of Nemateleotris decora (gobiiformes, gobiidae) Mitochondrial DNA A DNA Mapp Seq Anal. 2016;27(6):4274–5. doi: 10.3109/19401736.2015.1082091. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Z, Wang Z, Li S, Yuan X. Antimicrobial strategies for urinary catheters. J Biomed Mater Res A. 2019;107(2):445–67. doi: 10.1002/jbm.a.36561. [DOI] [PubMed] [Google Scholar]
- 54.Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(1):32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- 55.Chaudhari AA, Ashmore D, Nath SD, Kate K, Dennis V, Singh SR. et al. A novel covalent approach to bio-conjugate silver coated single walled carbon nanotubes with antimicrobial peptide. J Nanobiotechnology. 2016;14(1):58. doi: 10.1186/s12951-016-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar A, Dalal J, Dahiya S, Punia R, Sharma KD, Ohlan A. et al. In situ decoration of silver nanoparticles on single-walled carbon nanotubes by microwave irradiation for enhanced and durable anti-bacterial finishing on cotton fabric. Ceram Int. 2019;45(1):1011–9. doi: 10.1016/j.ceramint.2018.09.280. [DOI] [Google Scholar]
- 57.Karumuri AK, Oswal DP, Hostetler HA, Mukhopadhyay SM. Silver nanoparticles supported on carbon nanotube carpets: influence of surface functionalization. Nanotechnology. 2016;27(14):145603. doi: 10.1088/0957-4484/27/14/145603. [DOI] [PubMed] [Google Scholar]
- 58.Barbinta-Patrascu ME, Ungureanu C, Iordache SM, Iordache AM, Bunghez IR, Ghiurea M. et al. Eco-designed biohybrids based on liposomes, mint-nanosilver and carbon nanotubes for antioxidant and antimicrobial coating. Mater Sci Eng C Mater Biol Appl. 2014;39:177–85. doi: 10.1016/j.msec.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 59.Chang YN, Gong JL, Zeng GM, Ou XM, Song B, Guo M. et al. Antimicrobial behavior comparison and antimicrobial mechanism of silver coated carbon nanocomposites. Process Saf Environ Prot. 2016;102:596–605. doi: 10.1016/j.psep.2016.05.023. [DOI] [Google Scholar]
- 60.Mohammad MR, Ahmed DS, Mohammed MKA. Synthesis of Ag-doped TiO2 nanoparticles coated with carbon nanotubes by the sol–gel method and their antibacterial activities. J Solgel Sci Technol. 2019;90(3):498–509. doi: 10.1007/s10971-019-04973-w. [DOI] [Google Scholar]
- 61.Park SB, Steadman CS, Chaudhari AA, Pillai SR, Singh SR, Ryan PL. et al. Proteomic analysis of antimicrobial effects of pegylated silver coated carbon nanotubes in Salmonella enterica serovar Typhimurium. J Nanobiotechnology. 2018;16(1):31. doi: 10.1186/s12951-018-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A, Goswami A, Nain S. Enhanced antibacterial activity and photo-remediation of toxic dyes using Ag/SWCNT/PPy based nanocomposite with core–shell structure. Appl Nanosci. 2020;10(7):2255–68. doi: 10.1007/s13204-020-01394-y. [DOI] [Google Scholar]
- 63.Zhu Y, Xu J, Wang Y, Chen C, Gu H, Chai Y. et al. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020;13(2):389–400. doi: 10.1007/s12274-020-2621-3. [DOI] [Google Scholar]
- 64.Yun H, Kim JD, Choi HC, Lee CW. Antibacterial activity of CNT-Ag and GO-Ag nanocomposites against gram-negative and gram-positive bacteria. Bull Korean Chem Soc. 2013;34(11):3261–4. doi: 10.5012/bkcs.2013.34.11.3261. [DOI] [Google Scholar]
- 65.Leid JG, Ditto AJ, Knapp A, Shah PN, Wright BD, Blust R. et al. In vitro antimicrobial studies of silver carbene complexes: activity of free and nanoparticle carbene formulations against clinical isolates of pathogenic bacteria. J Antimicrob Chemother. 2012;67(1):138–48. doi: 10.1093/jac/dkr408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levashov PA, Sedov SA, Shipovskov S, Belogurova NG, Levashov AV. Quantitative turbidimetric assay of enzymatic gram-negative bacteria lysis. Anal Chem. 2010;82(5):2161–3. doi: 10.1021/ac902978u. [DOI] [PubMed] [Google Scholar]
- 67.Aslan S, Deneufchatel M, Hashmi S, Li N, Pfefferle LD, Elimelech M. et al. Carbon nanotube-based antimicrobial biomaterials formed via layer-by-layer assembly with polypeptides. J Colloid Interface Sci. 2012;388(1):268–73. doi: 10.1016/j.jcis.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 68.Tiraferri A, Vecitis CD, Elimelech M. Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties. ACS Appl Mater Interfaces. 2011;3(8):2869–77. doi: 10.1021/am200536p. [DOI] [PubMed] [Google Scholar]
- 69.Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457–65. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 71.Murugan E, Vimala G. Effective functionalization of multiwalled carbon nanotube with amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J Colloid Interface Sci. 2011;357(2):354–65. doi: 10.1016/j.jcis.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Neelgund GM, Oki A. Deposition of silver nanoparticles on dendrimer functionalized multiwalled carbon nanotubes: synthesis, characterization and antimicrobial activity. J NanosciNanotechnol. 2011;11(4):3621–9. doi: 10.1166/jnn.2011.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neelgund GM, Oki A, Luo Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf B Biointerfaces. 2012;100:215–21. doi: 10.1016/j.colsurfb.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding L, Wang H, Liu D, Zeng XA, Mao Y. Bacteria capture and inactivation with functionalized multi-walled carbon nanotubes (MWCNTs) J NanosciNanotechnol. 2020;20(4):2055–62. doi: 10.1166/jnn.2020.17332. [DOI] [PubMed] [Google Scholar]
- 75.Levi-Polyachenko N, Young C, MacNeill C, Braden A, Argenta L, Reid S. Eradicating group A streptococcus bacteria and biofilms using functionalised multi-wall carbon nanotubes. Int J Hyperthermia. 2014;30(7):490–501. doi: 10.3109/02656736.2014.966790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bai Y, Park IS, Lee SJ, Bae TS, Watari F, Uo M. et al. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon. 2011;49(11):3663–71. doi: 10.1016/j.carbon.2011.05.002. [DOI] [Google Scholar]
- 77.Khazaee M, Ye D, Majumder A, Baraban L, Opitz J, Cuniberti G. Non-covalent modified multi-walled carbon nanotubes: dispersion capabilities and interactions with bacteria. Biomed Phys Eng Express. 2016;2(5):055008. doi: 10.1088/2057-1976/2/5/055008. [DOI] [Google Scholar]
- 78.Akhavan O, Azimirad R, Safa S. Functionalized carbon nanotubes in ZnO thin films for photoinactivation of bacteria. Mater Chem Phys. 2011;130(1-2):598–602. doi: 10.1016/j.matchemphys.2011.07.030. [DOI] [Google Scholar]
- 79.Amiri A, Zare Zardini H, Shanbedi M, Maghrebi M, Baniadam M, Tolueinia B. Efficient method for functionalization of carbon nanotubes by lysine and improved antimicrobial activity and water-dispersion. Mater Lett. 2012;72:153–6. doi: 10.1016/j.matlet.2011.12.114. [DOI] [Google Scholar]
- 80.Venkatesan J, Jayakumar R, Mohandas A, Bhatnagar I, Kim SK. Antimicrobial activity of chitosan-carbon nanotube hydrogels. Materials (Basel) 2014;7(5):3946–55. doi: 10.3390/ma7053946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohamed NA, Al-Harby NF, Almarshed MS. Synthesis and characterization of novel trimellitic anhydride isothiocyanate-cross linked chitosan hydrogels modified with multi-walled carbon nanotubes for enhancement of antimicrobial activity. Int J Biol Macromol. 2019;132:416–28. doi: 10.1016/j.ijbiomac.2019.03.195. [DOI] [PubMed] [Google Scholar]
- 82.Mocan T, Matea CT, Pop T, Mosteanu O, Buzoianu AD, Suciu S. et al. Carbon nanotubes as anti-bacterial agents. Cell Mol Life Sci. 2017;74(19):3467–79. doi: 10.1007/s00018-017-2532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Shi J, Zhang H, Li H, Gao Y, Wang Z. et al. Synergistic anticancer effect of RNAi and photothermal therapy mediated by functionalized single-walled carbon nanotubes. Biomaterials. 2013;34(1):262–74. doi: 10.1016/j.biomaterials.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 84.Huang YP, Lin IJ, Chen CC, Hsu YC, Chang CC, Lee MJ. Delivery of small interfering RNAs in human cervical cancer cells by polyethylenimine-functionalized carbon nanotubes. Nanoscale Res Lett. 2013;8(1):267. doi: 10.1186/1556-276x-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martincic M, Tobias G. Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin Drug Deliv. 2015;12(4):563–81. doi: 10.1517/17425247.2015.971751. [DOI] [PubMed] [Google Scholar]
- 86.Zhang B, Wang H, Shen S, She X, Shi W, Chen J. et al. Fibrin-targeting peptide CREKA-conjugated multi-walled carbon nanotubes for self-amplified photothermal therapy of tumor. Biomaterials. 2016;79:46–55. doi: 10.1016/j.biomaterials.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 87.Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, Selegue JP. et al. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005;5(12):2448–64. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zare Zardini H, Davarpanah M, Shanbedi M, Amiri A, Maghrebi M, Ebrahimi L. Microbial toxicity of ethanolamines--multiwalled carbon nanotubes. J Biomed Mater Res A. 2014;102(6):1774–81. doi: 10.1002/jbm.a.34846. [DOI] [PubMed] [Google Scholar]
- 89.Gilbertson LM, Goodwin DG Jr, Taylor AD, Pfefferle L, Zimmerman JB. Toward tailored functional design of multi-walled carbon nanotubes (MWNTs): electrochemical and antimicrobial activity enhancement via oxidation and selective reduction. Environ Sci Technol. 2014;48(10):5938–45. doi: 10.1021/es500468y. [DOI] [PubMed] [Google Scholar]
- 90.Seo Y, Hwang J, Kim J, Jeong Y, Hwang MP, Choi J. Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int J Nanomedicine. 2014;9:4621–9. doi: 10.2147/ijn.s69561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jung JH, Hwang GB, Lee JE, Bae GN. Preparation of airborne Ag/CNT hybrid nanoparticles using an aerosol process and their application to antimicrobial air filtration. Langmuir. 2011;27(16):10256–64. doi: 10.1021/la201851r. [DOI] [PubMed] [Google Scholar]
- 92.Rusen E, Mocanu A, Nistor LC, Dinescu A, Călinescu I, Mustăţea G. et al. Design of antimicrobial membrane based on polymer colloids/multiwall carbon nanotubes hybrid material with silver nanoparticles. ACS Appl Mater Interfaces. 2014;6(20):17384–93. doi: 10.1021/am505024p. [DOI] [PubMed] [Google Scholar]
- 93.Mohan R, Shanmugharaj AM, Sung Hun R. An efficient growth of silver and copper nanoparticles on multiwalled carbon nanotube with enhanced antimicrobial activity. J Biomed Mater Res B Appl Biomater. 2011;96(1):119–26. doi: 10.1002/jbm.b.31747. [DOI] [PubMed] [Google Scholar]
- 94.Sui M, Zhang L, Sheng L, Huang S, She L. Synthesis of ZnO coated multi-walled carbon nanotubes and their antibacterial activities. Sci Total Environ. 2013;452-453:148–54. doi: 10.1016/j.scitotenv.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 95.Karthika V, Arumugam A. Synthesis and characterization of MWCNT/TiO2/Au nanocomposite for photocatalytic and antimicrobial activity. IET Nanobiotechnol. 2017;11(1):113–8. doi: 10.1049/iet-nbt.2016.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grover N, Borkar IV, Dinu CZ, Kane RS, Dordick JS. Laccase- and chloroperoxidase-nanotube paint composites with bactericidal and sporicidal activity. Enzyme Microb Technol. 2012;50(6-7):271–9. doi: 10.1016/j.enzmictec.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 97.Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D. et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42(18):4591–602. doi: 10.1016/j.watres.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 98.Deryabin DG, Vasilchenko AS, Aleshina ES, Tlyagulova AS, Nikiyan HN. An investigation into the interaction between carbon-based nanomaterials and Escherichia coli cells using atomic force microscopy. Nanotechnol Russ. 2010;5(11):857–63. doi: 10.1134/S1995078010110169. [DOI] [Google Scholar]
- 99.Liu S, Wei L, Hao L, Fang N, Chang MW, Xu R. et al. Sharper and faster “nano darts” kill more bacteria: a study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano. 2009;3(12):3891–902. doi: 10.1021/nn901252r. [DOI] [PubMed] [Google Scholar]
- 100.Wang X, Liu X, Han H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf B Biointerfaces. 2013;103:136–42. doi: 10.1016/j.colsurfb.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 101.Raghunath A, Perumal E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int J Antimicrob Agents. 2017;49(2):137–52. doi: 10.1016/j.ijantimicag.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 102.Akasaka T, Watari F. Capture of bacteria by flexible carbon nanotubes. Acta Biomater. 2009;5(2):607–12. doi: 10.1016/j.actbio.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 103.Simon-Deckers A, Loo S, Mayne-L’hermite M, Herlin-Boime N, Menguy N, Reynaud C. et al. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ Sci Technol. 2009;43(21):8423–9. doi: 10.1021/es9016975. [DOI] [PubMed] [Google Scholar]
- 104.Singha P, Locklin J, Handa H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017;50:20–40. doi: 10.1016/j.actbio.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. BiochimBiophys Acta. 1999;1462(1-2):11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 106.Wang X, Jiao C, Wang T, Yu Z. Study on DNA damage induced by the reactive oxygen species generated in situ based on the multi-walled carbon nanotubes and hemoglobin. J Electroanal Chem. 2016;767:182–7. doi: 10.1016/j.jelechem.2016.02.030. [DOI] [Google Scholar]
- 107.Lynch I, Dawson KA. Protein-nanoparticle interactions. Nano Today. 2008;3(1-2):40–7. doi: 10.1016/s1748-0132(08)70014-8. [DOI] [Google Scholar]
- 108.Albini A, Pagani A, Pulze L, Bruno A, Principi E, Congiu T. et al. Environmental impact of multi-wall carbon nanotubes in a novel model of exposure: systemic distribution, macrophage accumulation, and amyloid deposition. Int J Nanomedicine. 2015;10:6133–45. doi: 10.2147/ijn.s85275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saleemi MA, Kong YL, Yong PVC, Wong EH. An overview of recent development in therapeutic drug carrier system using carbon nanotubes. J Drug Deliv Sci Technol. 2020;59:101855. doi: 10.1016/j.jddst.2020.101855. [DOI] [Google Scholar]
- 110.Firme CP 3rd, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine. 2010;6(2):245–56. doi: 10.1016/j.nano.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 111.Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharmacol. 2012;261(2):121–33. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fujita K, Fukuda M, Endoh S, Maru J, Kato H, Nakamura A. et al. Size effects of single-walled carbon nanotubes on in vivo and in vitro pulmonary toxicity. InhalToxicol. 2015;27(4):207–23. doi: 10.3109/08958378.2015.1026620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee S, Khang D, Kim SH. High dispersity of carbon nanotubes diminishes immunotoxicity in spleen. Int J Nanomedicine. 2015;10:2697–710. doi: 10.2147/ijn.s80836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Amiri A, Zare Zardini H, Shanbedi M, Kazi SN, Taheri-Kafrani A, Chew BT, et al. Microbial toxicity of different functional groups-treated carbon nanotubes. In: Grumezescu AM, ed. Surface Chemistry of Nanobiomaterials. William Andrew Publishing; 2016. p. 33-70. 10.1016/b978-0-323-42861-3.00002-9 [DOI]
- 115.Bose S, Khare RA, Moldenaers P. Assessing the strengths and weaknesses of various types of pre-treatments of carbon nanotubes on the properties of polymer/carbon nanotubes composites: a critical review. Polymer. 2010;51(5):975–93. doi: 10.1016/j.polymer.2010.01.044. [DOI] [Google Scholar]
- 116.Andrews R, Weisenberger MC. Carbon nanotube polymer composites. CurrOpin Solid State Mater Sci. 2004;8(1):31–7. doi: 10.1016/j.cossms.2003.10.006. [DOI] [Google Scholar]
- 117.Gibson RF. A review of recent research on mechanics of multifunctional composite materials and structures. Compos Struct. 2010;92(12):2793–810. doi: 10.1016/j.compstruct.2010.05.003. [DOI] [Google Scholar]
- 118.Rahmat M, Hubert P. Carbon nanotube–polymer interactions in nanocomposites: a review. Compos Sci Technol. 2011;72(1):72–84. doi: 10.1016/j.compscitech.2011.10.002. [DOI] [Google Scholar]
- 119.Kaseem M, Hamad K, Ko YG. Fabrication and materials properties of polystyrene/carbon nanotube (PS/CNT) composites: a review. Eur Polym J. 2016;79:36–62. doi: 10.1016/j.eurpolymj.2016.04.011. [DOI] [Google Scholar]
- 120.Fernandes RM, Abreu B, Claro B, Buzaglo M, Regev O, Furó I. et al. Dispersing carbon nanotubes with ionic surfactants under controlled conditions: comparisons and insight. Langmuir. 2015;31(40):10955–65. doi: 10.1021/acs.langmuir.5b02050. [DOI] [PubMed] [Google Scholar]
- 121.Ma PC, Siddiqui NA, Marom G, Kim JK. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: a review. Compos Part A Appl Sci Manuf. 2010;41(10):1345–67. doi: 10.1016/j.compositesa.2010.07.003. [DOI] [Google Scholar]
- 122.Mensah B, Kim HG, Lee JH, Arepalli S, Nah C. Carbon nanotube-reinforced elastomeric nanocomposites: a review. Int J Smart Nano Mater. 2015;6(4):211–38. doi: 10.1080/19475411.2015.1121632. [DOI] [Google Scholar]
- 123.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G. et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 124.Moro ML. Health care-associated infections. Surg Infect. 2006;7(Suppl 2):S21–S3. doi: 10.1089/sur.2006.7.s2-21. [DOI] [PubMed] [Google Scholar]
- 125.Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJ, Zaat SA. et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci Transl Med. 2012;4(153):153rv10. doi: 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- 126.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 127. Mandakhalikar KD. Medical biofilms. In: Introduction to Biofilm Engineering. American Chemical Society; 2019. p. 83-99. 10.1021/bk-2019-1323.ch004 [DOI]
- 128.Darouiche RO. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis. 2001;33(9):1567–72. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 129.Pavithra D, Doble M. Biofilm formation, bacterial adhesion and host response on polymeric implants--issues and prevention. Biomed Mater. 2008;3(3):034003. doi: 10.1088/1748-6041/3/3/034003. [DOI] [PubMed] [Google Scholar]
- 130.Aubert D. [Vesico-ureteric reflux treatment by implant of polydimethylsiloxane (Macroplastique): review of the literature] Prog Urol. 2010;20(4):251–9. doi: 10.1016/j.purol.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 131. Kaali P, Strömberg E, Karlsson S. Prevention of biofilm associated infections and degradation of polymeric materials used in biomedical applications. In: Laskovski A, ed. Biomedical Engineering, Trends in Materials Science. IntechOpen; 2011. 10.5772/12983 [DOI]
- 132.Castonguay MH, van der Schaaf S, Koester W, Krooneman J, van der Meer W, Harmsen H. et al. Biofilm formation by Escherichia coli is stimulated by synergistic interactions and co-adhesion mechanisms with adherence-proficient bacteria. Res Microbiol. 2006;157(5):471–8. doi: 10.1016/j.resmic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 133.Trautner BW, Lopez AI, Kumar A, Siddiq DM, Liao KS, Li Y. et al. Nanoscale surface modification favors benign biofilm formation and impedes adherence by pathogens. Nanomedicine. 2012;8(3):261–70. doi: 10.1016/j.nano.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koseoglu H, Aslan G, Esen N, Sen BH, Coban H. Ultrastructural stages of biofilm development of Escherichia coli on urethral catheters and effects of antibiotics on biofilm formation. Urology. 2006;68(5):942–6. doi: 10.1016/j.urology.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 135.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–32. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 136. Saleemi MA, Palanisamy NK, Wong EH. Alternative approaches to combat medicinally important biofilm-forming pathogens. In: Kırmusaoğlu S, ed. Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods. IntechOpen; 2018. p. 29. 10.5772/intechopen.80341 [DOI]
- 137.Syron E, Casey E. Model-based comparative performance analysis of membrane aerated biofilm reactor configurations. BiotechnolBioeng. 2008;99(6):1361–73. doi: 10.1002/bit.21700. [DOI] [PubMed] [Google Scholar]
- 138.Syron E, Casey E. Performance of a pilot scale membrane aerated biofilm reactor for the treatment of landfill leachate. Procedia Eng. 2012;44:2082–4. doi: 10.1016/j.proeng.2012.09.052. [DOI] [Google Scholar]
- 139.Moreira JMR, Ponmozhi J, Campos JBLM, Miranda JM, Mergulhão FJ. Micro- and macro-flow systems to study Escherichia coli adhesion to biomedical materials. Chem Eng Sci. 2015;126:440–5. doi: 10.1016/j.ces.2014.12.054. [DOI] [Google Scholar]
- 140.Ercan D, Demirci A. Current and future trends for biofilm reactors for fermentation processes. Crit Rev Biotechnol. 2015;35(1):1–14. doi: 10.3109/07388551.2013.793170. [DOI] [PubMed] [Google Scholar]
- 141.Qureshi N, Annous BA, Ezeji TC, Karcher P, Maddox IS. Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Fact. 2005;4:24. doi: 10.1186/1475-2859-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vagos MR, Moreira JMR, Soares OSGP, Pereira MFR, Mergulhão FJ. Incorporation of carbon nanotubes in polydimethylsiloxane to control Escherichia coli adhesion. Polym Compos. 2019;40(Suppl 2):E1697–E704. doi: 10.1002/pc.25125. [DOI] [Google Scholar]
- 143.Spitalsky Z, Tasis D, Papagelis K, Galiotis C. Carbon nanotube–polymer composites: chemistry, processing, mechanical and electrical properties. Prog Polym Sci. 2010;35(3):357–401. doi: 10.1016/j.progpolymsci.2009.09.003. [DOI] [Google Scholar]
- 144.Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A. et al. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 2014;9(1):393. doi: 10.1186/1556-276x-9-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li X, Liu X, Huang J, Fan Y, Cui FZ. Biomedical investigation of CNT based coatings. Surf Coat Technol. 2011;206(4):759–66. doi: 10.1016/j.surfcoat.2011.02.063. [DOI] [Google Scholar]
- 146.Matsuoka M, Akasaka T, Totsuka Y, Watari F. Strong adhesion of Saos-2 cells to multi-walled carbon nanotubes. Mater Sci Eng B. 2010;173(1-3):182–6. doi: 10.1016/j.mseb.2009.12.044. [DOI] [Google Scholar]
- 147.Matsuoka M, Akasaka T, Totsuka Y, Watari F. Carbon nanotube-coated silicone as a flexible and electrically conductive biomedical material. Mater Sci Eng C. 2012;32(3):574–80. doi: 10.1016/j.msec.2011.12.011. [DOI] [Google Scholar]
- 148.Newman P, Minett A, Ellis-Behnke R, Zreiqat H. Carbon nanotubes: their potential and pitfalls for bone tissue regeneration and engineering. Nanomedicine. 2013;9(8):1139–58. doi: 10.1016/j.nano.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 149.Fabbro A, Prato M, Ballerini L. Carbon nanotubes in neuroregeneration and repair. Adv Drug Deliv Rev. 2013;65(15):2034–44. doi: 10.1016/j.addr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 150.Hirata E, Akasaka T, Uo M, Takita H, Watari F, Yokoyama A. Carbon nanotube-coating accelerated cell adhesion and proliferation on poly (L-lactide) Appl Surf Sci. 2012;262:24–7. doi: 10.1016/j.apsusc.2012.01.012. [DOI] [Google Scholar]
- 151.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28(2):344–53. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 152.Tenke P, Köves B, Nagy K, Hultgren SJ, Mendling W, Wullt B. et al. Update on biofilm infections in the urinary tract. World J Urol. 2012;30(1):51–7. doi: 10.1007/s00345-011-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34(34):8533–54. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 154. Teixeira-Santos R, Gomes M, Mergulhão FJ. Carbon nanotube-based antimicrobial and antifouling surfaces. In: Snigdha S, Thomas S, Radhakrishnan EK, Kalarikkal N, eds. Engineered Antimicrobial Surfaces. Singapore: Springer; 2020. p. 65-93. 10.1007/978-981-15-4630-3_4 [DOI]
- 155. Gomes M, Gomes LC, Teixeira-Santos R, Mergulhão FJ. PDMS in urinary tract devices: applications, problems and potential solutions. In: Carlsen PN, ed. Polydimethylsiloxane: Structure and Applications. 1st ed. Hauppauge, NY, USA: Nova Science Publishers; 2020. p. 95-144.
- 156.Shen Q, Shan Y, Lü Y, Xue P, Liu Y, Liu X. Enhanced antibacterial activity of poly(dimethylsiloxane) membranes by incorporating SiO2 microspheres generated silver nanoparticles. Nanomaterials. 2019;9(5):705. doi: 10.3390/nano9050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Keskin D, Mokabbar T, Pei Y, Van Rijn P. The relationship between bulk silicone and benzophenone-initiated hydrogel coating properties. Polymers (Basel) 2018;10(5):534. doi: 10.3390/polym10050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ji Y, Sun Y, Lang Y, Wang L, Liu B, Zhang Z. Effect of CNT/PDMS nanocomposites on the dynamics of pioneer bacterial communities in the natural biofilms of seawater. Materials (Basel) 2018;11(6):902. doi: 10.3390/ma11060902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun Y, Zhang Z. Anti-biofouling property studies on carboxyl-modified multi-walled carbon nanotubes filled PDMS nanocomposites. World J Microbiol Biotechnol. 2016;32(9):148. doi: 10.1007/s11274-016-2094-4. [DOI] [PubMed] [Google Scholar]
- 160.Hong J, Lee J, Hong CK, Shim SE. Effect of dispersion state of carbon nanotube on the thermal conductivity of poly(dimethyl siloxane) composites. Curr Appl Phys. 2010;10(1):359–63. doi: 10.1016/j.cap.2009.06.028. [DOI] [Google Scholar]
- 161.Kim T, Kim H. Effect of acid-treated carbon nanotube and amine-terminated polydimethylsiloxane on the rheological properties of polydimethylsiloxane/carbon nanotube composite system. Korea Aust Rheol J. 2010;22(3):205–10. [Google Scholar]
- 162.Lee JB, Khang DY. Electrical and mechanical characterization of stretchable multi-walled carbon nanotubes/polydimethylsiloxane elastomeric composite conductors. Compos Sci Technol. 2012;72(11):1257–63. doi: 10.1016/j.compscitech.2012.04.012. [DOI] [Google Scholar]
- 163.Kim TA, Kim HS, Lee SS, Park M. Single-walled carbon nanotube/silicone rubber composites for compliant electrodes. Carbon. 2012;50(2):444–9. doi: 10.1016/j.carbon.2011.08.070. [DOI] [Google Scholar]
- 164.So HM, Sim JW, Kwon J, Yun J, Baik S, Chang WS. Carbon nanotube based pressure sensor for flexible electronics. Mater Res Bull. 2013;48(12):5036–9. doi: 10.1016/j.materresbull.2013.07.022. [DOI] [Google Scholar]
- 165.Sepúlveda AT, Fachin F, Villoria RGd, Wardle BL, Viana JC, Pontes AJ. et al. Nanocomposite flexible pressure sensor for biomedical applications. Procedia Eng. 2011;25:140–3. doi: 10.1016/j.proeng.2011.12.035. [DOI] [Google Scholar]
- 166.Manawi YM, Samara A, Al-Ansari T, Atieh MA. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials (Basel) 2018;11(5):822. doi: 10.3390/ma11050822. [DOI] [PMC free article] [PubMed] [Google Scholar]


