Abstract
Toona ciliata Roem is an important timber species in the Toona genus of the Meliaceae family and an endangered species due to over-cutting and a low rate of natural regeneration in China. Although molecular markers have been applied to studying population genetic diversity, the absence of a reliable reference genome limits in-depth genetic conservation and evolutionary studies of this species. Here, we reported a high-quality assembly of the whole genome sequence of T. ciliata. The total assembled genome has 520.64 Mb in length anchored on 28 chromosomes (contig N50 = 4.48 Mb). A total of 42,159 genes were predicted after the ab initio, homology-based, and transcriptome analyses. A total of 41,284 protein-encoding genes (97.92%) were functionally annotated and 1,246 non-coding RNAs were identified in the T. ciliata genome. Phylogenomic analysis showed that T. ciliata was divergent at 15.06 (6–25) Ma from T. sinensis of the same genus Toona. This whole genome sequence provides a valuable resource to study the genetic conservation and molecular evolution of T. ciliata in the future.
Keywords: Meliaceae, Toona ciliata, Nanopore, Hi-C, phylogenetic evolution
Significance.
Toona ciliata is an important timber species in the genus Toona of the Meliaceae family and an endangered species at Grade II in China. Previous molecular and evolutionary studies of this species were restricted due to the absence of reference genome sequence. In this study, we sequenced the whole genome of T. ciliata, which would provide a valuable resource to study the evolution of T. ciliata and develop molecular markers for studying genetic conservation of this endangered species in the future.
Introduction
Toona ciliata belongs to the monophyletic genus Toona of the Meliaceae family (Sun et al. 2014). The species, aka Chinese mahogany, is an important tropical and sub-tropical species and has great socio-economic values, such as the high-quality wood for furniture and the leaves for medicinal material (Edmonds 1993; Liao et al. 2009). It is naturally distributed in Pakistan and western India, Southeast Asia, southern China, Malaysia, and eastern Australia, and considered as an endangered species due to over-cutting and a low rate of natural regeneration (inbreeding depression) in China (Liang et al. 2011).
Previous studies on genetic diversity and molecular evolution of T. ciliata were based on molecular markers. Muellner et al. (2010) used the sequences of nuclear ITS and cpDNA segments (trnS-trnG, psbB, psbT, and psbN genes) to infer the evolutionary relationship of T. ciliata with other species of the Meliaceae family. Other molecular marker studies included the use of the sequence-related amplified polymorphisms and simple sequence repeats to analyze population genetic structure and mating systems. These studies indicated that T. ciliata had a high level of population genetic differentiation and significant effects of isolation by distance in its natural distribution in China (Li et al. 2015; Zhan et al. 2019), and a predominant outcrossing system, with selfing and inbreeding (Zhou et al. 2020). However, exploration of molecular markers is limited for our in-depth understanding of the molecular evolution of this species. Here, we reported the high-quality chromosome-level sequences of T. ciliata genome assembled by combining nanopore and Hi-C sequencing analyses. This is alternative to T. sinensis in the genus Toona whose genome sequence was recently assembled (Ji et al. 2021).
For a phylogenetic comparison, we selected four species used by Ji et al. (2021), including Arabidopsis thaliana, Eucalyptus grandis, Salix purpurea, and Prunus persica, and six different angiosperm plant species (Citrus maxima, Citrus reticulata, Populus tremula, Glycine max, Amborella trichopoda, and T. sinensis) for providing the further context of analysis. Although two species in the Meliaceae family, Azadirachta indica (Krishnan et al. 2012) and Xylocarpus granatum (GenBank accession: GCA_019650275.1), were sequenced, the downstream genomic analysis was limited because gene annotations (gff files) were not provided. These two species were not included for phylogenetic analysis. Our phylogenetic analysis helps to view the evolutionary divergence of T. ciliata from T. sinensis and other land plant species.
Results and Discussion
Genome Assembly
Genome survey was performed with K-mer analysis (K = 21) using three 350 bp-library datasets. The haploid genome size was estimated to be 253.36 Mb in length, and repetitive sequences accounted for 35.89% of the genome size. Genomic heterozygosity was estimated to be 11.90% and the GC content was 34.15%. The karyotype study confirmed that the sample tree has 56 chromosomes 2n = 56 (supplementary fig. S1, Supplementary Material online), consistent with the previous findings (Singh 1951; Styles and Vosa 1971; Mehra et al. 1972).
With the Nanopore sequencing platform (Biomarker Biotechnology Company, Beijing), we obtained 66.02 Gb raw sequence data. After filtering, we obtained 62.85 Gb clean data, with the sequencing depth of about 120.72×, the length of reads N50 of 26.95 kb, and the average read length of 20.25 kb. Distribution of the read sizes was summarized in supplementary table S1, Supplementary Material online.
After corrections of the clean data with Canu and the assembly contigs with Racon and Pilon, we obtained the genome of T. ciliata, which contained 324 contigs, with the N50 length of 4,484,018 bp and a total length of 520,643,266 bp. The GC content was 32.73% (Table 1).
Table 1.
Summary of Genome Sequencing, Assembly, and Gene Annotations
| Genome Assembly and Gene Annotations | Statistics |
|---|---|
| Genome assembly | |
| Number of contigs | 349 |
| Contig N50 (bp) | 4,331,427 |
| Contig N90 (bp) | 600,000 |
| Maximum contig size (bp) | 12,652,477 |
| Number of scaffolds | 153 |
| Scaffold N50 (bp) | 17,615,381 |
| Scaffold N90 (bp) | 15,085,962 |
| Maximum scaffold size (bp) | 27,075,645 |
| Genome size (bp) | 520,643,266 |
| Number of chromosomes | 28 |
| Total length of chromosomes (bp) | 518,944,513 |
| GC content (%) | 32.73 |
| Gene annotations | |
| Total number of genes | 42,159 |
| Number of GO annotation | 34,439 |
| Number of KEGG annotation | 31,047 |
| Number of KOG annotation | 23,236 |
| Number of Pfam annotation | 34,769 |
| Number of Swissprot annotation | 33,407 |
| Number of TrEMBL annotation | 41,159 |
| Number of eggNOG annotation | 35,589 |
| Number of NR annotation | 41,216 |
| Number of all protein-coding genes | 41,284 |
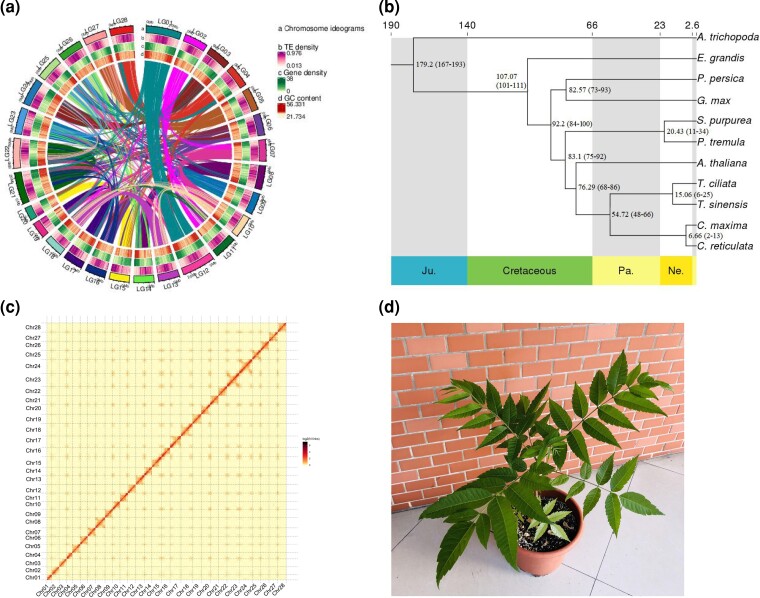
Hi-C assembly with LACHESIS and manual adjustment and inspection were showed in supplementary table S2, Supplementary Material online. A total of 518,944,513 bp (99.67% of the total assembled genome) was anchored on 28 chromosome groups, with the scaffold N50 of 17,615,381 bp in length. Among the sequences located on chromosomes, the sequence length with the order and direction determined was 497,824,081 bp, accounting for 95.93% of the total length of the mapped chromosomes. Figure 1a shows the distribution of gene density, repeat sequence density, GC content, and collinearity within and among chromosomes of T. ciliata.
Fig. 1.
Chromosomal synteny, Hi-C heap map, phylogeny, and the sequenced plant clone of Toona ciliata. (a) A general view of T. ciliata genome and syntenic relationships within the genome. a, circular maps of 28 pseudochromosomes; b, the density distributions of TEs; c, distribution of gene density; d, GC distribution. (b) Heat map of the Hi-C interaction density among 28 pseudochromosomes. The chromosomal-level assembled genome of T. ciliata was segmented into 100-kb bins. The heatmap was used to visualize the number of interactions reported by Hi-C read pairs between each pair of bins. (c) Phylogenetic relationships among 11 species based on 1,276 single-copy genes. Species divergent times (95% CI) were estimated based on the phylogenetic relationships of 11 species, given the fossil records of 168–194 Ma of divergent time between A. trichopoda and S. purpurea, 100–111 Ma between E. grandis and A. thaliana, 51–85 Ma between T. sinensis and C. maxima, and 12–48 Ma between P. tremula and S. purpurea. These divergent times were derived from TIMETREE (http://www.timetree.org/) by loading the list of 11 species. (d) The sample clone of T. ciliata cultivated in a pot from which leaf samples were collected for genome sequencing.
Figure 1 b shows the heat map analysis where 28 chromosomes were clearly distinguished. The completeness of genome assembly was assessed using CEGMA v2.5 and BUSCO v4.0 software with the eukaryotic core gene database and embryophyta_odb data set 9. A total of 450 CEGs were assembled with 98.25% completeness, and a total of 1,360 BUSCOs were assembled with 94.44% completeness, 1.11% fragmentation, and 4.44% missing.
Gene Prediction and Annotations
Repeat annotation analysis showed a total of 253,150,116 bp of transposable elements (TEs) in the T. ciliata genome (supplementary table S3, Supplementary Material online), comprising 48.62% of the whole genome. Among all the classifications of TEs, long terminal repeats (LTRs) accounted for 42.13% of the whole genome and was the largest part of repeats. The length of tandem repeat sequences was 90,986,870 bp, accounting for 17.84%.
From the gene predictions of three approaches (ab initio, homology-based, and transcriptome), we obtained 42,159 coding genes, and most of them were derived from homologous and transcriptome prediction (supplementary table S4 and fig. S2, Supplementary Material online). The gene annotations were also evaluated by BUSCO analysis with embryophyta data set 9. Overall, 1,572 complete BUSCOs (97.40%) were identified in gene annotation, including 1,045 single-copy (64.75%), 527 duplicated BUSCOs (32.65%), and 22 fragmented BUSCOs (3.6%). In total, 20 genes (1.24%) were recognized as missing BUSCOs in our genome. The transcriptome data were evaluated by Hisat2, and 90.74% of RNA-seq clean data were mapped to our predicted exons.
A total of 41,284 protein-encoding genes (97.92%) were functionally annotated in the T. ciliata genome from alignment against public databases [NR, EggNOG, gene ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), Swiss-Prot, Pfam, KOG, and TrEMBL] (Table 1). In addition, 1,246 non-coding RNAs were identified, including 676 tRNAs, 218 rRNAs, 149 miRNA, 72 snRNA and 131 snoRNA. Finally, a total of 148 pseudogenes were predicted, with 242,503 bp in total.
Gene Family and Phylogenetic Analyses
A total of 37,030 gene families, containing 42,159 genes, were clustered in the T. ciliata genome together with the genomes of other 10 species (supplementary table S5, Supplementary Material online). A total of 4,517 gene families were common to all species. Arabidopsis thaliana had the most unique gene families (2,408), while T. sinensis had the least (442). Toona ciliata had 463 unique gene families (supplementary fig. S3, Supplementary Material online). Analysis of the copy number of gene families showed substantial differences among 11 species genomes (supplementary fig. S4, Supplementary Material online). Toona ciliata had more genes that had four or more copy numbers than T. sinensis.
A phylogenetic tree was constructed using 1,276 single-copy genes from the whole genome where divergent times among species were estimated using MCMCTree, calibrated with the known fossil records of three pairs of plant species divergent times (fig. 1c). The divergent time was 15.06 (6–25) Ma between T. ciliata and T. sinensis, and 76.29(75–92) Ma between T. ciliata and A. thaliana. Our estimates of the divergent times between T. ciliata and T. sinensis were overlapped with the previous results (7–49 Ma) derived from genetic markers (Muellner et al. 2010; Cavers et al. 2013; Koecke et al. 2013; Koenen et al. 2015). The divergent time was generally longer between T. ciliata and T. sinensis in genus Toona than between C. maxima and C. reticulata in genus Citrus, 6.66 (2–13) Ma. The divergent times between T. sinensis and E. grandis (101–111 Ma) were comparable with the results (107.7–111.9 Ma) of Ji et al. (2021). However, the divergent times between T. sinensis and A. thaliana were less than those obtained by Ji et al. (2021). As expected, A. trichopoda, the earliest divergent species in angiosperms, had the largest divergent times from all other species investigated. Toona ciliata was divergent at more than 80 Ma but <100 Ma from P. tremula, S. purpurea, G. max, and P. persica.
Materials and Methods
Sample Collection, DNA Extraction, and Genome Sequencing
The sample for genome sequencing was collected from one clone of the individual growing in Pupiao, Baoshan City, Yunnan Province, China (25.04N, 99.06E) and identified as T. ciliata var. ciliata. Figure 1d shows the individual cultivated in a pot in South China Agricultural University. Young and healthy leaves of the 1-year-old plant were collected for DNA isolation. Genomic DNA (gDNA) was extracted using the cetyltrimethylammonium bromide method (Doyle 1987). The concentration and purity of the gDNA were determined using a Nanodrop 2000 spectrophotometer and a Qubit fluorometer. DNA integrity was evaluated on a 0.5% agarose gel.
Genome sequencing and assembly were carried out by Biomarker Biotechnology Company in Beijing. The Nanopore reads were filtered and corrected using Canu (Koren et al. 2017). Nanopore sequencing library was constructed using a total of 9 μg gDNA to select larger fragment sizes (>10 kb) using a Blue Pippin Automatic Nucleic Acid Recovery System. The standard ONT library prep protocol was applied with a Ligation Sequencing Kit (SQK-LSK109) (Deamer et al. 2016). The raw reads were filtered with the thresholds of Q-value >7 and the minimum length of read fragments >500 bp. The high-quality reads were used to assemble the genome.
Genome Size and Assembly
Preliminary genome survey was performed with K-mer analysis using three 350 bp-library datasets, including estimation of haploid genome size, proportion of repetitive sequences, genomic heterozygosity, and GC content. Our karyotype study was done to determine chromosomes of the sample.
We constructed Hi-C fragment libraries from 300 to 700 bp insert sizes (Vaser et al. 2017), and sequenced through Illumina platform. Adapter sequences of raw reads were trimmed, and low-quality pair-end reads were removed for clean data. The final valid reads were selected after the removal of the invalid read pairs, including dangling-end and self-cycle, re-ligation, and dumped products using Hic-Pro v2.10.0 (Servant et al. 2015).
The Hi-C data were mapped to these segments using BWA v0.7.10-r789 software. The uniquely mapped data were retained to perform assembly using LACHESIS (Burton et al. 2013) software. Parameters for running LACHESIS included: CLUSTER_MIN_RE_SITES = 100; CLUSTER_MAX_LINK_DENSITY = 2; ORDER_MIN_N_RES_IN_TRUNK = 110; ORDER_MIN_N_RES_IN_SHREDS = 104. After this step, placement and orientation errors exhibiting obvious discrete chromatin interaction patterns were manually adjusted.
The assembly results were assessed from three aspects: (1) the mapped rate (%) of clean reads on the reference genome sequence with bwa-mem software (Li 2013) for double-ended sequencing and bwa software for shorter sequences (Li and Durbin 2009); (2) the CEGMA(Core Eukaryotic Genes Mapping Approach) v2.5 (default parameters) database that contained 458 conserved key genes in eukaryotes (Parra et al. 2007) was used to evaluate the integrity of the final genome assembly; (3) BUSCOv4.0 software (Simão et al. 2015) was used to evaluate the integrity of the genome assembly by using OrthoDB V9 embryophyta database containing 1,440 conserved core genes. Parameters used with BUSCO were: –evalue 1e−03 (E-value cutoff for BLAST searches), -sp arabidopsis (reference species).
Gene Annotations
We first customized a de novo repeat library of the genome using RepeatModeler, which can automatically execute two de novo repeat finding programs, including RECON v1.08 (Bao and Eddy 2002) and RepeatScout (Price et al. 2005). Then the full-length LTR retrotransposons (fl-LTR-RTs) were identified using both LTRharvest (Ellinghaus et al. 2008) (-minlenltr 100 -maxlenltr 40,000 -mintsd 4 -maxtsd 6 -motif TGCA -motifmis 1 -similar 85 -vic 10 -seed 20 -seqids yes) and LTR_finder (Xu and Wang 2007) (-D 40,000 -d 100 -L 9,000 -l 50 -p 20 -C -M 0.9). The high-quality intact fl-LTR-RTs and non-redundant LTR library were then produced by LTR_retriever (Ou and Jiang 2018). Non-redundant species-specific TE library was constructed by combining the de novo TE sequences library with the known Repbase v19.06 (Jurka et al. 2005), REXdb v3.0 (Neumann et al. 2019), and Dfam v3.2 (Wheeler et al. 2013) database. Final TE sequences were identified and classified by homology search against the library using RepeatMasker v4.10 (Tarailo-Graovac and Chen 2009). Tandem repeats were annotated by Tandem Repeats Finder (Benson 1999) and MIcroSAtellite identification tool (MISA v2.1) (Beier et al. 2017).
The tRNAscan-SE v1.3.1 (Lowe and Eddy 1997) was used to predict tRNA with eukaryote parameters. Identification of the rRNA genes was conducted by barrnap v0.9 (Loman 2017) with Rfam v12.0 (Griffiths-Jones et al. 2005). MiRNA was identified by searching miRBase (release 21) databases (Griffiths-Jones et al. 2006). The snoRNA and snRNA genes were predicted using INFERNAL (Nawrocki and Eddy 2013) against the Rfam (release 12.0) database.
Gene structure was predicted using three strategies: de novo, homologue-based, and transcriptomic analysis. The de novo gene models were predicted using two ab initio gene-prediction software tools, Augustus v2.4 (Stanke et al. 2008) and SNAP (Korf 2004). For the homolog-based approach, GeMoMa v1.7 (Keilwagen et al. 2016) software was performed by using reference gene model from the T. sinensis, A. thaliana, Camellia sinensis, Acer yangbiense, and Pistacia vera. For the transcript-based prediction, RNA-sequencing data were mapped to the reference genome using Hisat v2.0.4 (Kim et al. 2015) and assembled by Stringtie v1.2.3 (Pertea et al. 2015). GeneMarkS-T v5.1 (Tang et al. 2015) was used to predict genes based on the assembled transcripts. The PASA v2.0.2 (Haas et al. 2003) software was used to predict genes based on the unigenes [and full-length transcripts from the PacBio (ONT) sequencing] assembled by Trinity v2.11. Gene models from these different approaches were combined using the EVM v1.1.1 (Haas et al. 2008) and updated by PASA. The gene annotations were obtained with BLASTv2.2.31 (E-value = 1e−5) by aligning against the GenBank Non-Redundant (NR, 20200921), TrEMBL (202005), Pfam (33.1), SwissProt (202005), eukaryotic orthologous groups (KOG, 20110125), (GO, 20200615), KEGG (20191220) databases.
GenBlastA v1.0.4 program (She et al. 2009) was used to scan the whole genome after masking the predicted functional genes. Putative candidates were then analyzed by searching for non-mature mutations and frame-shift mutations using GeneWise v2.4.1 (Birney et al. 2004).
Gene Family and Phylogenetic Analyses
We compared genomic sequences of 11 species (A. thaliana, A. trichopoda, C. maxima, C. reticulata, E. grandis, G. max, P. persica, P. tremula, S. purpurea, T. sinensis, and T. ciliata), with an emphasis on the evolutionary divergence between T. ciliata and T. sinensis. Genome sequences of these species except T. ciliata were downloaded from different databases (supplementary table S6, Supplementary Material online).
The protein sequences of 11 species were classified by gene family with Orthofinder V2.4 software (Emms and Kelly 2019), and the comparison method was diamond while E-value was 0.001. PANTHER V15 database (Mi et al. 2019) was used to annotate the obtained gene families. GO and KEGG enrichment analyses were carried out for the gene family unique to T. ciliata by clusterProfile v3.14.0 (Yu et al. 2012).
The predicted protein sets were condensed to include a single peptide sequence for each gene by filtering out redundant alternative splicing events with Gblocks V0.91 (Talavera and Castresana 2007). The single-copy genes were used to construct phylogenetic tree by the ModelFinder (Kalyaanamoorthy et al. 2017), and the optimal model was JTT + F+I + G4 with the maximum likelihood (ML) method. The number of bootstraps was set to 1,000. By combining the known divergent times of multiple species derived from TIMETREE (http://www.timetree.org/), the divergent times among species were calculated using the MCMCTREE module in PAML v4.9 (Yang 1997; Puttick 2019).
Supplementary Material
Acknowledgments
We appreciate Dr. Maud Tenaillon for helpful comments that improved the presentation of this paper. This study is supported jointly by the following organizations: National Natural Science Foundation of China (32171819), Guangdong Basic and Applied Basic Research Foundation (2021A1515010534), South China Agricultural University (4400-K16013), Science and Technology Project of Guangzhou (202102080217), and Characteristic Innovation Projects of Department of Education of Guangdong Province (2019KTSCX017).
Contributor Information
Xi Wang, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Yu Xiao, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Zi-Han He, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Ling-Ling Li, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Hui Yun Song, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Jun-Jie Zhang, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Xiang Cheng, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Xiao-Yang Chen, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Pei Li, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Xin-Sheng Hu, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China; Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, Guangzhou, 510642, China.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Author contributions
X.-S.H. and P.L. conceived and designed the study; X.W. analyzed data and drafted the manuscript; Y.X. analyzed data; L.-L.L., Z.-H.H., H.-Y.S., and X.C. participated in logistic assistance; J.-J.Z. participated in karyotype experiment; X.-Y.C. provided sampling support; X.-S.H. revised and finalized the manuscript.
Data availability
The data reported in this study are available under accession no. CNP0001985 in the CNGB Nucleotide Sequence Archive (CNSA: https://db.cngb.org/search/project/CNP0001985/), including raw sequence reads, genome assembly files, gene annotations, pseudogene predictions, and ncRNA files.
Literature Cited
- Bao Z, Eddy SR. 2002. Automated de novo identification of repeat sequence families in sequenced genomes. Genome Res. 12:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier S, Thiel T, Münch T, Scholz U, Mascher M. 2017. MISA-web: a web server for microsatellite prediction. Bioinformatics 33:2583–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E, Clamp M, Durbin R. 2004. Genewise and genomewise. Genome Res. 14:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JN, et al. 2013. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat Biotechnol. 31:1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavers S, et al. 2013. Cryptic species and phylogeographical structure in the tree Cedrela odorata L. throughout the netotropics. J Biogeogr. 40:732–746. [Google Scholar]
- Deamer D, Akeson M, Branton D. 2016. Three decades of nanopore sequencing. Nat Biotechnol. 34:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ. 1987. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15. [Google Scholar]
- Edmonds JM. 1993. The potential value of Toona species (Meliaceae) as multipurpose and plantation trees in Southeast Asia. Commonw For Rev. 72:181–186. [Google Scholar]
- Ellinghaus D, Kurtz S, Willhoeft U. 2008. LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinform. 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, et al. 2005. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 33:D121–D124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock RJ, Van Dongen S, Bateman A, Enright AJ. 2006. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34:D140–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, et al. 2003. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 31:5654–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, et al. 2008. Automated eukaryotic gene structure annotation using evidence modeler and the program to assemble spliced alignments. Genome Biol. 9:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y-T, et al. 2021. Long read sequencing of Toona sinensis (A. Juss) Roem: a chromosome-level reference genome for the family Meliaceae. Mol Ecol Res. 21:1243–1255. [DOI] [PubMed] [Google Scholar]
- Jurka J, et al. 2005. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 110:462–467. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TK, Haeseler VA, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilwagen J, et al. 2016. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res. 44:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 12:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koecke V, Muellner-Riehl AN, Pennington TD, Schorr G, Schnitzler J. 2013. Niche evolution through time and across continents: the story of neotropical Cedrela (Meliaceae). Am J Bot. 100:1800–1810. [DOI] [PubMed] [Google Scholar]
- Koenen EJ, Clarkson JJ, Pennington TD, Chatrou LW. 2015. Recently evolved diversity and convergent radiations of rainforest mahoganies (Meliaceae) shed new light on the origins of rainforest hyperdiversity. New Phytol. 207:327–339. [DOI] [PubMed] [Google Scholar]
- Koren S, Walenz BP, Berlin K, Miller JR, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27:722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf I. 2004. Gene finding in novel genomes. BMC Bioinform. 5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan NM, et al. 2012. A draft of the genome and four transcriptomes of a medicinal and pesticidal angiosperm Azadirachta indica. BMC Genomics 13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Genomics arXiv:1303.3997. doi: 10.48550/arXiv.1303.3997. [DOI] [Google Scholar]
- Li P, et al. 2015. Genetic diversity and population structure of Toona ciliata Roem. based on sequence-related amplified polymorphism (SRAP) markers. Forests 6:1094–1106. [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang RL, Liao RY, Dai J. 2011. Endangered causes and protection strategy of Toona ciliata. Guangxi For Sci. 40:201–203. [Google Scholar]
- Liao J-W, Yeh J-Y, Lin Y-C, Wei M-M, Chung Y-C. 2009. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis Rom or leaves. J Food Sci. 74:T7–T13. [DOI] [PubMed] [Google Scholar]
- Loman T. 2017. A novel method for predicting ribosomal RNA genes in prokaryotic genomes. http://lup.lub.lu.se/student-papers/record/8914064.
- Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra PN, Sareen TS, Khosla PK. 1972. Cytological studies on Himalayan Meliaceae. J Arnold Arbor. 53:558–568. [Google Scholar]
- Mi HY, Muruganujan A, Ebert D, Huang XS, Thomas PD. 2019. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 47:D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellner AN, Pennington TD, Koecke AV, Renner SS. 2010. Biogeography of Cedrela (Mellaceae, Sapindales) in central and south America. Am J Bot. 97:511–518. [DOI] [PubMed] [Google Scholar]
- Nawrocki EP, Eddy SR. 2013. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 29:2933–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P, Novák P, Hoštáková N, Macas J. 2019. Systematic survey of plant LTR-retrotransposons elucidates phylogenetic relationships of their polyprotein domains and provides a reference for element classification. Mobile DNA 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S, Jiang N. 2018. LTR_retriever: a highly accurate and sensitive program for identification of long terminal-repeat retrotransposons. Plant Physiol. 176:1410–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra G, Bradnam K, Korf I. 2007. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics 23:1061–1067. [DOI] [PubMed] [Google Scholar]
- Pertea M, et al. 2015. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 33:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Jones NC, Pevzner PA. 2005. De novo identification of repeat families in large genomes. Bioinformatics 21(Suppl 1):i351–i358. [DOI] [PubMed] [Google Scholar]
- Puttick MN. 2019. MCMCtreeR: functions to prepare MCMCtree analyses and visualize posterior ages on trees. Bioinformatics 35:5321–5322. [DOI] [PubMed] [Google Scholar]
- Servant N, et al. 2015. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She R, Chu JS-C, Wang K, Pei J, Chen N. 2009. GenBlastA: enabling BLAST to identify homologous gene sequences. Genome Res. 19:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. 2015. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31:3210–3212. [DOI] [PubMed] [Google Scholar]
- Singh B. 1951. Chromosome numbers in some flowering plants. Curr Sci. 20:105. [PubMed] [Google Scholar]
- Stanke M, Diekhans M, Baertsch R, Haussler D. 2008. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics 24:637–644. [DOI] [PubMed] [Google Scholar]
- Styles BT, Vosa CG. 1971. Chromosome numbers in the Meliaceae. Taxon 20:485–492. [Google Scholar]
- Sun Y-L, et al. 2014. Genetic diversity of Toona sinensis in Korea and the phylogenetic relationship of this species based on chloroplast DNA and ribosomal DNA sequences. Res J Biotechnol. 9:1–14. [Google Scholar]
- Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 56:564–577. [DOI] [PubMed] [Google Scholar]
- Tang S, Lomsadze A, Borodovsky M. 2015. Identification of protein coding regions in RNA transcripts. Nucleic Acids Res. 43:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarailo-Graovac M, Chen NS. 2009. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinform. 25:4.10.1–4.10.14. [DOI] [PubMed] [Google Scholar]
- Vaser R, Sović I, Nagarajan N, Šikić M. 2017. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 27:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler TJ, et al. 2013. Dfam: a database of repetitive DNA based on profile hidden Markov models. Nucleic Acids Res. 41:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wang H. 2007. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucl Acids Res. 35(Web Server issue):W265–W268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Bioinformatics 13:555–556. [DOI] [PubMed] [Google Scholar]
- Yu G, Wang L-G, Han Y, He Q-Y. 2012. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, et al. 2019. Genetic diversity and population structure of Toona ciliata revealed by simple sequence repeat markers. Biotechnol Biotechnol Equip. 33:214–222. [Google Scholar]
- Zhou W, et al. 2020. Mating system and population structure in the natural distribution of Toona ciliata (Meliaceae) in South China. Sci Rep. 10(1):16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this study are available under accession no. CNP0001985 in the CNGB Nucleotide Sequence Archive (CNSA: https://db.cngb.org/search/project/CNP0001985/), including raw sequence reads, genome assembly files, gene annotations, pseudogene predictions, and ncRNA files.