Summary
Background
The Endocrine Society Clinical Practice Guidelines recommend the avoidance of medications that may cause weight gain (i.e., obesogenic medications) in individuals with overweight or obesity. Obesity disproportionately affects people with lower socioeconomic status (SES); however, it is unknown whether the use of obesogenic medications differs by SES.
Methods
We included adults with overweight or obesity and used prescription medications from 2009-2018 of the US National Health and Nutrition Examination Survey. We examined the associations between a composite measure of SES and use of obesogenic medications and anti-obesity medications. The composite SES included <high school education (1 point), household income below federal poverty level (1 point), no insurance (2 points), and public health insurance only (1 point). We defined 3 composite SES groups (0 [high], 1 [intermediate], and ≥2 points [low]).
Findings
Among 10,673 US adults with overweight or obesity, 20.0% had low SES. Use of obesogenic medications was common (37.7%). Low (vs. high) SES was associated with greater obesogenic medication use, independent of demographic characteristics, prescription medication burden, and comorbidities (OR 1.3 [1.2-1.5]). Among 12,133 eligible participants, utilization of anti-obesity medications was very low overall (0.5%) and within all SES groups (low 0.27%, intermediate 0.71, and high 0.65%).
Interpretation
Our findings highlight common and modifiable risk factors for obesity. Clinicians should screen patient medications for those that may cause weight gain and increase adoption of anti-obesity medications, especially among adults living in low SES.
Funding
The National Institute of Diabetes and Digestive and Kidney Disease (R01DK115534, K24HL155861, and K01DK121825).
Keywords: Obesity, Obesogenic medications, Socioeconomic status, Disparities, Pharmacoequity
Research in context.
Evidence before this study
We searched PubMed and Google scholar for relevant articles in English, published since 2000, using the search terms “obesogenic medication”, “medications that cause weight gain”, “antiobesity medication”, “antiobesity pharmacotherapy”, “anti-obesity medication”, “anti-obesity phamarcotherapy”. To our knowledge, there is only one study on the use of obesogenic medication in the US by Hales et al. There are several studies on the trend of use of anti-obesity medication in the US. There is no study on the association between socioeconomic status and use of obesogenic medication or anti-obesity medication.
Added value of this study
We systematically assessed the use of obesogenic medication and anti-obesity medication among US adults with overweight or obesity. We found that among US adults with body-mass indices over 25 kg/m2, use of obesogenic medications was more common among people with lower socioeconomic status, even after accounting for differences in the numbers of prescription medications and comorbidities. Utilization of anti-obesity medications was limited, irrespective of socioeconomic status, with less than 1% of eligible adults receiving these medications.
Implications of all the available evidence
There are substantial gaps between guideline recommendations and the real-world use of obesogenic medications and anti-obesity medications in the US. There are socioeconomic disparities in use of obesogenic medications in the US. Clinicians should carefully screen patients' medical regimens for obesogenic medications that can be replaced by other medications that do not cause weight gain, and increase utilization of anti-obesity medications, especially among adults with low socioeconomic status.
Alt-text: Unlabelled box
Introduction
Obesity is a serious and growing public health challenge in the US and worldwide.1,2 In the US, nearly 1 in 3 adults are overweight (body mass index [BMI] 25.0-29.9 kg/m2), 42.4% of adults have obesity (BMI ≥30 kg/m2), and nearly 1 in 10 have severe obesity (BMI ≥40 kg/m2).3 Overweight and obesity are associated with higher risk of mortality, an expanding set of chronic diseases, reduced quality of life, and social disadvantages.4, 5, 6, 7 Halting and reversing the obesity epidemic has been a top national priority for decades yet the epidemic continues to worsen.
The cause of obesity is multifactorial and includes genetic, physiological, behavioral, sociocultural and environmental factors.8 Emerging evidence suggests that commonly prescribed medications can also cause weight gain (i.e., obesogenic medications) and may contribute to the obesity epidemic.9,10 Many of these medications have alternatives without obesogenic effects. In 2015, the Endocrine Society Clinical Practice Guidelines recommended the avoidance of obesogenic medications in patients with overweight or obesity, if possible.11 Weight gain secondary to medications is potentially avoidable and could be an important target of action to control the obesity epidemic. For the large population who are overweight in the US, avoidance of obesogenic medications may lower the risk of additional weight gain and lower the burden of obesity in the population. Recently, Hales et al. showed that use of obesogenic medications was common and increasing over time in the US.12
Inequalities in access to high quality medical care are documented across a wide range of diseases.13, 14, 15, 16, 17 Lower socioeconomic status (SES) is associated with lower immunizations,15 lower cancer screening,16 and lower quality of ambulatory and hospital care.17 However, it is unknown whether there are inequalities in the use of obesogenic medications. As obesity disproportionally affects people with lower SES,18 identifying and reducing potential disparities in obesogenic medication use could be an important step to reduce obesity. Further, appropriate use of anti-obesity medications is another important aspect in obesity management as these medications produce durable weight loss over and above behavioral interventions.19
In the current study, we sought to examine whether the use of obesogenic medications differs by SES among US adults with overweight or obesity. We also examined the use of anti-obesity medications and examined potential difference by SES. We hypothesized that the population with lower SES is more likely to use obesogenic medications and less likely to use anti-obesity medications.
Methods
Data source and study population
The National Health and Nutrition Examination Survey (NHANES) data is a nationally representative sample of the noninstitutionalized civilian residents of the US population.20 The NHANES uses a complex, stratified, multistage probability-cluster sampling design and collected information on demographic, socioeconomic, dietary, and health-related questions. The National Center for Health Statistics (NCHS) Research Ethics Review Board approved NHANES. Written informed consent was obtained from all adult participants.
The population for the primary analysis included NHANES participants from 2009 through 2018 who were 20 years of age or older, nonpregnant, had overweight (BMI 25-29.9 kg/m2) or obesity (BMI ≥30 kg/m2), took at least one prescription medication, and had complete information on education, income, and insurance (Supplemental Figure 1). We limited the study population to those with overweight or obesity because clinical guidelines recommended avoidance of obesogenic medications in this population and not in the population with under/normal weight.11
Exposure variables
Participants self-reported education, annual household income, and insurance status. Education was classified as less than high school, high school graduate, some college, or college graduate or above. Household income was classified as below vs. equal or above federal poverty level. Health insurance status was classified as no insurance, public insurance only (including Medicare, Medicaid, State Children's Healthcare Plan, military healthcare, Indian Health Service, State Sponsored Health Plan, or other government program) or full or partial private insurance (including any private health insurance, Medi-Gap, or single-service plan). We defined a composite SES score using the following indicators of low SES: less than high school education (1 point), household income below federal poverty level (1 point), no insurance (2 points) and public health insurance only (1 point). No insurance was given an extra point because no insurance may directly impact access to prescription medication and have a larger impact on appropriate medication management. We categorized participants into 3 composite SES groups (0, 1, and ≥2 low SES points as high, intermediate, and low SES, respectively). The cutoffs of composite SES scores were chosen based on the approximately 50th (0) and 75th (2) percentiles of the scores.
Outcome variables
Prescription medication information was extracted from prescription medication data according to the 3-level nested category system of Multum Lexicon.21 Participants were asked during the home interview if they had taken any prescription medications in the past month. Those who answered “yes” were asked to show the containers of all medications to interviewers. When a container was unavailable, participants reported the name of the medication. All medications were converted to a standard generic drug name. Obesogenic medications were identified from current Endocrine Society Clinical Practice Guidelines, further limited to those that has potential non-obesogenic alternatives,11,12,22 and included certain anticonvulsants, antidepressants/antianxiety drugs, antipsychotics, beta-blockers, corticosteroids, and antidiabetic medications (see detailed obesogenic medication list and potential non-obesogenic alternatives in Supplemental Table 1).
Covariates
Participants reported age, sex, and race/ethnic group (classified as non-Hispanic White, non-Hispanic Black, Mexican American, or other). BMI was calculated from height and weight (kg/m2). A priori, we chose to evaluate comorbidities that were the most common indications for obesogenic medication use.12 Hypertension, diabetes, cardiovascular disease (coronary heart disease, stroke, or heart failure), and arthritis were defined as a self-reported physician diagnosis of the conditions. The presence of depressive symptoms was defined as having five or more symptoms from the Patient Health Questionnaire, a nine-item instrument (PHQ-9) to screen depression.23
Statistical analysis
We first examined whether the mean BMI levels differed by the composite SES groups as well as individual SES indicators, using one-way Analysis of variance (ANOVA) tests. Adults with normal BMI or underweight were further included with our primary study population (i.e., adults with overweight or obesity) for this analysis.
Continuous variables were presented as mean (95% confidence interval [CI]) and categorical variables were presented as percentage (95% CI). Differences in characteristics across SES groups in our primary study population were compared using ANOVA test or Chi-square test, as appropriate.
We used logistic regression models to assess the associations between SES and obesogenic medication use. We fit an unadjusted model, a model adjusted for age, sex, race/ethnicity, and number of prescription medications (Model 1), and a model further adjusted for comorbidities (Model 2). We hypothesized that the associations between SES and obesogenic medication use were mainly mediated through two pathways. First, people with lower SES may have greater burden of comorbidities such as diabetes and hypertension. These comorbidities result in greater use of prescription medications and therefore a higher likelihood of obesogenic medication use. Second, people with lower SES may have more limited access to optimal care and may have fewer opportunities to switch to non-obesogenic alternatives (Supplemental Figure 2). To test the latter hypothesis (i.e., to determine if limited access to optimal care partly explained obesogenic medication use among adults living in lower SES, independent of comorbidities burden), we adjusted for number of prescription medications (Model 1) and further adjusted for comorbidities (Model 2). Based on the Model 2 (i.e., fully adjusted model), we plotted predicted probability of obesogenic medication use by SES. The predicted probability of use of a specific class of obesogenic medication was examined in the same way. Finally, since SES and race/ethnicity are closely intertwined in the US and race/ethnicity is a social construct, we repeated the analysis without adjusting for race/ethnicity in sensitivity analysis. We also stratified the analysis by race/ethnicity to test if the associations between SES and obesogenic medication use were consistent across race/ethnicity groups.
We examined a specific example of obesogenic medication use, sulfonylureas, one of the most common obesogenic antidiabetic medications. We chose this example because obesogenic antidiabetic medications have the largest impact on weight control compared with other classes of obesogenic medications.24 We evaluated for differences in sulfonylureas use by SES groups using Chi-square tests among people with diabetes, overweight or obesity, and received non-insulin antidiabetic medications.
Analysis of anti-obesity medication use
Anti-obesity medications were defined as any one of the FDA-approved anti-obesity medications (Supplemental Table 1). The population eligible for anti-obesity medications included participants with a BMI ≥30 kg/m2, or with a BMI 27-29.9 kg/m2 and at least one obesity-related comorbidity (hypertension, diabetes, dyslipidemia, or cardiovascular disease, Supplemental Figure 1).25 Trends of prevalence in population eligible for anti-obesity medications were assessed using logistic regression with time being modeled as a continuous variable. We compared the use of anti-obesity medications across SES groups using Chi-square tests.
All analyses accounted for the complex survey design of NHANES and incorporated survey weights.26 We used the Taylor series (linearization) method to obtain standard error estimates and corresponding confidence intervals.27 All statistical analyses were conducted with the use of SAS 9.4 (SAS Institute, Cary, NC) and R (www.R-project.org/).28 A two-sided p value <0.05 was considered statistically significant.
Role of the funding source
The funders have no role in study design, data collection, data analysis, interpretation, or writing of the report.
Results
Distribution of BMI by SES
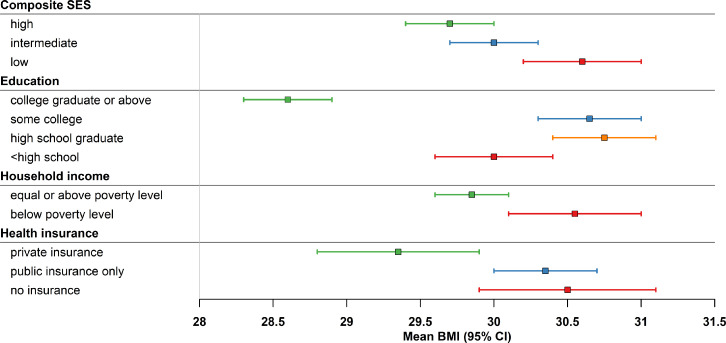
Among 14,154 US adults who took at least one prescription medication (including all BMI levels), 58.0% (95% CI: 56.0, 59.9) were classified as high SES, 22.0% (20.9, 23.1) as intermediate SES, and 20.0% (18.4, 21.7) as low SES. The mean BMI was significantly higher among people with lower composite SES: 29.7 (29.4, 30.0) kg/m2 in the high SES group, 30.0 (29.7, 30.3) kg/m2 in the intermediate SES, and 30.6 (30.2, 31.0) kg/m2 in the low SES group (p < 0.01, Figure 1). Similarly, the mean BMI was significantly higher among people with lower education, lower household income, and public insurance only or no insurance (p < 0.01 for all).
Figure 1.
Mean (95% confidence interval) body mass index (BMI) by socioeconomic status, NHANES 2009-2018.
Significant differences in BMI across composite SES and individual SES groups (p < 0.05 for all).
Use of obesogenic medications by SES
Among 10,673 US adults with overweight or obesity and on prescription medications, people with lower SES were older, more likely to be female, and more likely to be Mexican American or Black (Table 1). During the study period, 37.7% (36.3, 39.0) used obesogenic medications. Use of obesogenic medications was higher among people with lower composite SES (low composite SES, 44.8% [42.6, 46.9]; intermediate composite SES, 45.6% [43.3, 47.9]; high composite SES, 32.3% [30.6, 34.0], p < 0.01). Commonly used obesogenic medication classes were beta-blockers (18.2% [17.0, 19.3]), antidiabetic medications (10.7% [9.9, 11.5], and antidepressant/antianxiety drugs (9.3% [8.6, 10.1]).
Table 1.
Characteristics of US adults with overweight or obesity according to socioeconomic status category, NHANES 2009-2018.
| Total | High SES | Intermediate SES | Low SES | Overall p value | |
|---|---|---|---|---|---|
| Unweighted No. of participants | 10,673 | 4737 | 2677 | 3259 | |
| Age, years | 54.4 (53.8, 54.9) | 53.6 (52.9, 54.2) | 58.4 (57.6, 59.4) | 52.2 (51.4, 53.1) | <0.001 |
| Age group, years, % | <0.001 | ||||
| 18-44 | 26.9 (25.5, 28.3) | 27.4 (25.5, 29.2) | 20.4 (18.3, 22.5) | 32.7 (30.5, 35.0) | |
| 45-64 | 44.7 (43.4, 46.1) | 48.2 (46.2, 50.2) | 36.0 (33.3, 38.7) | 44.2 (42.1, 46.4) | |
| ≥65 | 28.4 (26.9, 29.8) | 24.4 (22.5, 26.3) | 43.6 (40.7, 46.5) | 23.1 (20.7, 25.4) | |
| Female, % | 53.7 (52.6, 54.8) | 51.4 (49.9, 53.0) | 54.0 (51.7, 56.2) | 60.1 (57.9, 62.3) | 0.04 |
| Race, % | <0.001 | ||||
| Mexican American | 10.2 (8.4,12.0) | 5.9 (4.7, 7.1) | 10.7 (8.7, 12.7) | 22.1 (18.1, 26.2) | |
| Non-Hispanic White | 73.1 (70.2, 76.1) | 81.1 (78.6, 83.6) | 70.5 (66.8, 74.1) | 52.9 (47.8, 58.0) | |
| Non-Hispanic Black | 10.9 (9.2, 12.6) | 8.0 (6.5, 9.4) | 12.2 (10.0, 14.4) | 17.9 (14.9, 20.9) | |
| Other | 5.8 (4.9, 6.7) | 5.0 (3.9, 6.1) | 6.6 (5.1, 8.2) | 7.1 (5.7, 8.5) | |
| BMI, kg/m2 | 32.5 (32.3, 32.8) | 32.3 (32.0, 32.5) | 32.5 (32.2, 32.8) | 33.5 (33.1, 33.8) | <0.001 |
| BMI group, kg/m2, % | <0.001 | ||||
| 25-30 | 42.1 (40.5, 43.7) | 43.8 (41.6, 46.0) | 41.0 (38.3, 43.6) | 38.6 (36.0, 41.2) | |
| 30-40 | 45.8 (44.5, 47.2) | 45.2 (43.1, 47.3) | 47.8 (45.2, 50.5) | 45.4 (42.9, 48.0) | |
| ≥40 | 12.0 (11.1, 13.0) | 11.0 (9.8, 12.2) | 11.2 (9.5, 12.9) | 16.0 (14.1, 17.9) |
Continuous variables were presented as mean (95% confidence interval [CI]) and categorical variables were presented as percentage (95% CI).
BMI, body mass index; SES, socioeconomic status.
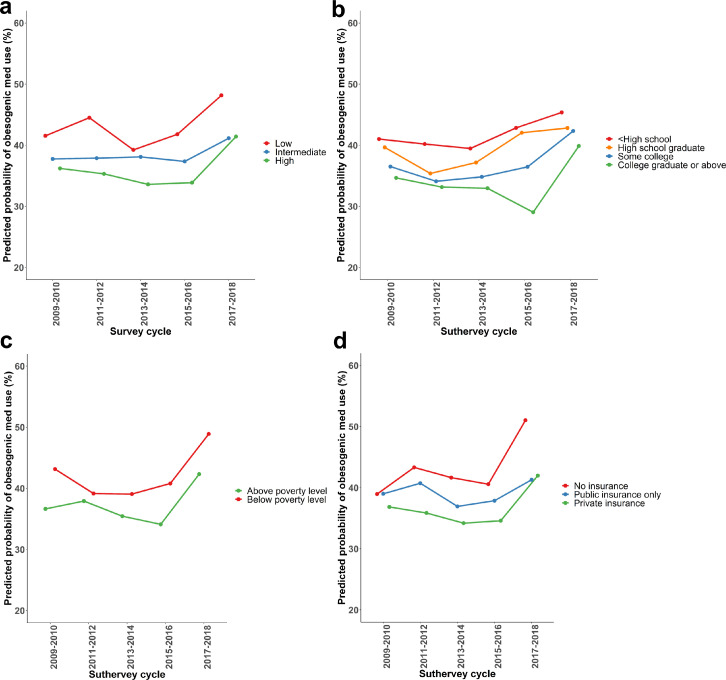
After adjusting for demographic characteristics, number of prescription medications, and comorbidities, greater predicted probability of obesogenic medication use was observed among people with lower SES in all survey years (Figure 2). Consistently, after adjusting for all the covariates, low composite SES was significantly associated with greater use of obesogenic medications (compared to high composite SES, odds ratio [OR]=1.3, 95% CI: 1.2–1.5 for low composite SES, Table 2). Similar patterns were observed when individual SES components were analyzed separately.
Figure 2.
Adjusted* predicted probability of use of obesogenic medications among US adults who had overweight or obesity and took at least one prescription medication by (a) composite SES, (b) education, (c) household income, and (d) type of health insurance, NHANES 2009-2018.
*The model adjusted for age, sex, race/ethnicity, number of prescription medications, diabetes, depression, hypertension, cardiovascular disease, and arthritis.
Table 2.
Odds ratio (95% confidence intervals) of use of obesogenic medications by socioeconomic status, NHANES 2009-2018.
| Unweighted N | Prevalence (95% CI), % | Odds ratio (95% confidence interval) |
|||
|---|---|---|---|---|---|
| Crude | Model 1 | Model 2 | |||
| Composite SES | |||||
| Low | 4737 | 44.8 (42.6, 46.9) | 1.7 (1.5, 1.9) | 1.5 (1.4, 1.8) | 1.3 (1.2, 1.5) |
| Intermediate | 2677 | 45.6 (43.3, 47.9) | 1.8 (1.6, 2.0) | 1.2 (1.03,1.4) | 1.1 (0.96, 1.3) |
| High | 3259 | 32.3 (30.6, 34.0) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Education | |||||
| <High school | 2455 | 45.9 (43.0, 48.9) | 2.0 (1.7, 2.3) | 1.4 (1.2, 1.7) | 1.2 (1.02, 1.4) |
| High school graduate | 2452 | 41.4 (38.7, 44.1) | 1.7 (1.4, 2.0) | 1.4 (1.2, 1.7) | 1.2 (0.99, 1.4) |
| Some college | 3378 | 38.8 (36.3, 41.2) | 1.5 (1.3, 1.7) | 1.3 (1.1, 1.6) | 1.2 (1.01, 1.5) |
| College graduate or above | 2388 | 29.8 (27.6, 32.1) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Household income | |||||
| Below poverty level | 2127 | 46.4 (43.6, 49.1) | 1.5 (1.3, 1.7) | 1.3 (1.1, 1.5) | 1.2 (1.04, 1.4) |
| Equal or above poverty level | 8546 | 36.5 (35.1, 38.8) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Insurance type | |||||
| No insurance | 1146 | 34.1 (30.4, 37.8) | 1.0 (0.9, 1.2) | 1.6 (1.4, 1.9) | 1.4 (1.2, 1.7) |
| Public insurance only | 3789 | 50.0 (47.6, 51.8) | 2.0 (1.7, 2.2) | 1.3 (1.1, 1.4) | 1.2 (1.02, 1.3) |
| Private insurance | 5738 | 33.3 (31.6, 35.0) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
Model 1 adjusted for age, sex, race/ethnicity, and number of prescription medications.
Model 2 further adjusted for diabetes, depression, hypertension, cardiovascular disease, and arthritis.
Greater predicted probabilities of obesogenic antidiabetic medication, antipsychotic medication, and anticonvulsant medication use were demonstrated among people with lower composite SES whilst similar use of beta-blockers, antidepressants, and corticosteroids were observed across SES levels, after adjusting for patient characteristics (Supplemental Figure 3).
SES and use of sulfonylureas
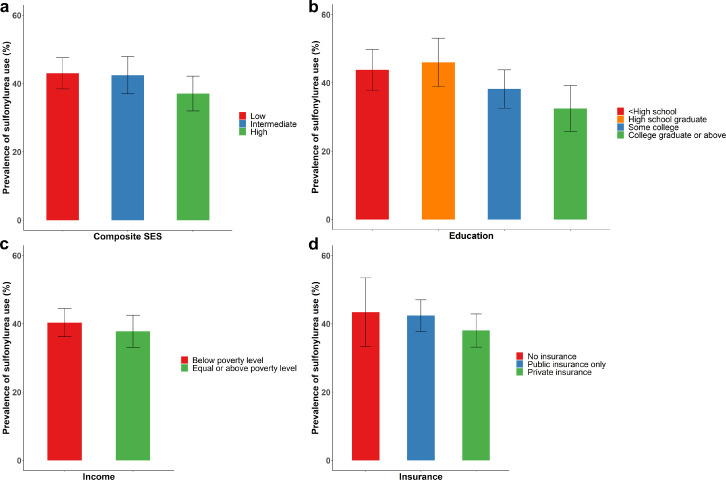
Among 1992 adults with self-reported diabetes, overweight or obesity, and who took non-insulin antidiabetic medications, the overall prevalence of sulfonylurea use was 40.0% (36.3, 43.7), with greater use among people with lower composite SES (43.0% [38.4, 47.6]; intermediate SES 41.5% [37.0, 47.9]; and high composite SES 37.1% [32.0, 42.2], p=0.04, Figure 3a). Greater use of sulfonylureas was observed among people with lower education (Figure 3b, p=0.004). No significant difference in use of sulfonylureas was observed across income or insurance (Figure 3c, d).
Figure 3.
Prevalence (95% confidence interval) of sulfonylurea use among US adults with diabetes, overweight/obesity, and on non-insulin antidiabetic medications by (a) composite SES, (b) education, (c) household income, and (d) type of health insurance, NHANES 2009-2018.
Significant difference in prevalence of sulfonylurea use by composite SES (p=0.03) and education (p=0.004).
SES and use of anti-obesity medications
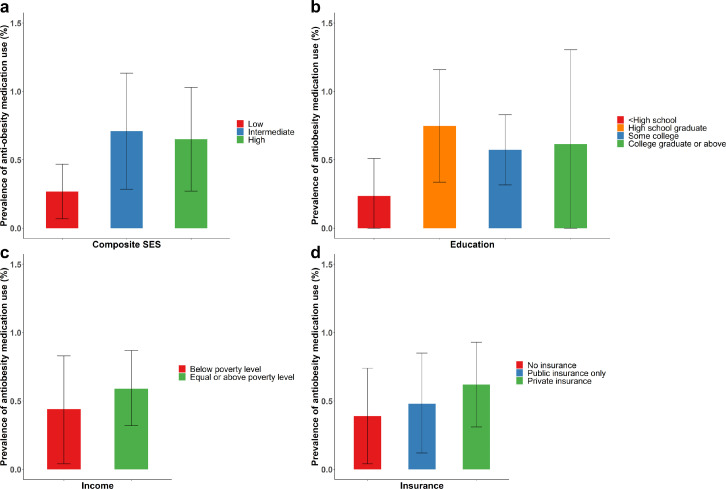
Among non-pregnant US adults, 17.7% (16.9, 18.6) had BMI between 27 and 29.9 kg/m2 and 41.6% (40.2, 43.0) had BMI ≥30 kg/m2. After accounting for obesity-related comorbidities, 54.6% (53.2, 55.9) were eligible for anti-obesity medication use. The prevalence of eligible population increased from 53.0% (51.1, 54.8) from 2009-2010 to 57.7% (54.0, 61.4) in 2017-2018 (p for linear trend=0.001, Supplemental Figure 4). However, use of anti-obesity medications remained very low: only 0.5% (0.3, 0.8) of eligible population used anti-obesity medication. The use was low irrespective of SES: 0.27% (0.07, 0.48) in the low SES group, 0.71% (0.29, 1.13) in the intermediate SES group, and 0.65% (0.28, 1.03) in the high SES group, and the differences were not statistically significant (Figure 4a). The results were consistent by individual SES components (Figure 4b–d).
Figure 4.
Prevalence (95% confidence interval) of anti-obesity use among US adults who were eligible for treatment by (a) composite SES, (b) education, (c) household income, and (d) type of health insurance, NHANES 2009-2018.
Sensitivity analysis
The results were consistent when we did not adjust for race/ethnicity (Supplemental Table 2) and consistent across race/ethnicity groups (p for interaction=0.39, Supplemental Table 3).
Discussion
In this nationally representative study, use of obesogenic medications was common among US adults with overweight or obesity whilst the use of anti-obesity medications was extremely low. Use of obesogenic medications was more common among people with lower SES, even after adjusting for differences in number of prescription medications and comorbidities. Our results suggest that clinicians need to carefully screen patients’ medications for those that may cause weight gain and increase prescription of anti-obesity medications, especially among adults living with low SES.
Overweight and obesity are associated with higher risk of chronic diseases such as hypertension, diabetes, and cardiovascular disease.8 Some of the most frequently prescribed medications for these conditions, such as beta-blockers and sulfonylureas, are obesogenic.11 Use of obesogenic medications is associated with lower chance of achieving successful weight loss after both behavioral interventions and bariatric surgery and may contribute to the obesity epidemic.9,10,24,29,30 In response to these data, the Endocrine Society Clinical Practice Guidelines recommend leptogenic medications (i.e., medications that promote weight loss) and weight-neutral medications as alternatives to obesogenic medications for people with overweight or obesity.11 However, our results showed that almost 40% of people with overweight or obesity took at least one obesogenic medication, even when there were non-obesogenic alternatives. Indeed, we found that sulfonylureas remained commonly used among people with diabetes and overweight or obesity, despite there being myriad non-obesogenic alternatives such as dipeptidyl peptidase-4 inhibitors, sodium glucose co-transporter 2 inhibitors (SGLT2i), and glucagon-like peptide 1 receptor agonists (GLP1RA). Even though others have shown a decrease in the use of sulfonylureas and an increase in SGLT2i and GLP1RA over time,31 we found that sulfonylurea use was still common among people with overweight or obesity, suggesting better pharmacotherapy strategies are needed. Our work should raise awareness about the obesogenic effect of some commonly used medications and call for closer scrutiny of medical regimens. The work also calls for efforts to increase utilization of weight-neutral or leptogenic alternatives and to minimize the dose and duration of obesogenic medication, if possible, when alternatives are not available.
In contrast to the use of obesogenic medications, the utilization of anti-obesity medications was extremely low. This finding is consistent with a previous study using outpatient visit data in the US.32 Our findings highlight substantial gaps between guideline recommendations and the real-world use of anti-obesity medications. A previous survey suggested that physicians may have a limited knowledge of evidence-based guideline recommendations for obesity management, including pharmacotherapy, and the limited knowledge may contribute to the low utilization of anti-obesity medications.33 Other factors that may have contributed to the low use of anti-obesity medications may include obesity-related bias and stigma, limited resources, and the competing demands of managing other chronic conditions common in obesity.34 Our work emphasizes the need to increase the adoption of anti-obesity medications.
We demonstrated that use of obesogenic medications was more common in adults with lower SES, who have a disproportionately higher burden of obesity.18 Inequalities in quality of care as well as medication management by SES are well documented.13 To improve equity in health care, Essien et al.35 proposed the goal of “pharmacoequity” and called for access to the highest-quality of medications regardless of race and ethnicity, SES, or availability of resources.35 Our data emphasized potential socioeconomic disparities in obesogenic medication use. Factors including limited access to care, medication cost, and differential quality of care can drive disparities in medication management.35 For patients with low-quality insurance, increased administrative demands such as prior authorization requirements may result in prescriber's hesitation to use more non-obesogenic alternatives. In addition, alternatives are often more expensive for the patient: SGLT2i and GLP1RA resulted in annual out-of-pocket costs for those with Medicare Part D plans in 2019 of $1298 to $1615, and $2102 to $2520, respectively, compared to $31 for sulfonylureas.36 Pricing is further complicated by the use of rebates and discounts provided by the pharmaceutical industry.37 In addition, patients with lower SES tend to have lower health literacy and lower trust of the health care systems,38 while clinicians tend to believe that patients with lower SES are less likely to comply with medical advice.39 Similar barriers may drive the low uptake of anti-obesity medications.34 Innovative solutions that address access to care, cost, and quality of care are needed to achieve pharmacoequity.
Our study has strengths. The NHANES is a national representative survey and provides national representative estimates. To our knowledge, our study is the first to systematically evaluate use of obesogenic medications as well as anti-obesity medications by SES. Our study also has limitations. First, there is no consensus list of obesogenic medications. There may be misclassification of medication due to lack of evidence and may result in an underestimated obesogenic medication use.22,40,41 Similarly, there is no high-quality evidence on weight change effects of some leptogenic and weight-neutral alternatives.42 Future studies are needed to provide more high-quality evidence on weight change effect of commonly used medications. Second, it is worth emphasizing that some obesogenic medications may be unavoidable for certain therapeutic purposes, such as corticosteroids for autoimmune disease. We were not able to differentiate whether the use of obesogenic medications had non-obesogenic alternatives for each individual patient's clinical scenario. Nevertheless, the high prevalence of obesogenic medication use in people with overweight or obesity should prompt scrutiny of medications. Third, self-reported use of prescription medications was not necessarily verified with medication bottles for all NHANES participants. A previous study reported that 20% of prescription medications in NHANES were not confirmed with a medication bottles.43 Fourth, only prescription medication use in the prior 30 days of interview was asked. Therefore, we were not able to examine prescription medication before this period and may have underestimated obesogenic medication use. Fifth, information about over-the counter medications was not available. Sixth, comorbidities were self-reported in the survey, and we only adjusted for certain comorbidities due to data availability. Seventh, we may have underestimated populations eligible for anti-obesity medications as information about some obesity-related comorbidities was not available in NHANES data (e.g., obstructive sleep apnea). Eighth, the sample size for sulfonylurea and anti-obesity medication use analysis was relatively small and limited our ability to capture differences by SES. Ninth, we did not have information about prescription medication coverage by insurance, which may impact medication utilization patterns. In addition, we could not examine the association by states. The association between SES and use of obesogenic and anti-obesity medications may vary across states with different insurance policies.44
In conclusion, our study demonstrated substantial gaps between guideline recommendations and the real-world use of obesogenic medications and anti-obesity medications in the US. Obesogenic medications were used more often among adults living in lower SES, independent of comorbidity and medication burden. These results suggest the need for closer scrutiny of patient medications that may cause weight gain and increased adoption of anti-obesity medications, especially among adults living in low SES who bear a disproportionate burden of obesity.
Contributors
Both Beini Lyu and Jung-Im Shin take full responsibility for the work, including verification of the underlying data, the study design, access to data, and the decision to submit and publish the manuscript.
Beini Lyu: Conceptualization, data curation, formal analysis, methodology, verification, visualization, writing − original draft, writing − review & editing.
Alex R. Chang: Methodology, writing − review & editing.
Lesley A. Inker: Funding acquisition, methodology, writing − review & editing.
Elizabeth Selvin: Methodology, writing − review & editing.
Morgan E. Grams: Conceptualization, funding acquisition; methodology, project administration, resources, supervision, writing − review & editing.
Jung-Im Shin: Conceptualization, data curation, funding acquisition, methodology, verification, project administration, supervision, writing − original draft, writing- review & editing.
Funding
Dr. Morgan E. Grams was supported by grant number R01DK115534 and K24HL155861 (Principal Investigator: M.G.) from the National Institute of Diabetes and Digestive and Kidney Disease. Dr. Jung-Im Shin was supported by grant number K01DK121825 (Principal Investigator: J-I.S.) from the National Institute of Diabetes and Digestive and Kidney Disease.
Data sharing statement
NHANES data are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm. Our analytic codes can be made available upon request.
Declaration of interests
Dr. Alex Chang has served as a scientific advisor to Reata, Amgen, and Novartis, and he receives research funding from Novo Nordisk. Dr. Jung-Im Shin received funding from Merck, outside of the submitted work. The other authors declared no competing financial interests in relation to the work.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100249.
Appendix. Supplementary materials
References
- 1.Roberto C.A., Swinburn B., Hawkes C., et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385:2400–2409. doi: 10.1016/S0140-6736(14)61744-X. [DOI] [PubMed] [Google Scholar]
- 2.Fryar C.D., Carroll M.D., Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Heal E-Stats. 2020 [Google Scholar]
- 3.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 Through 2017–2018. Natl Cent Heal Stat. 2021. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm. Accessed 26 January 2022.
- 4.Collaborators G.B.D. 2015 O. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chintam K., Chang AR. Strategies to treat obesity in patients with CKD. Am J Kidney Dis. 2021;77:427–439. doi: 10.1053/j.ajkd.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avgerinos K.I., Spyrou N., Mantzoros C.S., Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Taylor V.H., Forhan M., Vigod S.N., McIntyre R.S., Morrison K.M. The impact of obesity on quality of life. Best Pract Res Clin Endocrinol Metab. 2013;27:139–146. doi: 10.1016/j.beem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Bray G.A., Frühbeck G., Ryan D.H., Wilding J.P.H. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 9.Saunders K.H., Igel L.I., Shukla A.P., Aronne L.J. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780–788. [PubMed] [Google Scholar]
- 10.Choong E., Bondolfi G., Etter M., et al. Psychotropic drug-induced weight gain and other metabolic complications in a Swiss psychiatric population. J Psychiatr Res. 2012;46:540–548. doi: 10.1016/j.jpsychires.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Apovian C.M., Aronne L.J., Bessesen D.H., et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 12.Hales C.M., Gu Q., Ogden C.L., Yanovski S.Z. Use of prescription medications associated with weight gain among US adults, 1999-2018: a nationally representative survey. Obesity. 2022 doi: 10.1002/oby.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiscella K., Franks P., Gold M.R., Clancy C.M. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. Jama. 2000;283:2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 14.Essien U.R., Holmes D.N., Jackson L.R., et al. Association of Race/Ethnicity with oral anticoagulant use in patients with atrial fibrillation: findings from the outcomes Registry for better informed treatment of atrial fibrillation II. JAMA Cardiol. 2018;3:1174–1182. doi: 10.1001/jamacardio.2018.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucyk K., Simmonds K.A., Lorenzetti D.L., Drews S.J., Svenson L.W., Russell M.L. The association between influenza vaccination and socioeconomic status in high income countries varies by the measure used: a systematic review. BMC Med Res Methodol. 2019;19:1–23. doi: 10.1186/s12874-019-0801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen S.W., Blot W.J., Lipworth L., Steinwandel M., Murff H.J., Zheng W. Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in Southern US adults. JAMA Netw open. 2019;2 doi: 10.1001/jamanetworkopen.2019.17995. e1917995–e1917995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall I.J., Wang Y., Crichton S., McKevitt C., Rudd A.G., Wolfe C.D.A. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015;14:1206–1218. doi: 10.1016/S1474-4422(15)00200-8. [DOI] [PubMed] [Google Scholar]
- 18.Krueger P.M., Reither E.N. Mind the gap: race/ethnic and socioeconomic disparities in obesity. Curr Diab Rep. 2015;15:1–9. doi: 10.1007/s11892-015-0666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDaniels J.S., Schwartz T.L. Effectiveness, tolerability and practical application of the newer generation anti-obesity medications. Drugs Context. 2016;5 doi: 10.7573/dic.212291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Department of Health and Human Services, Centers for Disease Control and Preventio; Hyattsville, MD: U.S: 2021. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data.https://wwwn.cdc.gov/nchs/nhanes/about_nhanes.htm [Google Scholar]
- 21.Prevention C for DC and NHANES 2005-2006: prescription medications data documentation, codebook, and frequencies. 2019.
- 22.Domecq J.P., Prutsky G., Leppin A., et al. Drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363–370. doi: 10.1210/jc.2014-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desalermos A., Russell B., Leggett C., et al. Effect of obesogenic medications on weight-loss outcomes in a behavioral weight-management program. Obesity. 2019;27:716–723. doi: 10.1002/oby.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panel O.E., Cardiology A.C. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Socie. Obesity (Silver Spring) 2014;22:S5–39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 26.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey. Analytic guidelines, 1999-2010. 2013. [PubMed]
- 27.Statistics NC for H. Centers for disease control and prevention continuous NHANES tutorial on sampling design. Website accessed on January 2012;3.
- 28.Team RC. R: A language and environment for statistical computing. 2013.
- 29.Moon R.C., Almuwaqqat Z. Effect of obesogenic medication on weight-and fitness-change outcomes: evidence from the look AHEAD study. Obesity. 2020;28:2003–2009. doi: 10.1002/oby.22997. [DOI] [PubMed] [Google Scholar]
- 30.Leggett C.B., Desalermos A., Brown S.D., et al. The effects of provider-prescribed obesogenic drugs on post-laparoscopic sleeve gastrectomy outcomes: a retrospective cohort study. Int J Obes. 2019;43:1154–1163. doi: 10.1038/s41366-018-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang M., Wang D., Coresh J., Selvin E. Trends in diabetes treatment and control in US Adults, 1999–2018. N Engl J Med. 2021;384:2219–2228. doi: 10.1056/NEJMsa2032271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claridy MD, Czepiel KS, Bajaj SS, Stanford FC. Treatment of obesity: pharmacotherapy trends of office-based visits in the United States from 2011 to 2016. In: Mayo Clinic Proceedings. Elsevier, 2021:2991–3000. [DOI] [PMC free article] [PubMed]
- 33.Turner M., Jannah N., Kahan S., Gallagher C., Dietz W. Current knowledge of obesity treatment guidelines by health care professionals. Obesity. 2018;26:665–671. doi: 10.1002/oby.22142. [DOI] [PubMed] [Google Scholar]
- 34.Kushner R.F. Tackling obesity: Is primary care up to the challenge? Arch Intern Med. 2010;170:121–123. doi: 10.1001/archinternmed.2009.479. [DOI] [PubMed] [Google Scholar]
- 35.Essien U.R., Dusetzina S.B., Gellad W.F. A policy prescription for reducing health disparities-achieving pharmacoequity. JAMA. 2021;326(18):1793–1794. doi: 10.1001/jama.2021.17764. [DOI] [PubMed] [Google Scholar]
- 36.Dejong C., Masuda C., Chen R., Kazi D.S., Dudley R.A., Tseng C.W. Out-of-pocket costs for novel guideline-directed diabetes therapies under medicare Part D. JAMA Intern Med. 2020;180:1696–1699. doi: 10.1001/jamainternmed.2020.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor S.I. The high cost of diabetes drugs: disparate impact on the most vulnerable patients. Diabetes Care. 2020;43:2330–2332. doi: 10.2337/dci20-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rikard R.V., Thompson M.S., McKinney J., Beauchamp A. Examining health literacy disparities in the United States: a third look at the National Assessment of Adult Literacy (NAAL) BMC Public Health. 2016;16:1–11. doi: 10.1186/s12889-016-3621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arpey N.C., Gaglioti A.H., Rosenbaum M.E. How socioeconomic status affects patient perceptions of health care: a qualitative study. J Prim Care Community Health. 2017;8:169–175. doi: 10.1177/2150131917697439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhaegen A.A., Van Gaal L.F. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Invest. 2017;40:1165–1174. doi: 10.1007/s40618-017-0719-6. [DOI] [PubMed] [Google Scholar]
- 41.Wharton S., Raiber L., Serodio K.J., Lee J., Christensen R.A.G. Medications that cause weight gain and alternatives in Canada: a narrative review. Diabetes Metab Syndr Obes targets Ther. 2018;11:427. doi: 10.2147/DMSO.S171365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gill H., Gill B., El-Halabi S., et al. Antidepressant medications and weight change: a narrative review. Obesity. 2020;28:2064–2072. doi: 10.1002/oby.22969. [DOI] [PubMed] [Google Scholar]
- 43.Bertisch S.M., Herzig S.J., Winkelman J.W., Buettner C. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343–349. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jannah N., Hild J., Gallagher C., Dietz W. Coverage for obesity prevention and treatment services: analysis of Medicaid and state employee health insurance programs. Obesity. 2018;26:1834–1840. doi: 10.1002/oby.22307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.