Abstract
Background:
Adipose tissue (AT) expansion occurs by hypertrophy (increase in size) and hyperplasia (increase in number) of adipocytes. The AT expandability hypothesis postulates that impaired subcutaneous AT expansion leads to ectopic fat accretion, contributing to impaired metabolic health. The role of adipogenesis as a contributing factor is debatable.
Subjects/Methods:
In the present analysis, we assess changes in adipocyte size distribution in relation to changes in ectopic fat accretion in response to 8-weeks of overfeeding in 22 men (28 ± 5.4 years; BMI 25.5 ± 2.3kg/m2) who were fed 40% over their baseline energy requirements.
Results:
Participants gained 6.7 ± 2.1kg. The percentage of small adipocytes (p=0.03) and the peak diameter of large adipocytes (p=0.01) increased after overfeeding. At baseline, the percentage of small adipocytes was positively correlated with % body fat (p=0.03), SAT mass (p=0.01), VAT mass (p=0.02), VAT:TAT (p=0.05), and IHL (p=0.09; trend). The relative (percent) change in small adipocytes was positively associated with the increase in whole-body fat (p=0.001), VAT mass (p=0.0003), VAT:TAT (p=0.01), and IHL (p=0.007) in response to overfeeding.
Conclusions:
These findings, surprisingly, indicate that during substantial weight gain, an increase in small adipocytes (suggesting hyperplastic expansion) is associated with impaired (not improved) metabolic health outcomes, specifically visceral and ectopic fat accumulation.
ClinicalTrials.gov Identifier-NCT01672632
Introduction
White adipose tissue (AT) expands to accommodate changes in energy balance by enlargement of existing adipocyte size (hypertrophy) and increases in adipocyte number (hyperplasia or adipogenesis). The AT expandability hypothesis postulates that the limited expansion of subcutaneous AT may lead to visceral and ectopic fat deposition in the liver, skeletal muscle, and pancreas and contribute to the development of insulin resistance and type 2 diabetes mellitus (T2DM)[1, 2].
The role of adipogenesis in metabolic health as a contributing factor to lipid storage in visceral and ectopic depots remains debatable. Some studies reported a higher proportion of small adipocytes in insulin resistant and T2DM subjects compared to healthy individuals [3–5]. Our group [6] and others [7] have reported that individuals with smaller mean adipocyte size at baseline had worse health outcomes (e.g. decline in insulin sensitivity), than those with a larger mean adipocyte size in response to overfeeding.
Contrary to cross-sectional studies [3–5] and those that reported only mean adipocyte sizes [6], we have examined changes in adipocyte size distributions in relation to changes in clinical variables during weight gain in response to 8-weeks of 40% overfeeding in men (N=22) [6]. Our data show that an increased proportion of small adipocytes (i.e. hyperplasia) in subcutaneous AT is associated with impaired metabolic health outcomes, specifically visceral and ectopic fat accumulation, in response to overfeeding.
Materials/Subjects and Methods
Subject Characteristics
The study design and protocol have been previously described in detail [6]. Healthy individuals were recruited according to the following inclusion criteria: 20-40 years of age, body mass index (BMI) 22-32 kg/m2, normal laboratory tests, and weight stability (±2.5kg) for the previous 6-months. Exclusion criteria included a history of chronic disease, eating disorders, a BMI >32 kg/m2 at any point in life, HIV, and hepatitis B or C. Pennington Biomedical Research Center’s (PBRC) Institutional Review Board approved the protocol. All subjects gave written, informed consent.
Overfeeding Intervention
Participants completed a 2-week measurement of free-living energy expenditure using doubly labeled water to calculate baseline energy requirements [6]. Baseline energy requirements were multiplied by 1.4 to determine the 8-week overfeeding prescription. All meals (41% carbohydrate; 44% fat; 15% protein) were administered by PBRC’s Metabolic Kitchen. Consumption of breakfast, lunch, and dinner was supervised by staff.
Measurements at Baseline and Post-Overfeeding
Subjects consumed a weight-maintaining diet for 3-days before metabolic testing. Body fat was measured using dual x-ray absorptiometry (DXA; Hologics QDR 4500A). Scans were analyzed with QDR software v.11.1. Subcutaneous abdominal (SAT) and visceral (VAT) AT volumes were measured using a 3.0T scanner (General Electric, Excite HD System, Milwaukee, WI) by obtaining 240–340 images from the highest point of the liver through the pubic symphysis. Images were analyzed by a single technician using AnalyzeTM software (AnalyzeDirect, Overland Park, KS). VAT and SAT volumes were converted to mass using an assumed density of 0.92kg/L. Percent (%) VAT:TAT describes the ratio of VAT in proportion to total abdominal AT and was calculated as VAT/(SAT + VAT) x 100. IHL content was measured by 1H-MRS using jMRUI (Java-Based Magnetic Resonance User Interface) with IHL peak areas expressed relative to the peak area of an external oil phantom.
Insulin sensitivity (glucose infusion rate; GIR) was measured using a two-step hyperinsulinemic-euglycemic clamp as previously described [6]. The GIR (milligrams per minute per kilogram of estimated metabolic body size (FFM+ 17.7 kg) was calculated as the average amount of glucose required during the final 30 min of each insulin infusion step (10 and 50 mU/min·m2).
After anesthetizing the skin, AT biopsies were collected from the subcutaneous abdominal AT region using the Mercedes lipoaspirate technique and immediately fixed in osmium tetroxide. Adipocyte size was measured by a Multisizer-3 Coulter Counter (Beckman Coulter, Miami, FL) with a 400-μM aperture, which was set to count 6,000 particles and include a size range of 22 to 240μm [3]. Pulse sizes were collected, and data were expressed as particle diameters. Analysis of adipocyte size distribution for each subject entailed identification of the nadir, or the midway point where two cell populations were present. The percentage of cells with a diameter below the nadir represent the proportion of small adipocytes [3], and the diameter at which the Gaussian curve peaked was defined as the peak diameter of the large adipocytes (Supplementary Table 1). These measures have been shown to be more descriptive of adipocyte size distribution than mean cell size [3, 5].
Statistical Analysis
Statistical analyses were performed using SAS software 9.4 (SAS Institute Inc., Cary, NC). Data are presented as mean ± SD, with an α level of 0.05. Statistical tests were two-tailed. Comparisons of the covariates pre-and post-overfeeding were examined using paired t-tests. Simple associations between (a)baseline % small adipocytes versus measures of body composition and ectopic lipid and (b)the relative change in % small adipocytes versus relative changes in body composition and ectopic lipid were examined using Pearson’s correlation coefficients. Linear mixed effect models were used to adjust for other variables (% body fat, SAT mass, % VAT:TAT, and % IHL) in the analysis. Based on model assumptions, the final outcomes are expressed as relative (percent) change instead of change. Although the original study described 29 participants [6], this analysis includes a subset of men (n=22) who had adequate SAT samples available.
Results
Twenty-two men (28 ± 5.4 years; BMI 25.6 ± 2.3 kg/m2) who completed 8-weeks of 40% overfeeding and gained an average of 6.7 ± 2.1 kg were included in the analysis. Subjects had a higher proportion of small adipocytes (41 ± 21 vs. 56 ± 14; p=0.03) and an increased peak diameter of large adipocytes (87 ± 22 vs. 104 ± 14; p=0.01) post-overfeeding compared to baseline (Table 1). Mean relative (percent) changes of clinical characteristics with overfeeding are shown in Table 1. SAT mass increased by 32% (p<0.0001), VAT mass by 91% (p<0.0001), VAT:TAT by 38% (p=0.002), and IHL by 115% (p=0.001). At baseline, the percentage of small adipocytes was positively correlated with % body fat (R2=0.20; p=0.04), SAT mass (R2=0.29; p=0.01), VAT mass R2=0.26; p=0.02), and % VAT:TAT (R2=0.19; (p=0.05), and a trend with % IHL (R2=0.13; p=0.09) that did not reach statistical significance (data not shown). After adjusting for the other covariates (% body fat, SAT mass, % VAT:TAT, and % IHL), the percentage of small adipocytes was no longer correlated with % body fat, SAT mass, % VAT:TAT, or % IHL (p≥0.47). Figure 1 shows that the relative (percent) change in small adipocytes was positively associated with the percent change in body fat (A; R2=0.39; p=0.001), VAT mass (R2=0.57; p=0.0003; data not shown), VAT:TAT (B; R2=0.32; p=0.01), and IHL (C; R2=0.31; p=0.007), but not with the percent change in SAT mass (R2=0.03; p=0.47; data not shown). After adjusting for the other covariates, the percent change in small adipocytes remained positively associated with the percent change in body fat (p<0.0001), VAT:TAT (p=0.004), and IHL (p=0.01), but negatively associated with the percent change in SAT mass (p=0.003) (data not shown). There was a negative correlation (trend) between the percent change in small adipocytes and insulin sensitivity (GIR; 10 mU/min·m2 insulin; r=−0.42; p=0.06) that did not reach statistical significance. The percent change in small adipocytes was not associated with parameters of the lipid profile, or mean BP (data not shown; p≥0.84).
Table 1:
Adipose cell size distribution and clinical changes at baseline and post-overfeeding
| VARIABLE | BASELINE | POST-OVERFEEDING | |
|---|---|---|---|
|
| |||
| Mean ± SD | Mean ± SD | p value (% change) | |
|
| |||
| Small Adipocytes (%) | 41 ± 21 | 56 ± 15 | 0.03 |
|
|
|||
| Peak Diameter of Large Adipocytes | 87 ± 22 | 104 ± 14 | 0.01 |
|
| |||
| Body Weight (kg) | 81.7 ± 9.8 | 88.3 ± 10.1 | <.0001 |
|
|
|||
| BMI (kg/m2) | 25.6 ± 2.3 | 27.7 ± 2.5 | <.0001 |
|
|
|||
| Body Fat (%) | 18.2 ± 4.8 | 20.9 ± 4.8 | <.0001 |
|
|
|||
| SAT (kg) | 3.8 ± 1.6 | 5.0 ± 1.8 | <.0001 |
|
|
|||
| VAT (kg) | 0.56 ± 0.54 | 0.89 ± 0.66 | <.0001 |
|
|
|||
| VAT:TAT (%) | 11.5 ± 6.2 | 14.1 ± 6.3 | 0.002 |
|
| |||
| IHL (%) | 1.7 ± 3.9 | 2.5 ± 6.2 | 0.001 |
|
|
|||
| TRIG (mg/dL) | 89.7 ± 41.9 | 94.3 ± 73.9 | 0.77 |
|
| |||
| HDL (mg/dL) | 53.1 ± 11.4 | 55.9 ± 11.5 | 0.04 |
|
|
|||
| LDL (mg/dL) | 98.8 ± 26.27 | 119.9 ± 31.6 | <.0001 |
|
| |||
| Mean BP (mm Hg) | 85.4 ± 5.5 | 86.5 ± 4.1 | 0.27 |
|
|
|||
| Glucose Infusion (mg/min·[FFM+17.7]) | |||
|
| |||
| 10 (mU/min·m2 insulin) | 2.9 ± 1.0 | 2.4 ± 0.7 | 0.005 |
|
| |||
| 50 (mU/min·m2 insulin) | 11.5 ± 2.5 | 10.7 ± 2.6 | 0.03 |
Abbreviations: Adipose Tissue, AT; Body Mass Index, BMI; Blood Pressure, BP; High-Density Lipoprotein, HDL; Intrahepatic Lipid, IHL; Low-Density Lipoprotein, LDL; Subcutaneous Abdominal Adipose Tissue, SAT; Visceral Adipose Tissue, VAT; Visceral: Total Abdominal Adipose Tissue, VAT:TAT; Triglycerides, TG
Figure 1. The relative (%) change in small adipocytes in response to overfeeding was positively associated with the % changes in body fat (A), abdominal VAT:TAT (B), and IHL (C), but not SAT mass.
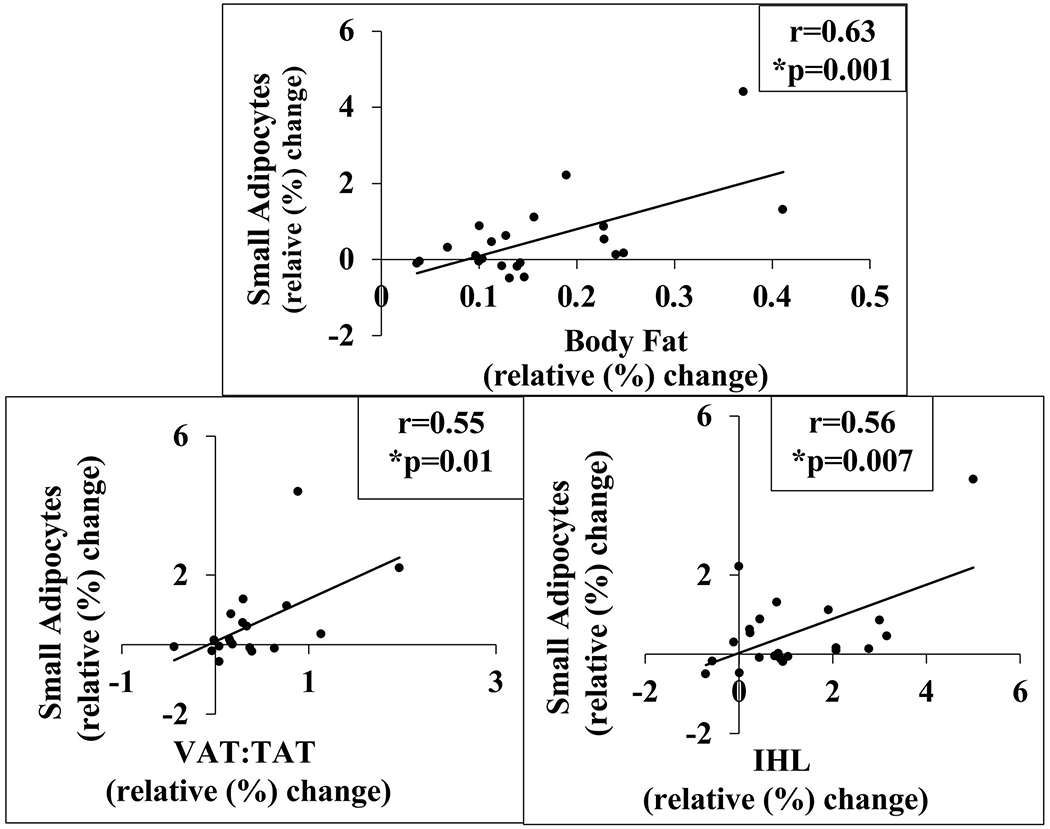
Simple associations between the % change in small adipocytes versus % changes in body composition and ectopic lipid were examined using Pearson’s correlation coefficients. The Pearson’s correlation between % change in small adipocytes and % change in body fat is 0.63 (R2=0.39; p=0.001) and is 0.17 (R2=0.03; p=0.47) between % change in small adipocytes and % change in SAT mass. The Pearson’s correlation between % change in small adipocytes and % change in VAT mass is 0.57 (R2=0.32; p=0.0003), is 0.55 (R2=0.32; p=0.01) between % change in VAT:TAT and % change in small adipocytes, and is 0.56 (R2=0.31; p=0.007) between % change in small adipocytes and % change in IHL. *Three participants did not complete the MRI, therefore, the total number of subjects with VAT data is N=19. The % change in IHL data was reanalyzed excluding data from two participants with % IHL of 17% and 10%. The Pearson correlation is 0.35 (R2=0.12; p=0.12).
Discussion
Some studies suggest that impaired hyperplasia is linked to metabolic dysfunction [8, 9], and a higher population of small adipocytes is associated with better glycemic and lipid profiles [10, 11]. Other studies demonstrate that individuals with insulin resistance or T2DM have a greater proportion of small adipocytes [3, 4]. We [6] and others [7] reported that individuals with smaller mean adipocyte sizes at baseline exhibited a greater decline in insulin sensitivity post-overfeeding. Given the limitations of measuring only mean adipocyte size, we have used a quantitative approach to estimate adipocyte size distribution and now show that an increased proportion of small adipocytes (hyperplasia) was associated with VAT and ectopic fat accumulation in the liver in response to overfeeding in men.
Our previous cross-sectional data from women demonstrated, for the first time, that higher in vivo adipogenesis was positively associated with VAT and decreased insulin sensitivity [12]. We now report longitudinal data during weight gain that also challenge the contribution of impaired adipogenesis to the AT expandability hypothesis. These findings imply the presence of small adipocytes with a decreased capacity to accommodate lipid, which may be influenced by changes in the extracellular matrix (ECM). In support of this hypothesis, our published data from this male cohort demonstrated that overfeeding induced a significant increase in the expression of ECM remodeling genes (COL1, COL3, and SPARC) and TGFβ, a fibrosis marker [13]. Together, ECM remodeling and impaired lipid handling of small adipocytes may contribute to ectopic lipid deposition.
Only one other overfeeding study has assessed changes in adipocyte size distribution in humans [7]. This study reported an increased peak diameter of large adipocytes post-overfeeding [7], as observed in our analysis (Table 1). Interestingly, the authors reported a decrease in the proportion of small adipocytes with overfeeding [7]. Notable differences between this study versus ours include the subject population (men and women; ~56yrs. vs. men; ~28yrs.), duration of overfeeding (4- vs. 8-weeks), and magnitude of weight gain (3.2kg vs. 6.7kg), which may explain the disparity in the results.
We observed a negative correlation (trend) between the increased proportion of small adipocytes with insulin sensitivity (GIR; 10 mU/min·m2 insulin; r=−0.42; p=0.06; trend). Though not statistically significant, these data suggest that an increase in the percentage of small adipocytes may be associated with a decline in insulin sensitivity, as previously suggested by other data [12]. Our data also showed that the increased peak diameter of large adipocytes was associated with the percent change (decline) in insulin sensitivity (GIR; r=−0.52; p=0.01), but was not associated with the percent change in VAT:TAT (r=0.14; p=0.55) or IHL (r=0.02; p=0.91). Hence, in this analysis, the index of hyperplasia (percentage of small adipocytes) was more closely related to VAT and ectopic lipid deposition than increased adipocyte size. Of note, it is well-accepted that greater hepatic and visceral lipid deposition are important markers for the development of insulin resistance and T2DM [14–16]. Interestingly, we also observed that the % change in small adipocytes was positively associated with the % change in leg fat (r=0.46; p=0.03) during overfeeding. Given the link between fat distribution and metabolic health, additional studies are needed to understand how mechanisms of AT expansion in one depot influence the functions of other depots.
In conclusion, our results from a well-controlled, longitudinal study demonstrate that an increase in the proportion of small adipocytes is associated with higher, not lower, VAT and ectopic fat accretion in the liver, indicating a worsened metabolic response to overfeeding. The use of osmium fixation (Multisizer analysis) is an accepted approach to estimate adipocyte size distribution [3–5, 17], and our data provide significant insight into the cellular mechanisms of adipocyte expansion during weight gain. Our analysis is limited by the inclusion of lean and overweight men; therefore, the results cannot be extrapolated to women or individuals with obesity. We analyzed only SAT biopsies; therefore, our results are not generalizable to all AT depots. Finally, it is difficult to establish causation for observed relationships in human studies. Nevertheless, numerous reports have suggested that mechanisms of AT expansion are key determinants of obesity-associated metabolic dysregulation [1]. Additional in vivo assessments during overfeeding interventions are necessary to further test the AT expandability hypothesis and better characterize the influence of adipocyte cellularity in the pathogenesis of metabolic disorders.
Supplementary Material
Acknowledgements
The author contributions are as follows. U.W. acquired the data, played an important role in interpreting the results, drafted and revised the manuscript, approved the final version, had full access to the data in the study, and agreed to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. R.A.B. acquired and analyzed experimental data, described all statistical methods, revised the manuscript, and approved the final version. E.R. conceived and designed the work that led to the submission, reviewed the data, played an important role in interpreting the results, revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work in ensuring the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R03DK112006 (U.W.) and R01DK060412 and P30DK072476 (E.R.) through the National Institutes of Health. R.A.B is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center.
Footnotes
Competing Interests
The authors declare no competing financial interests.
Parts of this work were presented at The Obesity Society Annual Meeting in Nashville, Tennessee (2018). The authors thank Caitlin Hebert (Pennington Biomedical Research Center) for technical assistance.
Data Availability
The data generated and/or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338–49. Epub 2010/01/09. [DOI] [PubMed] [Google Scholar]
- 2.Danforth E Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26(1):13. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50(8):1707–15. Epub 2007/06/06. [DOI] [PubMed] [Google Scholar]
- 4.Pasarica M, Xie H, Hymel D, Bray G, Greenway F, Ravussin E, et al. Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes care. 2009;32(5):900–2. Epub 2009/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring). 2014;22(3):673–80. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannsen DL, Tchoukalova Y, Tam CS, Covington JD, Xie W, Schwarz JM, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “adipose tissue expandability” hypothesis. Diabetes care. 2014;37(10):2789–97. Epub 2014/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin T, Craig C, Liu LF, Perelman D, Allister C, Spielman D, et al. Adipose Cell Size and Regional Fat Deposition as Predictors of Metabolic Response to Overfeeding in Insulin-Resistant and Insulin-Sensitive Humans. Diabetes. 2016;65(5):1245–54. Epub 2016/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–506. Epub 2001/01/11. [DOI] [PubMed] [Google Scholar]
- 9.Lessard J, Laforest S, Pelletier M, Leboeuf M, Blackburn L, Tchernof A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte. 2014;3(3):197–205. Epub 2014/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffstedt J, Arner E, Wahrenberg H, Andersson DP, Qvisth V, Lofgren P, et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia. 2010;53(12):2496–503. Epub 2010/09/11. [DOI] [PubMed] [Google Scholar]
- 12.White UA, Fitch MD, Beyl RA, Hellerstein MK, Ravussin E. Association of in vivo adipose tissue cellular kinetics with markers of metabolic health in humans. J Clin Endocrinol Metab. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CS, Covington JD, Bajpeyi S, Tchoukalova Y, Burk D, Johannsen DL, et al. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metab. 2014;99(5):1749–57. Epub 2014/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koska J, Stefan N, Permana PA, Weyer C, Sonoda M, Bogardus C, et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87(2):295–302. Epub 2008/02/09. [DOI] [PubMed] [Google Scholar]
- 15.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–75. Epub 2008/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bays H Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):345–51. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kursawe R, Eszlinger M, Narayan D, Liu T, Bazuine M, Cali AM, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59(9):2288–96. Epub 2010/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and/or analyzed during the study are available from the corresponding author on reasonable request.


