Although thiazide diuretics are common therapies for the treatment of high blood pressure, they are also associated with elevated blood lipid concentrations after initiation.1 Total cholesterol (TC) concentrations have been shown to increase by 4%, low density lipoprotein cholesterol (LDL-C) concentrations by 10%, and triglyceride (TG) concentrations by 5–15%; high density lipoprotein cholesterol (HDL-C) concentrations remain unaffected.1 Pharmacogenomics could potentially help identify mechanistic pathways underlying this observation. However, few well-powered studies examining variant-by-thiazide interactions on blood lipid concentrations have been conducted. The purpose of this study was to identify genetic variants that modify the effect of thiazide diuretics on LDL-C, TC, and TG using data from the UK Biobank (UKBB), a publicly available, longitudinal study of UK adults.
Due to small sample sizes for other populations, we restricted analyses to unrelated participants of European ancestry, defined by projecting the 1000G European reference panel dataset on previously calculated ancestral principal component loadings (PCs) and applying a k-means clustering approach defined by the first 4 PCs. Medications were recorded via nurse-led interview, mapped to corresponding active ingredients, and classified using the Anatomical Therapeutic Chemical (ATC) Classification system. We categorized participants as thiazide users if ATC codes at baseline visit included thiazides alone or in combination preparations; we excluded loop diuretic users. Participants taking neither thiazide nor loop diuretics were defined as unexposed. Lipid concentrations (mmol/l) were adjusted for statin use.
We conducted GWAS stratified by thiazide exposure, adjusting for age, sex, fasting status, BMI, study center, and 10 PCs using linear mixed models in SAIGE. After removing variants with minor allele frequency (MAF) < 0.01 and imputation quality < 0.60, we computed interaction p-values by testing for difference between and according to Winkler et al,2 using R version 4.0.3. Our conservative p-value threshold was 1.67 × 10−9 (i.e., 5×10−9/3 lipid traits).
After exclusions, a maximum of n=391,239 participants were available (mean age = 57 years; 54% female), of which 7.1% reported thiazide use. Thiazide users were older (mean age = 62 versus 56 years), with higher average LDL-C (4.55 versus 3.97 mmol/l), TG (2.42 versus 1.87 mmol/l), and TC (6.67 versus 6.13 mmol/l) than non-users. Statin use was higher among thiazide users than non-users (44% versus 14%); however, mean LDL-C concentrations among statin users and non-statin users were comparable across thiazide exposure strata.
The thiazide-LDL-C GWAS (n=9,680,686 variants) identified one chromosome 19 locus harboring statistically significant variants with larger effect size among thiazide users than non-users (Figure 1a). Lead variant rs2199576 (MAF = 0.18) had effect estimates (standard error) of 0.159 (0.013) and 0.083 (0.003) per A allele among thiazide users and non-users respectively, yielding an interaction effect estimate of 0.076 (0.013) (pinteraction=1.62 × 10−9). Sensitivity analyses excluding statin users decreased statistical power to 16%, but yielded effects consistent with primary analyses, as evidenced by overlapping 95% CIs for the interaction effect estimates. No genome-wide significant loci were identified for TC or TG (Figure 1b, 1c).
Figure 1. Manhattan plots for analysis of variant-thiazide interactions effects on LDL-C, total cholesterol, and natural log-transformed triglycerides concentrations, and LocusZoom plot and functional annotation of lead significant variant-thiazide interaction on LDL-C in European-ancestry UK Biobank participants.
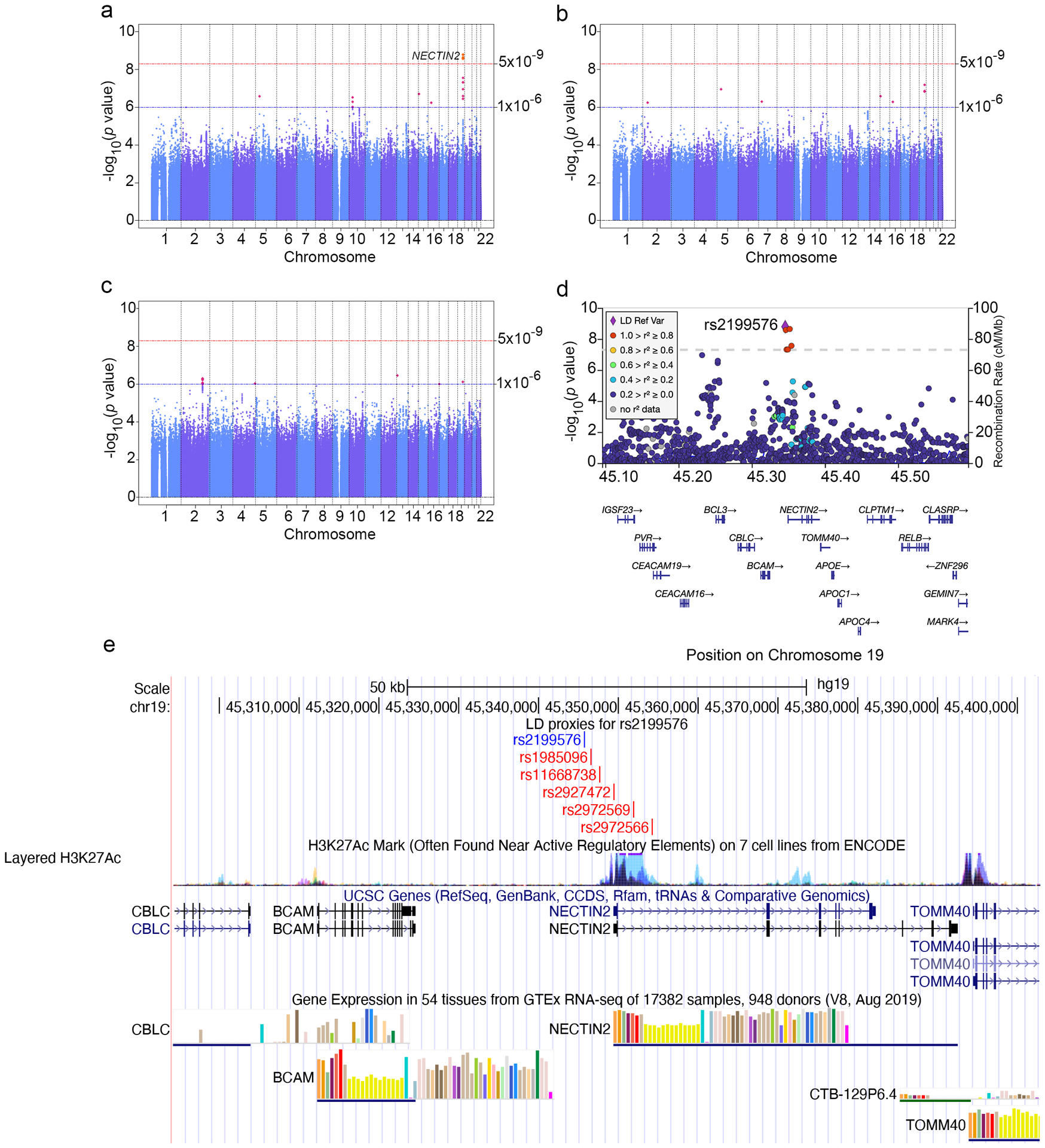
As done in previous publications, LDL-C was adjusted by +2.7695 mmol/l, TC was adjusted by +2.8916 mmol/l, and TG was adjusted by +1.0212 mmol/l among participants also using statins to account for their effect. TG concentrations were subsequently natural-log transformed. (a) Manhattan plot of variant-thiazide interaction on LDL-C concentration, (b) Manhattan plot of variant-thiazide interaction on TC concentration, (c) Manhattan plot of variant-thiazide interaction on natural-log transformed TG concentration, (d) LocusZoom plot of lead significant variant-thiazide interaction effect on LDL-C on chromosome 19. Linkage disequilibrium was generated in European ancestry, (e) functional annotation of lead significant variant rs2199576 (in blue) and high LD (r2 > 0.80) proxies (in red) from the UCSC Genome Browser.
Thiazide-LDL-C lead variant rs2199576 resides within 3.7 KB of NECTIN2, a cholesterol-responsive gene previously associated with TC3 (Figure 1d). Conditional analysis identified no genome-wide significant independent secondary signals. rs2199576 and proxies in high linkage disequilibrium (LD) (r2 > 0.80) are located near a H3K27Ac mark, which are often found near active regulatory elements and help enhance transcription (Figure 1e). In the GTEx database, rs2199576 and proxies have been identified as eQTLs with BCAM and NECTIN2 in lung and pancreas tissue respectively, as well as with RP11–15A1.8 in esophagus-mucosa tissue (LD proxy rs11668738).
Characterizing gene-by-environment effects for complex phenotypes has lagged behind main effect GWAS, limiting the ability to inform underlying biology. We attempted to address this limitation, providing sample sizes approximately 10-times larger than previous efforts.4 Nevertheless, we only had 74% power to detect lead variant rs2199576 at genome-wide significance thresholds. This study was also limited by its cross-sectional design, inability to account for medication duration and dosage effects, and restriction to European ancestry participants. Thus, this is a hypothesis-generating study, and future well-powered prospective analyses in diverse populations are necessary to replicate NECTIN2 and more completely characterize the genetic architecture underlying lipid response to thiazide diuretics.
Acknowledgements:
This research has been conducted using the UK Biobank Resource under Application Number 25953.
Funding:
CGD, HMH, CMS, and CLA are supported by R01HL14825. MLP is supported by F32HL149256. LMR is supported by KL2TR002490. BMP is supported by R01HL105756.
Footnotes
Disclosures: HMH is a Statistical Editor for Circulation Research. The remaining authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Data Availability:
Full summary statistics are available at NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics).
References:
- 1.Herink M, Ito MK. Medication Induced Changes in Lipid and Lipoproteins. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL and Wilson DP, eds. Endotext South Dartmouth (MA); 2000. [PubMed] [Google Scholar]
- 2.Winkler TW, Justice AE, Cupples LA, Kronenberg F, Kutalik Z, Heid IM and consortium G. Approaches to detect genetic effects that differ between two strata in genome-wide meta-analyses: Recommendations based on a systematic evaluation. PLoS One. 2017;12:e0181038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spracklen CN, Chen P, Kim YJ, Wang X, Cai H, Li S, Long J, Wu Y, Wang YX, Takeuchi F. Association analyses of East Asian individuals and trans-ancestry analyses with European individuals reveal new loci associated with cholesterol and triglyceride levels. Human molecular genetics. 2018;27:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Las Fuentes L, Sung YJ, Sitlani CM, Avery CL, Bartz TM, Keyser C, Evans DS, Li X, Musani SK, Ruiter R, et al. Genome-wide meta-analysis of variant-by-diuretic interactions as modulators of lipid traits in persons of European and African ancestry. Pharmacogenomics J. 2020;20:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full summary statistics are available at NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics).


