Abstract
Objective
Autologous haematopoietic cell transplantation (AHSCT) improves immunologic dysfunction in patients with SLE. However, the curative potential of this therapy remains uncertain. This study reports outcomes in SLE patients receiving a lymphodepleting, reduced intensity regimen for AHSCT in SLE.
Methods
Eight patients with SLE refractory to treatment, including i.v. cyclophosphamide (CYC), were enrolled. Five had LN and three CNS involvement as primary indications for transplant. Haematopoietic cell mobilization with CYC, G-CSF and rituximab was followed by collection of CD34+ positively selected cells. The conditioning regimen consisted of concurrent administration of CYC, fludarabine and rituximab. All immunosuppressive medications were discontinued at the start of mobilization and CS were rapidly tapered after the transplant.
Results
Five of eight patients achieved a complete response, including a decline in the SLEDAI to zero, which was sustained in four patients for a median of 165 months (range 138–191). One patient achieved a partial response, which was followed by relapse at month 18. Two patients with nephritis and underlying comorbidities in most organs had early deaths from infection and multiorgan failure. AHSCT resulted in profound lymphodepletion, followed by expansion of Treg cells and repopulation of naive T and B cells. Patients with a complete response showed a sustained suppression of the SLE-associated IFN-induced gene signature, marked depletion of memory and plasmablast B cells and resultant sustained elimination of anti-dsDNA antibody.
Conclusion
Durable clinical and serologic remissions with suppression in the IFN gene signature can be achieved in refractory SLE following lymphodepleting AHSCT.
Trial registration
ClinicalTrials.gov, https://clinicaltrials.gov, NCT00076752.
Keywords: SLE, stem cell transplantation, cytokine, interferon, lymphodepletion
Rheumatology key messages.
Autologous haematopoietic stem cell transplantation (AHSCT) is used increasingly to treat refractory SLE.
Durable remissions with suppression in the IFN gene signature can be achieved following lymphodepleting AHSCT.
Patients with high comorbidity indices are at higher risk for transplant complications.
Introduction
SLE is a chronic inflammatory autoimmune disorder with a broad spectrum of clinical presentations ranging from mild to severe multi-organ involvement. Current treatment regimens are effective in managing disease but are associated with both short- and long-term toxicities. Furthermore, a significant number of patients experience SLE flares and disease progression, leading to irreversible damage [1]. These observations have prompted a search for alternative treatment strategies that could durably reset the immune system and achieve sustained disease remissions without the need for chronic treatment.
Autologous haematopoietic cell transplant (AHSCT) has provided an alternative approach for patients with progressive SLE refractory to therapy. AHSCT conditioning regimens have been predicated on ablation of dysfunctional immune cells followed by infusion of haematopoietic stem cells to repopulate the immune system through naïve immune cells [2]. There is sufficient evidence to support the use of AHSCT as treatment option in patients with refractory SSc and multiple sclerosis [3, 4], however the role of AHSCT in SLE has been limited by the insufficient evidence of sustained responses, increased transplant-related toxicity and mortality.
The aim of this study was to determine the long-term efficacy of a newly developed targeted lymphodepleting, reduced intensity conditioning regimen for AHSCT in severe recalcitrant SLE. A conditioning regimen of rituximab and concurrent CYC and fludarabine was chosen based on profound T and B cell lymphodepletion after allogeneic HCT for haematologic malignancies [5]. This particular regimen used here administered about half of the dose of CYC from what has been typically administered for protocols for AHSCT in SLE along with concurrent administration with fludarabine, which has been shown to synergistically exert a lymphodepleting effect as opposed to sequential dosing [6]. The study goals were to assess the long-term disease ablating potential of this regimen in severe SLE and to characterize the extent of lymphodepletion and subsequent immune repopulation.
Methods
Patients
Subjects aged between 15 and 40 years were eligible for the study if they fulfilled at least 4 of 11 revised ACR SLE classification criteria [7], had severe, active SLE refractory to systemic immunosuppressive therapy involving at least one of four key organ manifestations (renal, CNS, pulmonary or haematologic disease). (Supplementary Data S1 for detailed inclusion criteria and Supplementary Protocol S1, both available at Rheumatology online). Subjects were treated at the National Institute of Health (NIH) Clinical Center in Bethesda, Maryland, USA after signing an informed consent. The study was approved by the NIH institutional review board under a Food and Drug Administration Investigational New Drug application sponsored by National Cancer Institute (ClinicalTrials.gov identifier: NCT00076752) and in compliance with the Helsinki Declaration.
Transplant treatment regimen
Priming and mobilization
To minimize reinfusion of B cells, patients received rituximab 375 mg/m2 (days 1 and 4), i.v. methylprednisolone 1000 mg (day 1), CYC 2000 mg/m2 (day 2) and s.c. G-CSF 10 µg/kg/day (starting on day 6). CD34 cells were positively selected by Isolex 300i (Nexell Therapeutics, Inc., Irvine, CA, USA), v 2.5. The target CD34+ cell infusion dose was 3 × 106/kg and the minimal dose was 1.5 × 106/kg (for details, see Supplementary Fig. S1, available at Rheumatology online).
Conditioning regimen
The conditioning regimen consisted of rituximab 750 mg/m2 (day –7), fludarabine 30 mg/m2 (days –6 to –3), CYC 1200 mg/m2 (days –6 to –3) with MESNA and normal saline hydration, followed by CD34+ stem cell infusion (day 0). G-CSF 5 μg/kg/day was started on day 1 until ANC >500/µL. For further details, please see Supplementary Fig. S1 and Supplementary Data S2, available at Rheumatology online.
Clinical efficacy endpoints
The primary efficacy endpoint was defined as continuous relapse-free complete clinical response at 24 months. Complete clinical response was based on predefined primary assessment of organ involvement, with no signs of active lupus in Systemic Lupus Erythematosus National Assessment-SLEDAI (SELENA-SLEDAI ≤3) [8], and ≤10 mg prednisone/day at 6 months and ≤5 mg/day at 12 months (for details and toxicity monitoring, see Supplementary Data S3, available at Rheumatology online).
Laboratory evaluation
Transplant regimen-induced lymphodepletion and subsequent immune reconstitution were assessed by flow cytometry. Nanostring analysis of monocyte and IFN gene expression signature at serial time points in the first year and annually up to 5 years. Monocytes sorted from peripheral blood mononuclear cells that were cryopreserved at pre-treatment and post-transplant were assessed for gene expression by a panel of type I IFN inducible genes (Supplementary Data S4, available at Rheumatology online).
Statistical methods
The primary efficacy endpoint of relapse-free complete clinical response sustained for 24 months was to be evaluated as a dichotomous variable. For secondary analyses of clinical and biological parameters, descriptive analyses were performed. Survival was evaluated beginning at the date of transplant until date of death or last follow-up, progression-free survival was evaluated from the transplant date the until date of progression, death or last follow-up using the Kaplan–Meier methods (Supplementary Data S5, available at Rheumatology online).
Results
Patient characteristics
Five SLE patients, age 15–40 years, who had failed multiple immunosuppressive treatment regimens received the AHSCT with International Society of Nephrology/Renal Pathology Society class IV LN [9] as the primary affected organ, and three with CNS lupus (two with transverse myelitis, one with retinal vasculitis) (Table 1 and Supplementary Table S1, available at Rheumatology online). Three patients were White (1 Hispanic), three were African American and two were Asian. Six of eight of patients were female.
Table 1.
Summary of the patient cohort
| Patient | Gender, age at AHSCT | Lupus manifestation | SLEDAI | HCT-CI score | HCT-CI factor | Prior systemic lupus therapy | Follow-up (months) | Clinical outcome | Most recent treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1* (diagnosed 1999, transplanted 4/1/2005) | 33, M, Caucasian | Transverse myelitis, skin rash | 10 | 5 | SLE, CVA history, FEV1 75% | CYC, MMF, HCQ, dapsone, MP, IVIG | 191+ |
|
HCQ |
| 2 (diagnosed 2001, transplanted 5/1/ 2005) | 20, F, African American | Transverse myelitis, arthritis | 14 | 2 | SLE | HCQ, CYC, prednisone, MP, RTX | 162 |
|
None for lupus |
| 3 (diagnosed 2000, transplanted 29/11/2005) | 19, F, Caucasian | Class IV nephritis, cerebritis, malar rash | 14 | 4 | SLE, TIA history, SVT treated with verapamil | CYC, MMF, HCQ, AZA, HCQ, MP, prednisone | 141+ |
|
None for lupus |
| 4 (diagnosed 2000, transplanted 20/12/2005) | 37, F, Asian | Class IV nephritis, malar rash, arthritis, | 12 | 7 | SLE, depression, DLCO 61%, hepatic comorbidity | MMF, HCQ, CYC, AZA, MP, prednisone | 6 |
|
N/A |
| 5 (diagnosed 2004, transplanted 10/2/2006) | 15, F, Caucasian | Vasculitis, CNS lupus with vision loss, chorea, JRA overlap, found have A20 Behçet’s | 8 | 6 | SLE, obesity, FEV1 71%, DLCO 66%, hepatic comorbidity | MMF, CYC, adalimumab, MTX, etanercept, infliximab, thalidomide, prednisone, MP, dexamethasone, RTX, IVIG | 160+ |
|
SLE controlled anakinra, 7.5 mg prednisone, AZA |
| 6 (diagnosed 2000, transplanted 14/3/2006) | 32, F, Asian | Class IV nephritis, serositis, haemolytic anaemia, | 14 | 2 | SLE | CYC, MMF, RTX, MP, prednisone | 168+ |
|
HCQ |
| 7 (diagnosed 1996, transplanted 16/5/2006) | 30, F, Hispanic | Class IV nephritis, arthritis, skin rash, alopecia | 14 | 4 | SLE, obesity, EF 50% | HCQ, MTX, CYC, MMF, prednisone, MP | 166+ |
|
SLE controlled prednisone (8 mg) |
| 8 (diagnosed 1998, transplanted 23/5/2006) | 23, M, African American | Class IV nephritis, CNS vasculitis, pancytopenia, pleuritis, arthritis | 14 | 7 | SLE, obesity, DLCO 62%, EF 50% | MMF, MTX, HCQ, CYC, prednisone, MP | 4 |
|
N/A |
AHSCT: autologous haematopoietic stem cell transplant; HCT-CI: Hematopoietic Cell Transplantation-specific Comorbidity Index; SVT: supraventricular tachycardia; MP: mercaptopurine; RTX: rituximab; DLCO: diffusing capacity for carbon monoxide; EF: ejection fraction; CVA: cerebrovascular acccident; TIA: transient ischemic attack; N/A: not applicable. *After acceptance of this manuscript, we learned that patient 1 who relapsed at 191 months passed away 200 months following AHSCT. We did not have access to medical records for further details.
The median SELENA-SLEDAI score at study entry was 14 (range 8–14) with average disease duration of 5.5 years (2–10) before AHSCT. Even though the main indication for transplant was either LN or CNS involvement, all the patients had multiorgan involvement at baseline. Median number of prior systemic immunosuppressive therapies was 6 (5–12). Median Haematopoietic Cell Transplantation—Comorbidity Index (HCT-CI) score was 5 (2–7) (Supplementary Table S1, available at Rheumatology online).
Clinical outcomes
Patients received a CD34-selected autologous AHSCT containing a median 4.94 × 106 CD34+ cells/kg (range 2.6–11.4 × 106), containing 0.001 × 106 CD3+ cells/kg (0–0.1 × 106) and 0 CD19+ cells/kg (0–0.007 × 106) (Supplementary Table S2, available at Rheumatology online). The median time to neutrophil recovery count was 9 days [6–9] and to platelet recovery (20 000/µl) was 11 days [9–12]. Median follow-up period for all eight transplanted patients was 161 months (4–191).
Individual clinical outcomes are summarized in Table 1. The SELENA-SLEDAI scores and organ-specific endpoints showed complete response (CR) in five patients at a median of 3 months [3–6] post-transplant (Supplementary Table S3, available at Rheumatology online). In four of these patients (two CNS, two nephritis), the primary efficacy endpoint (CR sustained at 24 months) was achieved, and they remained in CR for a median of 13 (11–15) years without any need for systemic immunosuppressive medications (Fig. 1). Patient 7 (severe LN) had non-renal progression (retinal vasculitis) at 6 months, which was treated with CS and remained controlled at 168 months. Patient 5 (CNS lupus) had a partial response initially and relapsed at 18 months post-transplant; the patient responded to MMF and her disease is well controlled at 160 months with low-dose prednisone, azathioprine and anakinra (Table 1). Patient 1 (CNS lupus) had non-CNS relapse at 191 months with nephritis and skin rash.
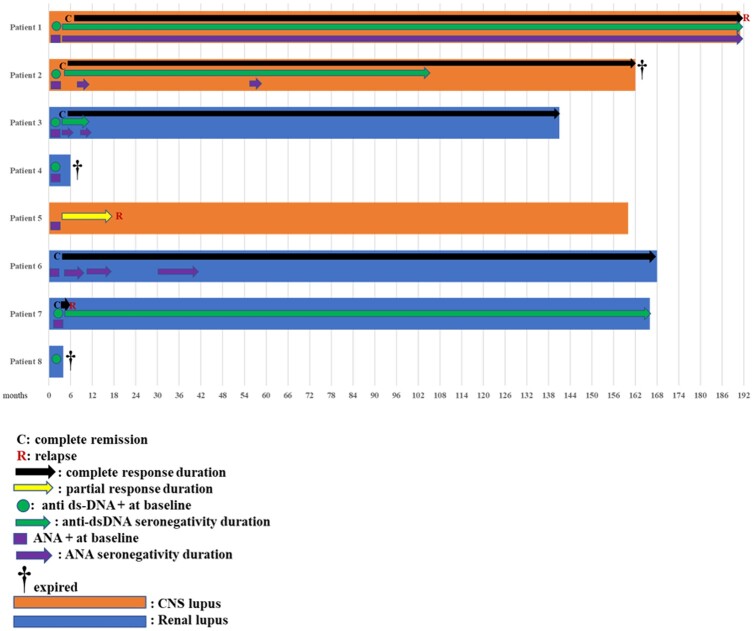
Fig. 1.
Swimmer plot for clinical and autoantibody responses
Swimmer plot summarizing the course of each individual patient over time including clinical responses and autoantibody (anti-dsDNA and ANA) status. The position of each letter (C and R) correspond to the time the event was first documented. Length of each bar represents the follow-up period for individual patients. Black arrows indicate the time when the patients remained in remission. Green and purple arrows indicate the time the patients were seronegative for anti-dsDNA and ANA, respectively. Patients 2, 3 and 6 shown were in sustained long-lasting complete remissions since AHSCT; patient 1 relapsed after 191 months. A colour version of the figure is available at Rheumatology online.
At study entry, all patients were seropositive for autoantibodies: eight for ANA, six for anti-dsDNA, four for anti-SmRNP, three for anti-SSA or anti-SSB, two for aCL IgG or IgM, one for anti-Smith. Anti-dsDNA titre dropped to zero in all six and did not reappear; ANA titre declined in the first 2 years, but none of the patients was negative at the last follow-up (Supplementary Fig. S2 and Supplementary Table S4, available at Rheumatology online).
Organ-specific responses
Nephritis
Five patients were transplanted for recalcitrant LN. Median glomerular filtration rate at baseline was 56 ml/min (range 38–115). Patient 3 achieved CR at 3 months post-transplant that was maintained until 141 months when she was lost to follow-up. Patient 6 achieved CR at 3 months post-transplant that was maintained until 168 months, with no need for systemic immunosuppression. Patient 7 achieved CR at 3 months but relapsed at 6 months with retinal vasculitis (Supplementary Fig. S3, available at Rheumatology online). Patients 4 and 8 died at 6 and 3 months, respectively, from infections preceded by multiple other adverse events and intensive care unit hospitalization for multiorgan failure. Of interest, renal histology at autopsy of patient 4 showed improvement in renal histology (Supplementary Fig. S4, available at Rheumatology online). The kidney biopsy (per protocol) of patient 6 at 27 months also revealed reduced glomerular inflammation compared with baseline.
CNS lupus
Three patients were transplanted for CNS lupus as a primary indication. Patient 1 with transverse myelitis had a CR at 6 months based on MRI and decreased cell counts in the CSF. Despite being paraplegic at baseline, patient 1 functionally improved, regaining the ability to use a walker, with corresponding improvement in Scripps Neurological Rating Scale score from 68 to 81 at 12 months (Supplementary Table S5, available at Rheumatology online). Patient 2 achieved a CR in transverse myelitis at 3 months by MRI and remained without need for immunosuppression until her death at 13 years post-transplant due to unknown causes. Paraplegia, caused by prior extensive spinal cord damage, however, never functionally improved (Supplementary Fig. S5, available at Rheumatology online). Patient 5 with CNS vasculitis was 15 years old at transplant and achieved a partial response but relapsed at 18 months. Vision loss at baseline mildly improved post-transplant, enabling her to recover social functioning, finish college and achieve employment.
Adverse events
Priming
Chemomobilization and the transplant conditioning regimen were well tolerated. There were a total of 13 grade 3 adverse events and 18 grade 4 events observed in seven patients (Supplementary Table S6, available at Rheumatology online). There were no deaths during this period.
Transplant
All grade 3–5 adverse events occurred in the first year after transplant (Table 2). Patient 1 had transient worsening of transverse myelitis-related neurological function during the period of haematological recovery [10]. Patient 2 developed appendicitis and had a laparoscopic appendectomy on day +20 after transplant. Patient 7 underwent a right hemicolectomy following persistent nausea, abdominal pain and pneumatosis intestinalis associated with protracted norovirus enteritis. Four patients (1, 3, 5 and 7) had early post-transplant CMV reactivation but no visceral disease. Two patients (6 and 7) had persistent hypogammaglobulinemia and received preventive IVIG replacement. There were two transplant-related deaths. Patient 4 had lung biopsy proven coronavirus HKU1 pneumonia treated with zanamivir and expired at day 186 [11]. Patient 8 was hospitalized with brain herniation and brain death at day 128, and the autopsy findings were consistent with mycobacterial meningoencephalitis. Notably, both patients who died were transplanted for LN and had very high HCT-CI scores (both scores being 7) (Table 1).
Table 2.
Adverse events after transplant (up to 1-year post-transplant)
| Category | No. of patients | Grade | Day of events following AHSCT |
|---|---|---|---|
| Neurologic | 2 | ||
| Sensory neuropathy | 1 | 4 | 17 |
| Motor neuropathy | 1 | 4 | 91 |
| Hydrocephalus and encephalopathy | 1 | 4a | 127 |
| Infectious | 6 | ||
| Tuberculous meningoencephalitis | 1 | 5 | 121 |
| Human coronavirus HKU1 pneumonia | 1 | 5 | 186 |
| CMV reactivation | 3 | 3 | 22, 23, 29, 190 |
| Febrile neutropenia | 1 | 4 | 8 |
| Catheter-related infection | 1 | 3 | 221 |
| Catheter-related infection | 1 | 4 | 25 |
| Aspergillus pneumonia | 1 | 3 | 170 |
| Influenza A pneumonia | 1 | 3 | 92 |
| Klebsiella pneumonia | 1 | 3 | 179 |
| Listeria bacteraemia | 1 | 3 | 56 |
| Pneumocystis carinii pneumonia | 1 | 3 | 152 |
| Norovirus gastroenteritis | 1 | 3 | 215 |
| Escherichia coli enterocolitis | 1 | 3 | 238 |
| Proteus UTI | 1 | 3 | 357 |
| Staphylococcus aureus bacteraemia | 1 | 3 | 357 |
| GI | 3 | ||
| Pneumatosis—hemicolectomy | 1 | 4 | 237 |
| Appendicitis | 1 | 4 | 20 |
| Anorexia | 1 | 3 | 4 |
| Diarrhoea | 1 | 3 | 182 |
| Nausea and vomiting | 1 | 3 | 182 |
| Pulmonary | 3 | ||
| Pneumonitis | 1 | 3 | 92 |
| Dyspnoea and hypoxia | 2 | 3 | 78, 92 |
| Ophthalmologic | 1 | ||
| Retinopathy | 1 | 4 | 29 |
| Blurred vision/retinopathy | 1 | 3 | 13 |
| Haematologic | 5 | ||
| Purpura | 1 | 3 | 11 |
| Thrombocytopenia | 3 | 3 | 4, 4, 4 |
| Thrombocytopenia | 5 | 4 | 2, 3, 6, 49, 308 |
| Leukopenia | 3 | 3 | 9, 10, 51 |
| Leukopenia | 3 | 4 | 1, 2, 148 |
| Lymphopenia | 3 | 3 | 14, 17, 21 |
| Lymphopenia | 4 | 4 | 12, 15, 28, 35 |
| Neutropenia | 3 | 3 | 9, 10, 107 |
| Neutropenia | 5 | 4 | 1, 1, 1, 2, 3 |
| Anaemia | 3 | 3 | 3, 9, 15 |
| Anaemia | 1 | 4 | 103 |
| Elevated aPTT | 1 | 3 | 188 |
| Cardiac | 1 | ||
| Hypertension | 1 | 4 | 2 |
| Pericardial effusion | 1 | 4 | 3 |
| Cardiac arrhythmia (AV block and asystole) | 1 | 5 | 188 |
| Metabolic | 3 | ||
| Hyperuricemia | 1 | 4 | 54 |
| Hyperkalaemia | 1 | 3 | 5 |
| Hyperkalaemia | 1 | 4 | 292 |
| Hypocalcaemia | 2 | 3 | 3, 6 |
| Hypophosphatemia | 2 | 3 | 56, 177 |
| Hyponatremia | 2 | 3 | 186, 334 |
| Hypermagnesemia | 1 | 3 | 78 |
| Elevated amylase/lipase | 1 | 4 | 7 |
| Elevated ALT/AST | 2 | 3 | 23, 269 |
| Hypoalbuminemia | 1 | 3 | 8 |
Patient died from tuberculous meningoencephalitis. UTI: urinary tract infection; AV: atrioventricular; ALT: alanine aminotransferase; AST: aspartate transaminase.
Survival
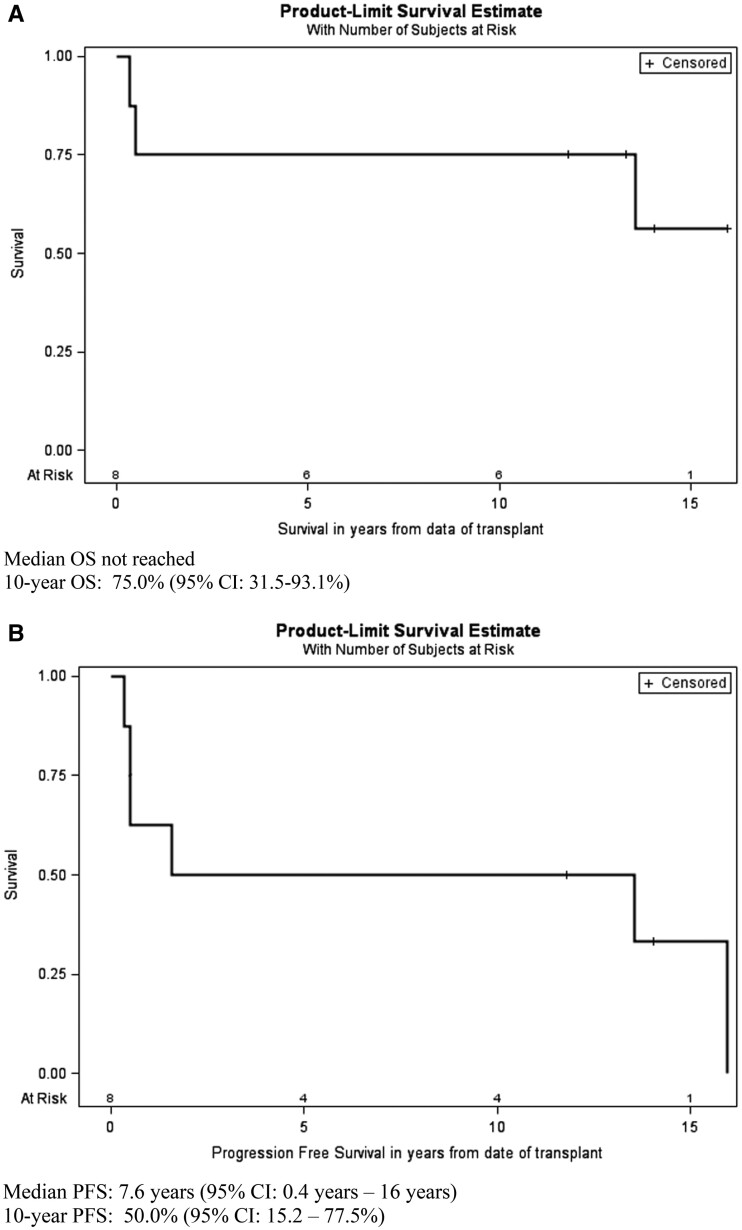
The median follow-up of five surviving patients was 169 months (range 141–191). Estimated 10-year overall survival (OS) and progression-free survival (PFS) are 75.0% (95% CI 31.5, 93.1%) and 50.0% (95% CI 15.2, 77.5%), respectively (Fig. 2). Four out of eight patients had a CR lasting for a median of 13 years (range 11–15) without any need for systemic immunosuppressive medications.
Fig. 2.
Overall survival and SLE relapse-free survival after AHSCT
Overall survival (OS) (A) and progression -free survival (PFS) (B) for all eight patients. Transplant-related mortality was 25%, whereas 50% (4/8) of patients had a complete response lasting for 11–15 years without any need for systemic immunosuppressive medications. Patient number 2 died >13 years after transplant from unknown causes. Patient number 1 relapsed after 16 years. For OS, five patients are censored, at 11.8, 13.3, 14.1, 14.1 and 16.0 years. For progression-free survival, two patients are censored at 11.8 and 14.1 years.
Immunological outcomes
Lymphocyte repopulation
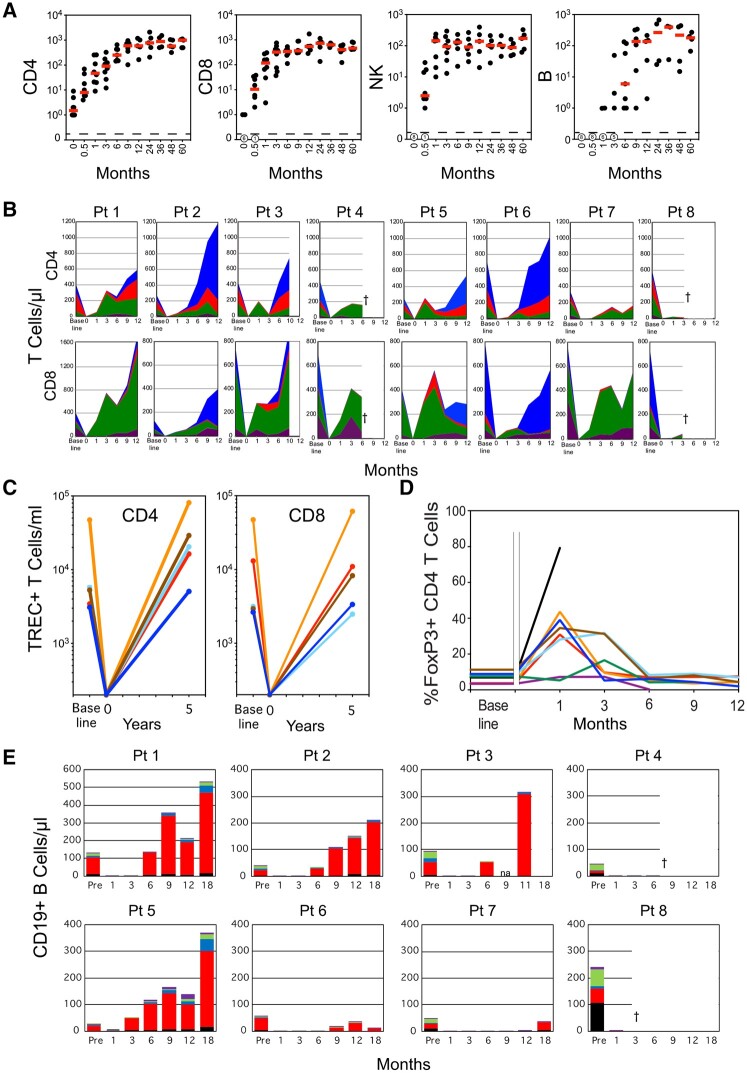
The lymphodepleting transplant regimen resulted in profound depletion of CD4+ and CD8+ T-cells at the day of transplant (DOT), with medians of 1.5 and 0.0 cells/µl, respectively (Fig. 3A). CD4+ and CD8+ T cells levels increased rapidly within the early months, returning to the normal range in all but one patient by 1 year, and remained in normal ranges for 5 years of follow-up. NK cells, absent at DOT, repopulated to a normal range within the first month (median 144 cells/µl; range 10–237) (Fig. 3A). Except for patient 5, CD19+ B cell recovery was delayed 6–9 months post-transplant. B cell numbers subsequently remained low (<50 B cells/µl) in two of five patients followed for 5 years (Fig. 3A).
Fig. 3.
Repopulation of lymphocytes after autologous lymphodepleting, reduced intensity conditioning transplant
(A) CD4, CD8, NK and B cells. Medians shown as red bars. (B) Quantitative area plots of T cell subsets. [Naïve: CCR7+CD45RA+, blue; central memory: CCR7+CD45RA–, red; effector memory: CCR7–CD45RA–, green; T effector memory RA+: CCR7–CD45RA+, purple.] †Deceased. (C) Thymic-dependent generation of T cells as calculated as total sjTREC+ CD4 or CD8 T cells/ml, based on cells/ml and sjTREC/105 T cells. Each line corresponds to a separate patient at baseline and at 5–6 years. TREC levels at DOT were estimated as <103 TREC+ T cells/ml. (D) Recovery of CD4 Treg after transplant. Time course plot of the percentage of FoxP3+CD25+ Treg cells within gated CD4+ T cells in individual patients. Gap separates baseline Treg levels from post-transplant. Patient key for both (C) and (D): patient 1, blue; patient 2, brown; patient 3, green; patient 4, purple; patient 5, turquoise; patient 6, orange; patient 7, red; patient 8, black. (E) Repopulation of B cells during the first 18 months after transplant. Double negative: IgD–CD27–, black. Naïve: IgD+CD27–, red; IgM memory: IgD+CD27+, blue; chain-switched memory: IgD–CD27+, green; plasmablasts/plasma cells: IgD–CD27+CD38++, purple. †Deceased. A colour version of the figure is available at Rheumatology online. sjTREC: signal joint T cell receptor rearrangement excision circles.
Repopulation of T cells by renewed thymopoiesis
In the first 3 months after transplant, naïve (CD45RA+CCR7+) T cells were absent, and the CD4 and CD8 T cells consisted predominately of central memory (CD45RA–CCR7+), effector memory (CD45RA–CCR7–) and T effector memory RA (TEMRA) (CD45RA+CCR7–) cells (Fig. 3B). In four patients (patients 2, 3, 5 and 6), naïve CD4+ T-cell subsets reappeared between 3 and 6 months, becoming the largest proportion of CD4+ T cells by 9–12 months and restoring normal CD4 numbers by 12 months; increases in proportion and absolute numbers of naïve CD8+ T cells concurrently occurred in three of these four patients (Fig. 3B). To evaluate renewal of thymopoiesis over a longer term, the number/ml of circulating CD4 and CD8+ T cells bearing signal joint T cell receptor rearrangement excision circles (sjTREC) was assessed at 5–6 years after transplant. All patients had recovered comparable or higher levels of sjTREC/ml than that observed prior to treatment. Considering the severely depleted T-cell numbers at DOT, all demonstrated effective thymopoietic renewal and naïve repopulation (Fig. 3C). In the first 3 months after transplant, marked increases in CD8+ T cells were associated with expansion of effector memory and TEMRA cells, consistent with CMV reactivation-driven lymphoproliferation (Fig. 3B).
Expansion of Treg cells
Because of the key role of regulatory T cells in controlling autoimmunity, early repopulation of Treg cells (CD4+FoxP3+CD25+) was examined. In six out of eight patients, Treg cells expanded disproportionally, constituting 30–44% of total CD4+ T cells at 1 month and persisting until 3 months in patients 2 and 5; in a seventh patient (patient 3) Treg frequency doubled from pretransplant, reaching 16% of CD4 at 3 months (Fig. 3D).
Early immune repopulation of B cells
B cell subsets were characterized over the first 18 months, including naïve (IgD+CD27–), IgM+ memory (IgD+CD27+), class-switched memory (IgD–CD27+), plasmablasts/plasma cells (CD27+CD38++) and double-negative (IgD–CD27–) B cells. Following a post-transplant delay in B lymphopoiesis, regenerating B cell populations consisted almost entirely of naïve (IgD+CD27–) B cells, with few IgM+ memory or class-switched memory cells, plasmablasts/plasma cells or double-negative B cells for the first year, with one exception (Fig. 3E). In patient 5, naïve B cells reappeared at 3 months, with memory cells including plasmablasts/plasma cells expanding from 6 months until a flare of lupus symptoms at 18 months. This raises the possibility of persistence of some autoimmune clones, or of conditions reinitiating autoantibody production in that patient.
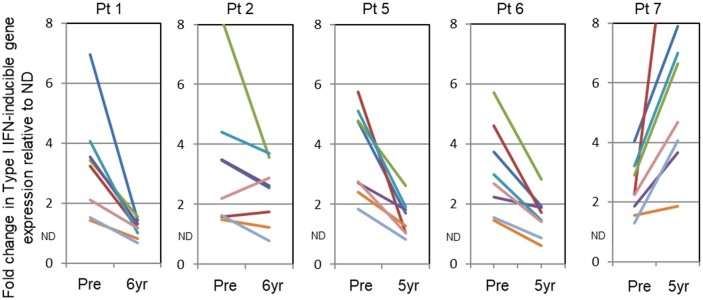
IFN-stimulated gene signature
Patients with SLE express a Type I IFN gene signature, that is, elevated expression of specific Type I IFN-induced genes, particularly in monocyte transcriptomes [12–14]. A marked reduction in expression of Type I IFN-inducible genes was found in three patients remaining in complete remission with no immunosuppressive/immunomodulating treatment over the long term after auto-HSCT (patients 1, 2 and 6), as well as in one patient who relapsed at 18 months (patient 5) but had a partial response (Fig. 4). In contrast, patient 7, who required ongoing immunosuppressive treatment after a lupus flare 6 months post-transplant, had an increase in the level of Type I IFN regulated gene expression 5 years post-transplant compared with her pre-transplant baseline.
Fig. 4.
Expression of Type I IFN-induced genes in circulating monocytes at baseline and 5–6 years after transplant.
Expressed as fold-change in gene expression as compared with median expression of these genes in 20 healthy control donors (ND). Patients in remission after AHSCT whether drug-free (patients 1, 2 and 6) or under maintenance dose steroids (patient 5) showed a significant decline in expression of Type I IFN-induced genes; patient 7, who continued to have lupus symptoms, had elevated expression of these genes. A colour version of the figure is available at Rheumatology online. (IFI44L, blue; IFIT1, red; IFITM3, green; IRF7, purple; MX1, turquoise; PLSCR1, orange; RSAD2, gray; XAF1, brown). AHSCT: autologous haematopoietic stem cell transplantation.
Discussion
In this study, eight treatment-refractory severe SLE patients were transplanted using a reduced intensity conditioning designed to specifically potentiate lymphodepletion without increasing overall chemotherapy intensity [5]. Following AHSCT, four (50%) patients remained free of lupus symptoms for over a decade without need for any post-transplant systemic immunosuppression. Two patients, who relapsed, responded to conventional therapies and returned to a functional life. Two patients with very high comorbidity indices succumbed to early infections and multiorgan failure. Earlier studies of AHSCT in SLE have reported comparable 5-year PFS in the range of 29–75% and treatment-related mortality of about 10–20%, including 110 patients transplanted in the European Group for Blood and Marrow Transplantation registry [15–18]. More recently, AHSCT studies in patients with refractory SLE have reported 5-year OS of 95.2%, and PFS of 67.9% [19], and 10-year OS and PFS rates of 86% [20]. Overall, these data demonstrate a significant therapeutic impact of AHSCT in patients who had previously failed multiple cycles of standard therapies.
A critical element in most AHSCT regimens for SLE has been depletion of mature T and B cells during conditioning through lymphodepleting antibodies such as alemtuzumab, rabbit ATG and/or horse ATG or by ex vivo T cell depletion. The fludarabine/CYC/rituximab/CD34-selection regimen used here has previously been shown to facilitate rapid donor engraftment and achieve effective lymphodepletion in haematologic malignancies [21, 22]. The effectiveness of this approach on T cells was evident here in the severely depleted T cell counts on DOT through 1 month. Additionally, B cells were depleted in the infusion product by rituximab administration during mobilization and ex vivo positive selection of CD34 cells as shown by post-appendectomy tissue at 3 weeks post-transplant in one patient (Supplementary Fig. S6, available at Rheumatology online). The dual rituximab treatment, at mobilization and at conditioning, may have contributed to delayed recovery of naïve B cells. Anti-dsDNA autoantibodies, a key element in SLE [23], were not detected after AHSCT in six out of six patients (as previously noted) [2] and did not reappear, suggesting that re-setting of mature B cell repertoire may cause durable responses. On the downside, however, in two patients with low pretransplant B cell levels (patients 6 and 7), overall first year B cell recovery was minimal (<50 B cells/µl). Over the longer term, both patients had recurrent problems in maintaining peripheral B cell populations, requiring treatment with IVIG to prevent infectious diseases.
A key presumption in AHSCT is the capacity for thymus-dependent repopulation by naïve CD45RA+CCR7+ T cells within the first year in patients under 40 years [24]. A rapid increase in naïve T cells was shown in serial flow cytometry studies, and was substantiated by molecular evidence of renewed thymopoiesis by repopulation of sjTREC expressing cells in all long-term survivors (Fig. 3C) [24]. Concurrent with recovery of naïve cells, the central memory population began to expand (Fig. 3B), consistent with functional activation and differentiation of newly generated T cells [24].
Another important factor in the re-setting of the immune system is the early expansion of Treg cells. Tregs have been identified as critical cells in the control of autoimmunity [25, 26]. Expansion of Treg numbers by low-dose IL-2 or by adoptive transfer of Treg cells has been able to control development of autoimmune disease [27]. Results presented here indicate a disproportionate early expansion of CD25+FoxP3+ cells in the first 3 months in the responders, consistent with re-establishment of a tolerant state.
Severe susceptibility to infections has been observed in SLE following lymphodepletion used to decrease autoreactive effectors in this study. Four patients had CMV reactivation, which was accompanied by rapid CD8 effector expansion in these patients and in a persistent population of CD8+ effector memory and TEMRA [28]. Of note, early expansion of effector memory and TEMRA cells, particularly in CD8 cells, did not preclude subsequent thymic-dependent generation of naïve cells in these patients.
Finally, this study is the first to report that innate immunity inflammatory pathways remain down-regulated following AHSCT for treatment refractory SLE. Patients with SLE and several other systemic autoimmune disorders have been demonstrated to have elevated expression of Type I IFN-inducible genes in circulating blood cells, as well as evidence of these pathways in affected tissues [13, 29]. In the current trial, it was shown that AHSCT leads to protracted reduction in Type I IFN-induced genes in most patients except for one. Even though this patient did not have return of high titre autoantibodies, there was persistent disease activity, suggesting that disease activity driven by Type I IFN might have contributed. This reduction was also reflected clinically since half of the patients maintained immunosuppression-free disease remission of their SLE for >12 years after AHSCT, providing a strong biological correlate of therapeutic efficacy of AHSCT. The IFN signature was not checked before relapse, but such studies would be worth pursuing in future trials.
Two patients who died at 6 and 4 months, respectively, had very high HCT-CI scores along with glomerular filtration rates of 56 and 32 ml/min, and markedly reduced diffusion capacity at baseline (both scores 7); comorbidity scores of 3 or more would often preclude transplantation for allogenic transplant indications [30]. Overall, all patients in this study had >4 years of disease activity, decreased end organ functions, history of multiple prior treatments including i.v. CYC, and severe disability or sequalae that may have predisposed them to higher rates of complications. Recruitment of patients earlier in the course of refractory SLE should help to increase the rates of survival and decrease adverse events after AHSCT [31]. In addition, alternative regimens using established drugs for HSCT conditioning that are not renally eliminated might be more suitable for future testing to be combined with CYC in SLE patients who have impaired renal function [32]. Reducing the dosage of steroids and immunosuppressants or biologicals long term is important in SLE patients to reduce the treatment associated morbidity. So even in patients with relapse, effects of AHSCT may have contributed to the ease of the control of disease activity that was resistant to therapy before transplant.
In conclusion, this study shows that AHSCT after a lymphodepleting, reduced intensity conditioning regimen can be an effective therapy for patients with organ threatening, treatment-refractory SLE. Half of patients demonstrated long-lasting CRs for more than a decade without the need for any disease-modifying or systemic immunosuppressive treatment despite post-transplant lymphocyte recovery. AHSCT resets the immune system to a more naïve state demonstrated by increased TRECs and repopulation of regulatory T cells. Sustained Type I IFN gene signature downregulation along with a decline in dsDNA autoantibodies provide a mechanistic correlate of therapeutic efficacy. Lymphodepleting AHSCT may be an effective one-time therapeutic intervention with potential to fundamentally alter the underlying disease mechanisms leading to long-term treatment-free complete clinical responses, and should be further explored in severe SLE, particularly for patients who have limited comorbidities.
Supplementary Material
Acknowledgements
The authors thank Najibah Rehman, Susan Leitman, Nikolay Nikolov, Juan Gea-Banacloche, Claude Sportes, Tom Hughes, Unsong Oh, Jeanette Nashed, clinical fellows and nurses for their support and contributions. Special thanks are given to all the patients and their families for their participation in the study and support. The views expressed in this work do not necessarily represent the views of the National Institutes of Health or the United States Government.
Funding: This research was supported by the Intramural Research Program of the National Institutes of Health, the Center for Cancer Research, the National Cancer Institute and the National Institute of Arthritis, Musculoskeletal and Skin Diseases.
Disclosure statement: S.G., S.H., F.T.H., J.B., F.P., J.R., S.M., S.M.S., E.H.B., S.R.P., R.G., S.Z.P., P.E.L., G.G.I. and D.F.F. do not declare any conflict of interest. P.A.M. reports consulting for Jasper Therapeutics and Magenta Therapeutics, unrelated to this work.
Contributor Information
Sencer Goklemez, Center for Cancer Research, National Cancer Institute.
Sarfaraz Hasni, Lupus Clinical Trials Unit, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA.
Frances T Hakim, Center for Cancer Research, National Cancer Institute.
Paolo A Muraro, Department of Brain Sciences, Faculty of Medicine, Imperial College London, London, UK.
Filip Pirsl, Center for Cancer Research, National Cancer Institute.
Jeremy Rose, Center for Cancer Research, National Cancer Institute.
Sarfraz Memon, Center for Cancer Research, National Cancer Institute.
Daniel F Fowler, Rapa Therapeutics, Rockville.
Seth M Steinberg, Center for Cancer Research, National Cancer Institute.
Eva H Baker, Department of Radiology and Imaging Services; Clinical Center.
Sandya R Panch, Center for Cellular Engineering, National Institutes of Health, Bethesda.
Ronald Gress, Center for Cancer Research, National Cancer Institute.
Gabor G Illei, Viela Bio, Gaithersburg, MD.
Peter E Lipsky, AMPEL Bio Solutions and the RILITE Research Institute, Charlottesville, VA, USA.
Steven Z Pavletic, Center for Cancer Research, National Cancer Institute.
Data availability statement
Data for this study are not publicly available. For access to data, please contact the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Lateef A, Petri M.. Biologics in the treatment of systemic lupus erythematosus. Curr Opin Rheumatol 2010;22:504–9. [DOI] [PubMed] [Google Scholar]
- 2. Alexander T, Thiel A, Rosen O. et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 2009;113:214–23. [DOI] [PubMed] [Google Scholar]
- 3. Sullivan KM, Goldmuntz EA, Keyes-Elstein L. et al. ; SCOT Study Investigators. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med 2018;378:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muraro PA, Pasquini M, Atkins HL. et al. ; Multiple Sclerosis–Autologous Hematopoietic Stem Cell Transplantation (MS-AHSCT) Long-term Outcomes Study Group. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol 2017;74:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salit RB, Fowler DH, Wilson WH. et al. Dose-adjusted EPOCH-rituximab combined with fludarabine provides an effective bridge to reduced-intensity allogeneic hematopoietic stem-cell transplantation in patients with lymphoid malignancies. J Clin Oncol 2012;30:830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petrus MJ, Williams JF, Eckhaus MA, Gress RE, Fowler DH.. An immunoablative regimen of fludarabine and cyclophosphamide prevents fully MHC-mismatched murine marrow graft rejection independent of GVHD. Biol Blood Marrow Transplant 2000;6:182–9. [DOI] [PubMed] [Google Scholar]
- 7. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 8. Petri M, Buyon J, Kim M.. Classification and definition of major flares in SLE clinical trials. Lupus 1999;8:685–91. [DOI] [PubMed] [Google Scholar]
- 9. Weening JJ, D’Agati VD, Schwartz MM. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004;15:241–50. [DOI] [PubMed] [Google Scholar]
- 10. Muraro PA, Nikolov NP, Butman JA. et al. Granulocytic invasion of the central nervous system after hematopoietic stem cell transplantation for systemic lupus erythematosus. Haematologica 2006;91:Ecr21. [PubMed] [Google Scholar]
- 11. Uhlenhaut C, Cohen JI, Pavletic S. et al. Use of a novel virus detection assay to identify coronavirus HKU1 in the lungs of a hematopoietic stem cell transplant recipient with fatal pneumonia. Transpl Infect Dis 2012;14:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgs BW, Zhu W, Richman L. et al. Identification of activated cytokine pathways in the blood of systemic lupus erythematosus, myositis, rheumatoid arthritis, and scleroderma patients. Int J Rheum Dis 2012;15:25–35. [DOI] [PubMed] [Google Scholar]
- 13. Catalina MD, Bachali P, Geraci NS, Grammer AC, Lipsky PE.. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Commun Biol 2019;2:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hakim FT, Memon S, Jin P. et al. Upregulation of IFN-inducible and damage-response pathways in chronic graft-versus-host disease. J Immunol 2016;197:3490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burt RK, Traynor A, Statkute L. et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA 2006;295:527–35. [DOI] [PubMed] [Google Scholar]
- 16. Illei GG, Cervera R, Burt RK. et al. Current state and future directions of autologous hematopoietic stem cell transplantation in systemic lupus erythematosus. Ann Rheum Dis 2011;70:2071–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexander T, Greco R, Snowden JA.. Hematopoietic stem cell transplantation for autoimmune disease. Annu Rev Med 2021;72:215–28. [DOI] [PubMed] [Google Scholar]
- 18. Alchi B, Jayne D, Labopin M. et al. ; EBMT Autoimmune Disease Working Party members. Autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: data from the European Group for Blood and Marrow Transplantation registry. Lupus 2013;22:245–53. [DOI] [PubMed] [Google Scholar]
- 19. Cao C, Wang M, Sun J. et al. Autologous peripheral blood haematopoietic stem cell transplantation for systemic lupus erythematosus: the observation of long-term outcomes in a Chinese centre. Clin Exp Rheumatol 2017;35:500–7. [PubMed] [Google Scholar]
- 20. Leng XM, Jiang Y, Zhou DB. et al. Good outcome of severe lupus patients with high-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation: a 10-year follow-up study. Clin Exp Rheumatol 2017;35:494–9. [PubMed] [Google Scholar]
- 21. Salit RB, Fowler DH, Dean RM. et al. Host lymphocyte depletion as a strategy to facilitate early full donor chimerism after reduced-intensity allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hardy NM, Mossoba ME, Steinberg SM. et al. Phase I trial of adoptive cell transfer with mixed-profile type-I/type-II allogeneic T cells for metastatic breast cancer. Clin Cancer Res 2011;17:6878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanaoka H, Okazaki Y, Satoh T. et al. Circulating anti-double-stranded DNA antibody-secreting cells in patients with systemic lupus erythematosus: a novel biomarker for disease activity. Lupus 2012;21:1284–93. [DOI] [PubMed] [Google Scholar]
- 24. Hakim FT, Memon SA, Cepeda R. et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest 2005;115:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weigert O, von Spee C, Undeutsch R. et al. CD4+Foxp3+ regulatory T cells prolong drug-induced disease remission in (NZBxNZW) F1 lupus mice. Arthritis Res Ther 2013;15:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev 2011;241:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He J, Zhang X, Wei Y. et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med 2016;22:991–3. [DOI] [PubMed] [Google Scholar]
- 28. Mackus WJ, Frakking FN, Grummels A. et al. Expansion of CMV-specific CD8+CD45RA+CD27- T cells in B-cell chronic lymphocytic leukemia. Blood 2003;102:1057–63. [DOI] [PubMed] [Google Scholar]
- 29. Higgs BW, Liu Z, White B. et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis 2011;70:2029–36. [DOI] [PubMed] [Google Scholar]
- 30. Sorror ML, Storb RF, Sandmaier BM. et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol 2014;32:3249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander T, Hiepe F.. Autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: time ready for a paradigm shift? Clin Exp Rheumatol 2017;35:359–61. [PubMed] [Google Scholar]
- 32. Atkins HL, Bowman M, Allan D. et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 2016;388:576–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study are not publicly available. For access to data, please contact the corresponding author.