Abstract
Aims:
In the era of precision medicine, accurate pathologic diagnoses are crucial for appropriate management.
Methods:
We herein described the histologic features and clinical impacts of 66 salivary gland epithelial neoplasms in which the diagnosis was altered after expert review.
Results:
The most common revised diagnosis was that of salivary duct carcinoma (SDC, n = 12), adenoid cystic carcinoma (n = 12), and myoepithelial carcinoma (n = 10). The most common initial diagnosis was mucoepidermoid carcinoma (n = 19) with SDC being the most common revised diagnosis (7/19). Thirteen salivary gland carcinomas were initially diagnosed as benign entities, whereas five benign tumors were initially interpreted as carcinoma. The change in diagnosis was considered to be clinically significant in 65 (97%) cases.
Conclusions:
Given their rarity, salivary gland neoplasms are prone to diagnostic inaccuracy and discrepancy. A constellation of histologic features and ancillary studies are useful in reaching the correct diagnosis, which can have significant clinical impacts.
Keywords: adenoid cystic carcinoma, mucoepidermoid carcinoma, quality assurance, salivary duct carcinoma, salivary gland carcinoma
1 |. INTRODUCTION
The era of personalized precision medicine has introduced several new treatment modalities to the management of salivary gland neoplasia (e.g., targeted therapy and androgen deprivation therapy), making their diagnosis an even more complex endeavor.1–3 For instance, the precise diagnosis of salivary secretory carcinoma, which harbors NTRK3 fusion, is necessary to be eligible for TRK inhibitor targeted therapy.4 On the other hand, the rise in patient awareness of various treatment options has resulted in an increase in second opinion consults for the pathologist and clinician. Thus, the accurate diagnosis and subtyping of salivary gland carcinomas is more necessary than ever.
In this study, we gathered a retrospective cohort of 66 patients over an 11-year span with primary epithelial neoplasms of salivary gland (major or minor) whose initial diagnosis was significantly different from the one rendered in our tertiary cancer center. The principal aims of this study were tiered: first, to identify the main reason behind the misdiagnosis; second, to identify helpful approaches to reach the correct diagnosis, and finally, to assess the clinical impact of the second opinion pathology consultation.
2 |. METHODS
After obtaining Institutional Review Board (IRB) approval, the pathology archives of Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, USA) were searched for cases diagnosed from 2009 to 2020 fulfilling the following criteria: (1) surgical consultation cases that were diagnosed at another institute and re-reviewed at MSKCC with a quality assurance indicator of minor or major discrepancy and (2) a revised (MSKCC) diagnosis of primary epithelial neoplasm originating from a major or minor salivary gland. Exclusion criteria included: (1) lesions representing metastasis or/and secondary extension to the major or minor salivary gland from a mucosal or cutaneous tumor were excluded; (2) cases in which the original pathologist did not offer a definite diagnosis and/or one of the differential diagnoses provided included the revised diagnosis; and (3) personal consultation cases initiated by another pathologist to seek expert second opinion.
Among the 749 cases of primary salivary gland neoplasm reviewed at our center, a total of 66 (9%) cases fulfilling the above criteria were included in this study. The site of origin, original diagnoses, and revised diagnoses were documented. Molecular and/or cytogenetic testing was available on 15 (22%) cases. All cases with available slides were reviewed by Nora Katabi and Bin Xu to confirm the diagnosis at consensus meetings and to assess the reason(s) that led to the re-classification (i.e., histologic features, immunohistochemistry, and molecular/cytogenetic findings).
Furthermore, the potential clinical impact of the change in histologic diagnoses was assessed by Jennifer R. Cracchiolo, Bin Xu, and Nora Katabi and classified into five groups: (1) impact on the initial treatment modality (e.g., surgery, chemotherapy, and/or radiation therapy) and the extent of the surgery (e.g., with or without neck dissection); (2) impact on chemotherapy regimens and adjuvant therapy decision making; (3) impact related to potential hormonal and/or targeted therapies; and 4) impact related to patients’ follow-up and surveillance strategy. A change in histologic diagnosis was considered to be clinically significant if there was a potential alteration in one or more of the above impact groups. Clinical impact was considered insignificant if the clinical management and follow-up strategy remained the same after the change in histologic diagnosis.
3 |. RESULTS
Among the 749 cases of salivary gland neoplasms reviewed at our center between 2009 and 2020, 66 (9%) consult cases of primary epithelial neoplasms of salivary gland fulfilling the criteria defined in the methods were included in this study. Details of each case are provided in Table 1. Twenty-three (35%) cases were biopsies, whereas the remaining 43 (65%) were resections. The anatomic sites were major salivary glands (n = 42, 64%), oral cavity (n = 12, 18%), sinonasal tract (n = 3, 5%), oropharynx (n = 2, 3%), nasopharynx (n = 2, 3%), orbit (n = 2, 3%), parapharyngeal space (n = 1, 2%), external ear canal (n = 1, 2%), and trachea (n = 1, 2%).
TABLE 1.
Original and reviewed diagnosis of the study cohort
| N | Site | Specimen Type | Original diagnosis | Reviewed diagnosis | Reason for discrepancy | Fusion results |
|---|---|---|---|---|---|---|
| 1 | Major salivary | Resection | Chronic sialoadenitis | AciCC | Histology | |
| 2 | Major salivary | Resection | SDC admixed with AciCC | AciCC, high grade | Histology, IHC | |
| 3 | Major salivary | Resection | Adenocarcinoma, NOS | ACC | Histology, IHC | |
| 4 | EAC | Biopsy | Basal cell carcinoma of skin | ACC | Histology | |
| 5 | Trachea | Biopsy | PA | ACC | Histology | |
| 6 | Major salivary | Resection | EMC | ACC | Histology | |
| 7 | Oral | Resection | PAC | ACC | Histology, IHC | |
| 8 | Oral | Biopsy | PAC | ACC | Histology | |
| 9 | Oral | Biopsy | PAC | ACC favored | Histology | |
| 10 | Oral | Biopsy | Adenosquamous carcinoma | ACC | Histology, IHC, molecular | MYB fusion |
| 11 | Oral | Biopsy | PAC | ACC | Histology, IHC | |
| 12 | SNT | Resection | PD ca favor SNUC | ACC | Histology | No MYB fusion |
| 13 | SNT | Resection | ACC and adenocarcinoma NOS | ACC with high-grade transformation | Histology | |
| 14 | Parapharynx | Resection | CA ex PA | ACC with high-grade transformation | Histology, IHC | |
| 15 | Major salivary | Resection | MEC, high grade | Adenosquamous carcinoma favored | Histology | |
| 16 | Major salivary | Resection | ACC | BCA | Histology | |
| 17 | Major salivary | Resection | BCA | BCAC | Histology | |
| 18 | Major salivary | Resection | BCA | BCAC and PA | Histology, IHC | |
| 19 | Nasopharynx | Biopsy | SqCC | CCC | Histology, molecular | EWSR1 fusion |
| 20 | Oropharynx | Biopsy | SqCC | CCC | Histology, IHC, molecular | EWSR1-ATF1 |
| 21 | Oral | Biopsy | Adenocarcinoma, suggestive of SC | MEC | Histology | |
| 22 | Nasopharynx | Biopsy | Carcinoma with squamous features and focal mucin, favor adenosquamous carcinoma | MEC | Histology, molecular | MAML2 fusion |
| 23 | Major salivary | Resection | ACC | MECA | Histology | |
| 24 | Major salivary | Resection | EMC | MECA | Histology | |
| 25 | Major salivary | Resection | Oncocytic carcinoma | MECA | Histology, IHC | |
| 26 | Major salivary | Resection | PA | MECA | Histology | |
| 27 | Major salivary | Resection | PA | MECA favored | Histology | |
| 28 | Major salivary | Resection | PA | MECA favored | Histology | |
| 29 | Major salivary | Resection | PA | MECA ex PA | Histology | |
| 30 | Major salivary | Resection | PA | MECA ex PA | Histology | |
| 31 | Major salivary | Resection | PA | MECA ex PA | Histology | |
| 32 | Major salivary | Resection | Adenocarcinoma ex PA | MECA ex PA | Histology, IHC | No MAML2 fusion |
| 33 | Major salivary | Resection | MEC, high grade | NUT carcinoma | Histology, IHC, molecular | BRD4-NUTM1 |
| 34 | Major salivary | Resection | CA ex PA | PA, recurrent | Histology | |
| 35 | Major salivary | Resection | MEC | PA | Histology, molecular | No MAML2 fusion |
| 36 | Major salivary | Resection | Metastatic carcinoma | PA | Histology | |
| 37 | Major salivary | Resection | PAC | PA | Histology | |
| 38 | Oral | Resection | ACC | PAC | Histology | |
| 39 | Oral | Biopsy | Benign salivary tumor, favor monomorphic adenoma | PAC favored | Histology | |
| 40 | Oropharynx | Biopsy | MEC, high grade | PAC with high-grade transformation | Histology, IHC | |
| 41 | Major salivary | Biopsy | PA | PAC/CASG | Histology, IHC | |
| 42 | Major salivary | Resection | AciCC | SC | Histology, IHC, molecular | ETV6 fusion |
| 43 | Major salivary | Resection | AciCC | SC | Histology, IHC | No ETV6 fusion |
| 44 | Major salivary | Biopsy | MEC | SC | Histology, IHC, molecular | ETV6 fusion |
| 45 | Major salivary | Resection | MEC | SC with high-grade transformation | Histology, IHC, molecular | ETV6-NTRK3 |
| 46 | SNT | Resection | Adenocarcinoma, non-intestinal type | SC with high-grade transformation | Histology, IHC, molecular | ETV6 fusion |
| 47 | Major salivary | Resection | Adenosquamous carcinoma | SDC | Histology | |
| 48 | Major salivary | Resection | MEC, high grade | SDC | Histology | |
| 49 | Major salivary | Resection | MEC, high grade | SDC | Histology, IHC | |
| 50 | Major salivary | Resection | MEC, high grade | SDC | Histology, IHC | |
| 51 | Major salivary | Resection | MEC, high grade | SDC | Histology, IHC | |
| 52 | Major salivary | Resection | MEC, high grade | SDC | Histology, IHC | |
| 53 | Oral | Biopsy | MEC, high grade | SDC | Histology, molecular | No MAML2 fusion |
| 54 | Major salivary | Resection | MEC, low grade | SDC | Histology, IHC | |
| 55 | Major salivary | Resection | SqCC | SDC | Histology, IHC | |
| 56 | Major salivary | Biopsy | SqCC | SDC | Histology, IHC | |
| 57 | Major salivary | Biopsy | Benign | SDC (small cluster) | Histology | |
| 58 | Orbit | Resection | CA ex PA | SDC ex PA | Histology | |
| 59 | Orbit | Biopsy | SqCC | Carcinoma with basaloid features of salivary gland origin | Histology, IHC | |
| 60 | Major salivary | Resection | MEC, high grade | Carcinoma with squamous and basaloid features | Histology, molecular | No MAML2 fusion |
| 61 | Major salivary | Biopsy | MEC, high grade | Carcinoma with squamous features | Histology | |
| 62 | Oral | Biopsy | MEC | Carcinoma, differential diagnoses include SC and MEC | Histology | |
| 63 | Major salivary | Resection | MEC, high grade | Salivary carcinoma with SDC and MECA features, high grade | Histology | |
| 64 | Oral | Biopsy | Adenocarcinoma NOS | Salivary gland neoplasm with ductal and myoepithelial features. Resection diagnosis: EMC | Histology | |
| 65 | Oral | Biopsy | PAC | Salivary gland neoplasm with ductal and myoepithelial differentiation. The differential diagnosis includes ACC | Histology | |
| 66 | Major salivary | Biopsy | Mucin-producing carcinoma. Differential diagnoses include mucinous adenocarcinoma of skin adnexal origin and ACC | SMARCB1-deficient carcinoma | Histology, IHC |
Abbreviations: ACC, adenoid cystic carcinoma; AciCC, acinic cell carcinoma; BAC, basal cell adenoma; BCAC, basal cell adenocarcinoma; CA ex PA, carcinoma ex pleomorphic adenoma; CASG, cribriform adenocarcinoma of salivary gland; CCC, clear cell carcinoma; EMC, epithelial myoepithelial carcinoma; IHC, immunohistochemistry; MEC, mucoepidermoid carcinoma; NOS, not otherwise specified; PA, pleomorphic adenoma; PAC, polymorphous adenocarcinoma; SC, secretory carcinoma; SDC, salivary duct carcinoma.
The histologic diagnosis was revised based on (1) the histologic features (n = 66, 100%); (2) immunohistochemical profile (n = 27, 41%), and/or (3) the molecular/cytogenetic findings (n = 12, 18%). Among the 15 cases tested for gene fusions, nine showed specific gene fusions that supported the histologic diagnosis, including four ETV6 fusion-positive secretory carcinomas, two EWSR1 fusion-positive clear cell carcinomas, one MAML2 fusion-positive mucoepidermoid carcinoma, one MYB-translocated adenoid cystic carcinoma (ACC), and one BRD4-NUTM1 fusion-positive NUT carcinoma.2
Changing the diagnosis upon review was considered to be clinically significant in 65 (97%) cases. The potential clinical impact categories were as follows: impact on the initial treatment modality/extent of the surgery in 22 (33%) cases; impact on potential specific hormonal and/or targeted therapy in 19 (29%) cases; impact on chemotherapy/adjuvant therapy decision making in 16 (24%) cases; and impact related to patients’ follow-up and surveillance strategy in seven (10%) cases.
The original and revised diagnoses of the entire cohort are plotted in Figure 1. The revised final diagnoses of salivary gland tumors were as follows: salivary duct carcinoma (SDC) (n = 12), ACC (n = 12), myoepithelial carcinoma (n = 10), secretory carcinoma (n = 5), pleomorphic adenoma (n = 4), polymorphous adenocarcinoma (n = 4), basal cell adenocarcinoma (n = 2), clear cell carcinoma (n = 2), mucoepidermoid carcinoma (n = 2), acinic cell carcinoma (n = 2), parotid NUT carcinoma (n = 1), adenosquamous carcinoma (n = 1), basal cell adenoma (n = 1), SMARCB1-deficient carcinoma of the submandibular gland (n = 1), salivary carcinoma with salivary duct and myoepithelial features (n = 1), and six cases with descriptive diagnoses.
FIGURE 1.
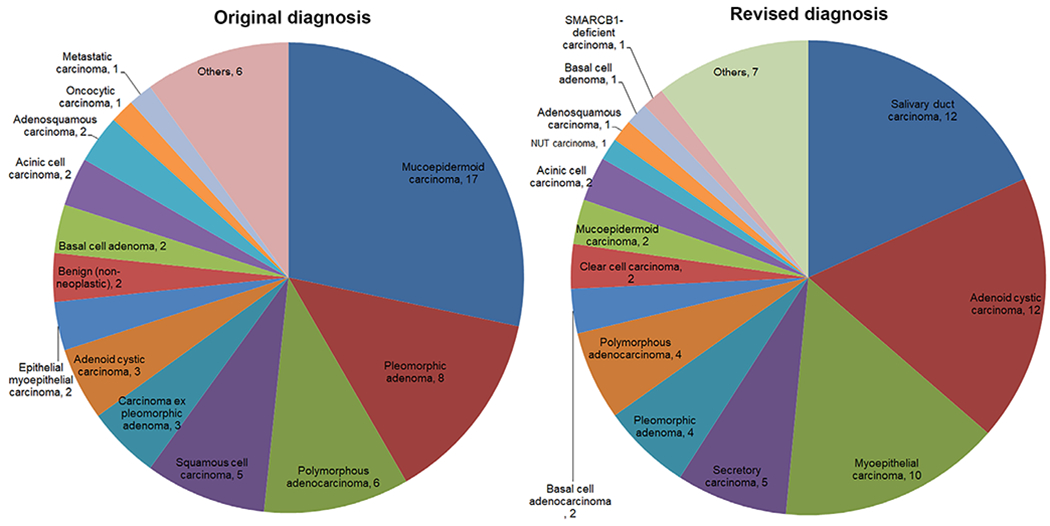
Pie charts of the original and revised diagnosis of the study cohort
The most common initial diagnosis was mucoepidermoid carcinoma (n = 19), including 12 cases that were diagnosed as high-grade mucoepidermoid carcinoma. The revised diagnoses for these 19 cases were: SDC (n = 7), carcinoma with squamous features (n = 3), secretory carcinoma with confirmed ETV6-NTRK3 fusion (n = 2), carcinoma with a differential diagnosis between secretory carcinoma and mucoepidermoid carcinoma (n = 1), pleomorphic adenoma (n = 1), adenosquamous carcinoma (n = 1), NUT carcinoma with confirmed BRD4-NUTM1 fusion (n = 1), polymorphous adenocarcinoma (n = 1), salivary carcinoma with SDC and myoepithelial carcinoma features (n = 1), and adenocarcinoma with apocrine features (n = 1).
Twelve cases had a revised diagnosis of SDC (Figure 2). The initial diagnoses for these tumors were mucoepidermoid carcinoma (n = 7, including six high grade and one low grade), squamous cell carcinoma (n = 2), adenosquamous carcinoma (n = 1), carcinoma ex pleomorphic carcinoma without further classification of the carcinoma component (n = 1), and a case that was called benign salivary gland but had a small cluster of SDC that was missed at the time of initial diagnosis (n = 1).
FIGURE 2.

Salivary duct carcinoma. (A and B) A salivary duct carcinoma was initially diagnosed as squamous cell carcinoma. Salivary duct carcinoma may appear squamoid showing solid architecture (A). However, it typically shows apocrine features with eosinophilic granular cytoplasm and prominent central nucleoli and diffuse/strong immunopositivity for AR (B). (C and D) A case of salivary duct carcinoma misinterpreted as high-grade mucoepidermoid carcinoma. As salivary duct carcinoma is a glandular tumor, it may contain mucocytes and intraluminal mucin (blue arrows) with solid (C) and cystic (D) architecture. Note the comedo-type tumor necrosis (N). Inset in C: mucicarmine stain shows mucocytes with intracellular mucin. Of note, this case was diffusely positive for AR immunohistochemical stain
Twelve cases had a revised diagnosis of ACC, two of which showed features of high-grade transformation. The initial diagnoses of these 12 cases were: polymorphous adenocarcinoma (n = 4), adenocarcinoma not otherwise specified (n = 1), cutaneous basal cell carcinoma (n = 1), pleomorphic adenoma (n = 1), epithelial myoepithelial carcinoma (n = 1), adenosquamous carcinoma (n = 1), poorly differentiated carcinoma favor sinonasal undifferentiated carcinoma (SNUC, n = 1), combined ACC with adenocarcinoma not otherwise specified (n = 1), and carcinoma ex pleomorphic adenoma without further classification of the carcinoma component (n = 1). All four ACCs misdiagnosed as polymorphous adenocarcinoma originated from the palate (Figure 3).
FIGURE 3.
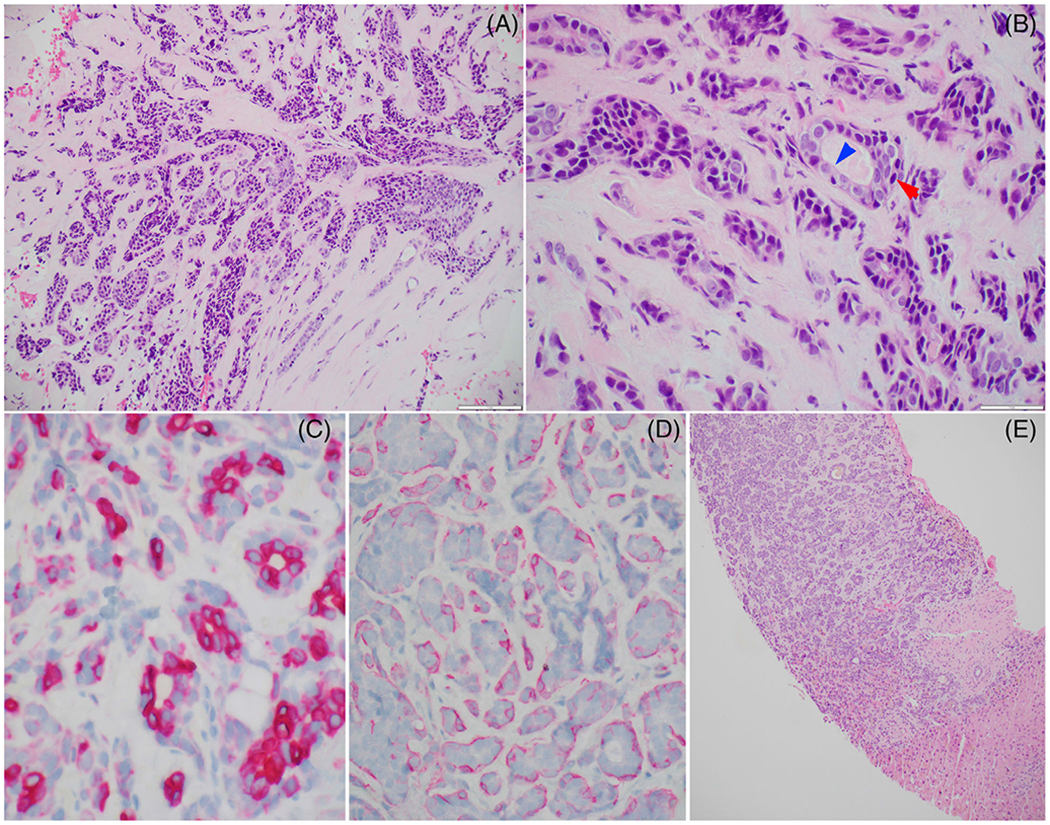
An adenoid cystic carcinoma of the palate initially misinterpreted as polymorphous adenocarcinoma. (A and B) The tumor shows predominant tubular architecture with biphasic ductal (blue arrow) and myoepithelial (red arrow) differentiation and contains dark angulated nuclei at the periphery of the tumor nests. (C) CK7 immunostain highlights the inner ductal cells, whereas smooth muscle actin (D) is positive in the outer myoepithelial cells. (E) The patient developed a liver metastasis
Four tumors had a revised diagnosis of polymorphous adenocarcinoma. The initial diagnoses were ACC, mucoepidermoid carcinoma, pleomorphic adenoma, and benign salivary neoplasm favor monomorphic adenoma.
A revised diagnosis of myoepithelial carcinoma was rendered in 10 cases. These tumors were initially diagnosed as pleomorphic adenoma (n = 6, Figure 4), epithelial myoepithelial carcinoma (n = 1), oncocytic carcinoma (n = 1), and adenocarcinoma ex pleomorphic adenoma (n = 1).
FIGURE 4.

Myoepithelial carcinoma (MECA) ex pleomorphic adenoma (PA) misdiagnosed as PA. (Top) At high power, the PA component contains ductal elements forming microcysts and myoepithelial component in the stroma. The MECA component is composed of monotonous myoepithelial cells with clear and epithelioid cytomorphology. (Bottom) The MECA shows expansile multinodular growth having a lobulated contour with alternating hypercellular (Hy) and hypocellular (H) zonal distribution
Five (5/66, 7.5%) cases with an initial diagnosis of squamous cell carcinoma were revised to salivary gland carcinomas, including SDC (n = 2), salivary clear cell carcinoma (n = 2), and salivary carcinoma with basaloid features (n = 1). The two cases of clear cell carcinoma originated from the nasopharynx and oropharynx. Both cases were positive for EWSR1 fusion (Figure 5(A),(B)).
FIGURE 5.

(A and B) A clear cell carcinoma of salivary gland originating from oropharynx initially diagnosed as a squamous cell carcinoma. Noted the relatively monotonous tumor cells, the nested growth, and the abundant clear cytoplasm. (C and D) Acinic cell carcinoma with prominent tumor-associated lymphoid stroma that was misdiagnosed as chronic lymphocytic sialadenitis. Acinic cell carcinoma lacks the lobulated architecture seen in normal salivary gland
Thirteen (13/66, 19.7%) revised salivary gland carcinomas were initially diagnosed as benign entities, including pleomorphic adenoma (n = 8), basal cell adenoma (n = 2), monomorphic adenoma (n = 1), chronic sialadenitis (n = 1), and benign salivary gland in which a focus of SDC was missed (n = 1). A case of acinic cell carcinoma with prominent tumor-associated lymphocytic stroma was misdiagnosed as chronic sialadenitis (Figure 5(C),(D)).
Five (5/66, 7.5%) cases had a final diagnosis of benign salivary gland neoplasms but were initially diagnosed as malignant. The initial diagnoses were ACC (n = 1), mucoepidermoid carcinoma (n = 1), metastatic carcinoma (n = 1), polymorphous adenocarcinoma (n = 1), and carcinoma ex pleomorphic adenoma (n = 1). The revised diagnoses were pleomorphic adenoma (n = 4) and basal cell adenoma (n = 1).
4 |. DISCUSSION
Documentation of diagnostic discordance is a major component of quality assurance in anatomic pathology aims to ensure patient safety. The reported discrepancy rate varies significantly from 1% to 14%.5–7 Furthermore, site-specific studies have suggested a higher frequency of discordance in head and neck pathology compared with other subspecialties.5–7 Within the field of head and neck pathology, the salivary gland is the most common subsite to trigger a second-opinion expert review.8 We herein studied a retrospective cohort of 66 discrepant cases of primary epithelial neoplasms of major and minor salivary gland with an aim to provide useful histologic features and diagnostic approaches to achieve accurate pathologic diagnosis.
In our cohort, SDC was the most common revised diagnosis. SDC is a highly aggressive tumor that requires a multimodal treatment approach, including androgen deprivation therapy and Her2 inhibitor-targeted therapy.9–11 Although uncommon, overt squamous differentiation may occur in SDC, but it is usually focal and associated with areas of typical SDC morphology. Moreover, the apocrine morphology of SDC with the eosinophilic cytoplasm can be misinterpreted as squamous cell carcinoma or high-grade mucoepidermoid carcinoma even without a clear-cut squamous differentiation, or when SDC displays an infiltrative solid pattern without obvious glandular formation. As over 90% of SDC is diffusely positive for androgen receptor (AR) by immunohistochemistry,12–14 utilizing AR immunohistochemical stain and recognizing the typical SDC apocrine cytomorphology with prominent central nucleoli and eosinophilic granular cytoplasm can help establish the diagnosis.14 Differentiating SDC from squamous cell carcinoma is critical as this differential often occurs in major salivary gland where most squamous cell carcinomas are metastatic and may require radiotherapy, whereas patients’ with SDC may benefit from androgen deprivation therapy and Her2 inhibitor targeted therapy instead.
Similar to recent reports,15,16 mucoepidermoid carcinoma was one of the commonly misdiagnosed tumors in our cohort, particularly when high grade. Various major or minor salivary gland carcinomas can show squamoid and/or mucinous features and therefore may be misinterpreted as mucoepidermoid carcinoma.14–22 SDC was the most common revised diagnosis of mucoepidermoid carcinomas. Finding areas showing typical lower-grade mucoepidermoid carcinoma, molecular/cytogenetic studies demonstrating MAML2 fusion and immunohistochemistry with positive staining for squamous markers (CK5/6, p63, and p40) and negative staining for AR, S100, and myoepithelial markers (smooth muscle actin and calponin) are important tools to establish the diagnosis of mucoepidermoid carcinoma and differentiate it from other entities.
ACC is a relatively aggressive salivary gland carcinoma characterized by late relapses and poor long-term prognosis.18,21 Postoperative radiotherapy is generally considered to be beneficial with improved local control rate.23 In our cohort, several ACCs were misclassified as polymorphous adenocarcinomas. It is important to differentiate polymorphous adenocarcinoma from ACC as the former has an overall excellent prognosis and commonly does not require adjuvant radiotherapy.24–26 These two tumors share certain clinicopathologic similarities: both tumors may show tubular, cribriform, and solid patterns, may involve the palate, and both have a propensity for perineural invasion.18,21,24,27 However, unlike polymorphous adenocarcinoma that is composed of one type of tumor cells with uniform open nuclei, ACC is biphasic, showing both ductal and myoepithelial elements and contains dark hyperchromatic nuclei. Additionally, polymorphous adenocarcinoma has a wide range of histologic appearances and may show other unique architectural patterns, for example, single-file arrangement, papillary, and trabecular growth.28 It is worthwhile to mention that the biphasic pattern of ACC may not be evident in the solid pattern, which is an architecture pattern that associated with adverse clinical outcome.21,29,30 In such cases, a meticulous search for the biphasic epithelial and myoepithelial population in the residual tubular and cribriform areas is helpful to establish the diagnosis of ACC. In challenging cases and small biopsy material, molecular testing for characteristic alterations, including MYB, MYBL1 and/or NFIB fusion in ACC31–33 or PRKD1 hotspot mutation in polymorphous adenocarcinoma,34–36 may provide clarification.
It may be difficult to rendering definite diagnosis of salivary gland neoplasm in small biopsy material, which constituted 35% of the study cohort. A constellation of histologic, immunohistochemistry, and molecular findings may be useful to establish the diagnosis.
In our cohort, myoepithelial carcinoma (n = 10) was the most commonly revised diagnosis among salivary gland tumors, with 60% initially misclassified as pleomorphic adenoma. Clinically, myoepithelial carcinoma is relatively aggressive, with approximately 25%−30% risk of distant metastasis.19,37 The pathologic diagnosis of myoepithelial carcinoma may be challenging and may be subjective even among expert head and neck pathologists, especially when arising in a pre-existing pleomorphic adenoma component (i.e., myoepithelial carcinoma ex pleomorphic adenoma). In our opinion, the diagnostic histologic features to recognize malignancy in myoepithelial tumors are the uniform clonal morphology of the myoepithelial proliferation, the presence of an invasive expansile nodular pattern, and the typical zonal cellular distribution with a necrotic/hypocellular center and a hypercellular peripheral zone.37 At the molecular level, PLAG1 fusion is the most common molecular alteration in myoepithelial carcinoma, in both de novo and ex pleomorphic adenoma setting.38 Nonetheless, the presence of PLAG1 fusion is not helpful in differentiating between pleomorphic adenoma and myoepithelial carcinoma.38,39
Tumor-associated lymphoid stroma is a common histologic finding in salivary gland neoplasms, in particular acinic cell carcinoma40 and mucoepidermoid carcinoma.41,42 The significance of recognizing tumor-associated lymphoid stroma is twofold: first, to histologically separate carcinomas from non-neoplastic conditions (e.g., chronic sialadenitis) on one hand, and from benign tumors with lymphoid stroma (e.g. Warthin tumor) on the other hand, and second, to distinguish primary tumor with lymphoid stroma from metastases to intra- or periparotid lymph nodes. In this study, a case of acinic cell carcinoma with prominent lymphoid stroma was misdiagnosed as chronic sialadenitis. The key histologic features to separate chronic sialadenitis from lymphoid stroma associated with acinic cell carcinoma are the lobulated architecture of normal salivary gland with acini and ductal system as compared with the neoplastic process of acinic cell carcinoma, in which various tumor cell types (zymogen granule-rich, vacuolated, and ductal cells) are arranged in unorganized sheets and clusters within the tumor mass.
NUT carcinoma characterized by NUTM1 gene fusion leading to immunoexpression of the NUT protein has been described in various head and neck anatomic regions,43,44 including major salivary gland.45 In this series, a case of NUT carcinoma of the parotid gland was initially misdiagnosed as mucoepidermoid carcinoma. The native entrapped salivary ducts were misinterpreted as the glandular component of mucoepidermoid carcinoma. NUT immunopositivity is essential to establish the diagnosis and guide therapy. Based on the limited data, the effect of chemotherapy may not last in NUT carcinomas.46
In our cohort, one case of salivary clear cell carcinoma was initially diagnosed as p16-positive oropharyngeal squamous cell carcinoma. As per National Comprehensive Cancer Network (NCCN) guidelines, definitive treatment for p16/HPV-positive oropharyngeal squamous cell carcinoma includes primary radiation or primary surgery approaches as equivalent,47 whereas the NCCN recommends primary surgery for the treatment of malignancies of salivary gland origins. Therefore, after the pathology review of that specific case, a recommendation for primary surgery was made.
Extent of primary surgery can also vary according to the histologic diagnosis. In primary salivary gland malignancies, elective neck dissection is indicated in high-grade and high-stage cancers.48,49 This represents a change in clinical care for cases initially interpreted as benign or low-grade neoplasm but was reclassified as - high-grade lesions.
A recommendation for adjuvant therapy can also vary. For example, in one case where we revised the diagnosis from metastatic carcinoma to the neck to a recurrent pleomorphic adenoma, the patient was seen with a multifocal neck recurrence and a prior history of submandibular gland excision 30 years ago. He underwent a modified radical neck dissection with plan for adjuvant therapy; however, after revision of the histologic diagnosis to pleomorphic adenoma, the management changed to close observation.
Additionally, accurate pathologic diagnosis can alter options for targeted and hormonal therapies. Of note, some of these therapies are both pursued in regular practice or in the context of a clinical trial. Emerging treatment options in clinical/clinical trial setting include TRK inhibitor for secretory carcinoma harboring NTRK3 fusion,4 androgen deprivation and Her2 inhibitor therapy for SDC,50 Bromodomain inhibitor for NUT carcinoma with BRD4-NUTM1 rearrangement,51 and EZH2 inhibitor for tumors with loss-of-function mutations in epigenetic factors (e.g., SMARCB1-deficient sinonasal carcinoma).52
Finally, surveillance schedules and location of possible recurrences would likely change based on pathologic re-review in some cases. NCCN recommends follow-up-based risk of relapse.49 In cases where the diagnosis changed from benign to malignant (e.g., from pleomorphic adenoma to carcinoma ex pleomorphic adenoma), more definitive therapy may be indicated. Furthermore, distant disease and patterns of spread also can be histologically dependent and possible location of recurrence is also dictated by accurate pathologic diagnosis. For instance, changing the diagnosis of polymorphous adenocarcinoma to ACC may modify the patients surveillance plan18 with closer follow-up and imaging in view of the higher local/distant recurrence rate and the neurotropic behavior of ACC compared to polymorphous adenocarcinoma.
In summary, by studying a large retrospective cohort of discrepant cases in our head and neck consultation service, we identified histologic pitfalls and key diagnostic features that can help pathologists make the correct diagnosis. We also underscored the potential clinical impact resulting from this change in diagnosis. The rarity and the clinicopathologic overlap of malignant salivary gland neoplasms make them especially prone to misdiagnosis, even by experienced pathologists. In our opinion, the pathologists may consider a second expert opinion by a Head and Neck Pathologist when facing a challenging salivary gland tumor.
ACKNOWLEDGMENTS
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Cancer Institute, Grant/Award Number: P30CA008748
Footnotes
CONFLICTS OF INTEREST
The authors have disclosed that they have no significant relationships with or financial interest in any commercial companies pertaining to this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.von der Grun J, Rodel F, Brandts C, et al. Targeted therapies and immune-checkpoint inhibition in head and neck squamous cell carcinoma: where do we stand today and where to go? Cancers. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Even C, Baste N, Classe M. New approaches in salivary gland carcinoma. Curr Opin Oncol. 2019;31(3):169–174. [DOI] [PubMed] [Google Scholar]
- 3.Kashat L, Le CH, Chiu AG. The role of targeted therapy in the management of sinonasal malignancies. Otolaryngol Clin North Am. 2017;50(2):443–455. [DOI] [PubMed] [Google Scholar]
- 4.Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol. 2016;27(5):920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strosberg C, Gibbs J, Braswell D, et al. Second opinion reviews for cancer diagnoses in anatomic pathology: a comprehensive cancer center’s experience. Anticancer Res. 2018;38(5):2989–2994. [DOI] [PubMed] [Google Scholar]
- 6.Kronz JD, Westra WH. The role of second opinion pathology in the management of lesions of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2005;13(2):81–84. [DOI] [PubMed] [Google Scholar]
- 7.Raab SS, Nakhleh RE, Ruby SG. Patient safety in anatomic pathology: measuring discrepancy frequencies and causes. Arch Pathol Lab Med. 2005;129(4):459–466. [DOI] [PubMed] [Google Scholar]
- 8.Mullin MH, Brierley DJ, Speight PM. Second opinion reporting in head and neck pathology: the pattern of referrals and impact on final diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(6):656–660. [DOI] [PubMed] [Google Scholar]
- 9.Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473–e476. [DOI] [PubMed] [Google Scholar]
- 10.Johnston ML, Huang SH, Waldron JN, et al. Salivary duct carcinoma: treatment, outcomes, and patterns of failure. Head Neck. 2016;38(Suppl 1):E820–E826. [DOI] [PubMed] [Google Scholar]
- 11.Yeoh CC, Dabab N, Rigby E, et al. Androgen receptor in salivary gland carcinoma: a review of an old marker as a possible new target. J Oral Pathol Med. 2018;47(7):691–695. [DOI] [PubMed] [Google Scholar]
- 12.Xu B, Dogan S, Haroon Al Rasheed MR, Ghossein R, Katabi N. Androgen receptor immunohistochemistry in salivary duct carcinoma: a retrospective study of 188 cases focusing on tumoral heterogeneity and temporal concordance. Hum Pathol. 2019;93:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39(5):705–713. [DOI] [PubMed] [Google Scholar]
- 14.Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11(3):288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seethala RR, Chiosea SI. MAML2 status in mucoepidermoid carcinoma can no longer be considered a prognostic marker. Am J Surg Pathol. 2016;40(8):1151–1153. [DOI] [PubMed] [Google Scholar]
- 16.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. [DOI] [PubMed] [Google Scholar]
- 17.Simpson RH. Salivary duct carcinoma: new developments – morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol. 2013;7 (Suppl 1):S48–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Naggar AKJC, Grandis JR, Takata T, Grandis J, Slootweg PP. WHO Classification of Head and Neck Tumours. 4th ed. Lyon: IARC; 2017. [Google Scholar]
- 19.Kong M, Drill EN, Morris L, et al. Prognostic factors in myoepithelial carcinoma of salivary glands: a clinicopathologic study of 48 cases. Am J Surg Pathol. 2015;39(7):931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinreb I Hyalinizing clear cell carcinoma of salivary gland: a review and update. Head Neck Pathol. 2013;7 (Suppl 1):S20–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu B, Drill E, Ho A, et al. Predictors of outcome in adenoid cystic carcinoma of salivary glands: a clinicopathologic study with correlation between MYB fusion and protein expression. Am J Surg Pathol. 2017;41(10):1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seethala RR, Hunt JL, Baloch ZW, Livolsi VA, Leon BE. Adenoid cystic carcinoma with high-grade transformation: a report of 11 cases and a review of the literature. Am J Surg Pathol. 2007;31(11):1683–1694. [DOI] [PubMed] [Google Scholar]
- 23.Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: a review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38(4):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Aneja A, Ghossein R, Katabi N. Predictors of outcome in the phenotypic spectrum of polymorphous low-grade adenocarcinoma (PLGA) and cribriform adenocarcinoma of salivary gland (CASG): a retrospective study of 69 patients. Am J Surg Pathol. 2016;40(11):1526–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elhakim MT, Breinholt H, Godballe C, et al. Polymorphous low-grade adenocarcinoma: a Danish national study. Oral Oncol. 2016;55:6–10. [DOI] [PubMed] [Google Scholar]
- 26.Evans HL, Batsakis JG. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 14 cases of a distinctive neoplasm. Cancer. 1984;53(4):935–942. [DOI] [PubMed] [Google Scholar]
- 27.Seethala RR, Johnson JT, Barnes EL, Myers EN. Polymorphous low-grade adenocarcinoma: the University of Pittsburgh experience. Arch Otolaryngol Head Neck Surg. 2010;136(4):385–392. [DOI] [PubMed] [Google Scholar]
- 28.Waldron CA, el-Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66(3):323–333. [DOI] [PubMed] [Google Scholar]
- 29.van Weert S, van der Waal I, Witte BI, Leemans CR, Bloemena E. Histopathological grading of adenoid cystic carcinoma of the head and neck: analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015;51(1):71–76. [DOI] [PubMed] [Google Scholar]
- 30.Zhang CY, Xia RH, Han J, et al. Adenoid cystic carcinoma of the head and neck: clinicopathologic analysis of 218 cases in a Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(3):368–375. [DOI] [PubMed] [Google Scholar]
- 31.Ferrarotto R, Heymach JV, Glisson BS. MYB-fusions and other potential actionable targets in adenoid cystic carcinoma. Curr Opin Oncol. 2016;28(3):195–200. [DOI] [PubMed] [Google Scholar]
- 32.Mitani Y, Li J, Rao PH, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16(19):4722–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106(44):18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sebastiao APM, Xu B, Lozada JR, et al. Histologic spectrum of polymorphous adenocarcinoma of the salivary gland harbor genetic alterations affecting PRKD genes. Mod Pathol. 2020;33(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreasen S, Melchior LC, Kiss K, et al. The PRKD1 E710D hotspot mutation is highly specific in separating polymorphous adenocarcinoma of the palate from adenoid cystic carcinoma and pleomorphic adenoma on FNA. Cancer Cytopathol. 2018;126(4):275–281. [DOI] [PubMed] [Google Scholar]
- 36.Weinreb I, Piscuoglio S, Martelotto LG, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu B, Mneimneh W, Torrence DE, et al. Misinterpreted myoepithelial carcinoma of salivary gland: a challenging and potentially significant pitfall. Am J Surg Pathol. 2019;43(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalin MG, Katabi N, Persson M, et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat Commun. 2017;8(1):1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52(7):675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michal M, Skalova A, Simpson RH, Leivo I, Ryska A, Starek I. Well-differentiated acinic cell carcinoma of salivary glands associated with lymphoid stroma. Hum Pathol. 1997;28(5):595–600. [DOI] [PubMed] [Google Scholar]
- 41.Bishop JA, Cowan ML, Shum CH, Westra WH. MAML2 rearrangements in variant forms of mucoepidermoid carcinoma: ancillary diagnostic testing for the ciliated and Warthin-like variants. Am J Surg Pathol. 2018;42(1):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishibashi K, Ito Y, Masaki A, et al. Warthin-like Mucoepidermoid carcinoma: a combined study of fluorescence in situ hybridization and whole-slide imaging. Am J Surg Pathol. 2015;39(11):1479–1487. [DOI] [PubMed] [Google Scholar]
- 43.Napolitano M, Venturelli M, Molinaro E, Toss A. NUT midline carcinoma of the head and neck: current perspectives. Onco Targets Ther. 2019;12:3235–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEvoy CR, Fox SB, Prall OWJ. Emerging entities in NUTM1-rearranged neoplasms. Genes Chromosomes Cancer. 2020;59(6):375–385. [DOI] [PubMed] [Google Scholar]
- 45.Agaimy A, Fonseca I, Martins C, et al. NUT carcinoma of the salivary glands: clinicopathologic and molecular analysis of 3 cases and a survey of NUT expression in salivary gland carcinomas. Am J Surg Pathol. 2018;42(7):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18(20):5773–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattox AK, Lee J, Westra WH, et al. PD-1 expression in head and neck squamous cell carcinomas derives primarily from functionally Anergic CD4(+) TILs in the presence of PD-L1(+) TAMs. Cancer Res. 2017;77(22):6365–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong JG, Harrison LB, Spiro RH, Fass DE, Strong EW, Fuks ZY. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990;116(3):290–293. [DOI] [PubMed] [Google Scholar]
- 49.Kasaian K, Wiseman SM, Walker BA, et al. The genomic and transcriptomic landscape of anaplastic thyroid cancer: implications for therapy. BMC Cancer. 2015;15:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakaguro M, Tada Y, Faquin WC, Sadow PM, Wirth LJ, Nagao T. Salivary duct carcinoma: updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. 2020;128(10):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison-Smith CD, Knox TM, Filic I, et al. Combined targeting of the BRD4-NUT-p300 axis in NUT midline carcinoma by dual selective bromodomain inhibitor, NEO2734. Mol Cancer Ther. 2020;19(7):1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamagishi M, Uchimaru K. Targeting EZH2 in cancer therapy. Curr Opin Oncol. 2017;29(5):375–381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


