Abstract
Although once considered a ‘counterfeit paradise’, the Amazon Basin is now a region of increasing interest in discussions of pre-colonial tropical land-use and social complexity. Archaeobotany, archaeozoology, remote sensing and palaeoecology have revealed that, by the Late Holocene, populations in different parts of the Amazon Basin were using various domesticated plants, modifying soils, building earthworks, and even forming ‘Garden Cities’ along the Amazon River and its tributaries. However, there remains a relatively limited understanding as to how diets, environmental management, and social structures varied across this vast area. Here, we apply stable isotope analysis to human remains (n = 4 for collagen, n = 17 for tooth enamel), and associated fauna (n = 61 for collagen, n = 28 for tooth enamel), to directly determine the diets of populations living in the Volta Grande do Rio Xingu, an important region of pre-Columbian cultural interactions, between 390 cal. years BC and 1,675 cal. years AD. Our results highlight an ongoing dietary focus on C3 plants and wild terrestrial fauna and aquatic resources across sites and time periods, with varying integration of C4 plants (i.e. maize). We argue that, when compared to other datasets now available from elsewhere in the Amazon Basin, our study highlights the development of regional adaptations to local watercourses and forest types.
Introduction
Archaeological and palaeoecological understanding of pre-colonial subsistence and population density in the Amazon Basin has undergone a major shift over the course of the last half a century [1]. In the second half of the 20th century, ethnographic observations of small, hunting and gathering communities, as well as human ecological theories rooted in environmental determinism, were used to argue that poor soils, high humidity, and hydrological activity prevented the development of cultivation that could support large sedentary and complex societies in this part of the Neotropics [2–4]. Since then, however, archaeobotanical work has demonstrated that the tropical forests of the Amazon were a major center of crop domestication, including manioc (Manihot esculenta ssp. Esculenta), pineapple (Ananas comosus), rice (Oryza sp.) and peach palm (Bactris gasipaes) [5–7]. Meanwhile, studies have tracked the arrival of maize (Zea mays) from North and Central America into the region, and its integration into swidden cultivation strategies [8, 9]. Not only that, but there is evidence for the active management of freshwater resources [10], the movement and arboriculture of tree species such as the Brazil Nut (Bertholletia excelsa) [11], and burning to manage ecological structure and dynamics [12]. Pre-colonial Indigenous impacts are now known to have been so significant that they led to the widespread formation of Anthropogenic Dark Earth (ADE) soils and a lasting legacy on the species composition of tropical forests across the Amazon Basin that are still visible in the 21st century [9, 13, 14]. Furthermore, mixed cultivation, fishing, and arboriculture seem to have sustained populations of up to 8–20 million at the time of European arrival [1, 15], and urban networks [16], earthworks [17], and other forms of landscape modification [10] have been documented across the basin.
Nevertheless, while the Amazon Basin has now been established as a key center of past human settlement and cultivation [15, 18–21], regional, multidisciplinary studies highlighting subsistence complexity are rare. As elsewhere in the Americas, there have been discussions of the importance of the expansion of maize in different parts of the Amazon Basin for the development of social stratification and complexity [22, 23]. However, detailed local studies have also highlighted human subsistence reliance on domesticated root crops such as manioc and non-domesticated plants and animals, including aquatic resources (e.g. [9, 24]). Indeed, given the vast area (7,500,000 km2) of the Amazon Basin and its forests, spanning eight countries, and a variety of different ecological zones, it is essential to develop more nuanced, contextual insights into variations in settlement distribution, geography, and human subsistence adaptations. This has traditionally been difficult, with warm, humid conditions often leading to poor preservation of organic plant and animal remains commonly used to reconstruct subsistence elsewhere [24]. Where such remains are preserved, supplemented by microbotanical approaches to studying plant use, it can still remain difficult to determine the degree to which human societies relied upon different food groups (e.g. [25]). This is significant when seeking to test hypotheses such as those which argues that differences between terra firme (upland habitats away from the floodplain) and várzea settings (seasonally flooded areas) would have dramatically shaped subsistence strategies, with the generally nutrient poor soils of the former necessitating use of forest resources or dramatic landscape modification, and the latter providing ample riverine products and rich soils for cultivation (e.g. [10, 26]). Similarly, while extensive archaeological sites are often associated with ADEs across the Amazon, there is remarkable diversity in pre-colonial ceramic typologies and funerary practices [27–34], though little is known about how this social and cultural diversity played out in terms of subsistence practices and economic adaptations.
Here, we undertake a detailed subsistence analysis of populations living at the Volta Grande do Rio Xingu (VGRX) between 390 cal. years BC and 1,675 cal. years AD (see Table 1) through the application of stable isotope analysis. The Upper Xingu is renowned for the discovery of so-called ‘Garden Cities’ that were fundamental in overturning ideas of the Amazon Basin as a ‘pristine wilderness’ [16, 35]. Although less known, archaeological research carried out in the middle and lower portions of the Xingu River have also uncovered an extremely rich, diverse and extended archaeological record [27, 28, 36–39], which in some cases presents well preserved organic remains. Despite its long history of application in archaeology, stable isotope analysis has rarely been applied across the vast area of the Amazon Basin [22, 40], although recent research at the Marajó Delta [24] and at the mouth of Mearim River (at the eastern limit of the Amazon Basin by the sea) [41] are highlighting its potential. In order to fill in gaps in the knowledge of dietary and cultural variability across this increasingly important region in the context of human-environment interactions, we apply stable isotope analysis to human and animal remains from twelve archaeological sites in the lower and middle portions Xingu River for the first time to explore the varying subsistence practices, such as degree of reliance upon wild and cultivated rainforest C3 plants, animal resources, and arriving C4 resources (i.e. maize), among groups with different cultural affiliations and micro-habitats within this part of the Amazon Basin (Fig 1).
Table 1. Radiocarbon analysis of archaeological sites from which isotope analysis were performed.
| Site and ceramic association | Material | Level (cm) | Lab number | Results | δ13C | Cal 2α 5 |
|---|---|---|---|---|---|---|
| Palhal 2 (Koriabo and Tupi) | Human Bone1 | Burial 2 | OxA-X-3050-26 | 424±25 BP | -16.8‰ | 1429 (92.8%) 1493calAD |
| 1602 (2.6%) 1611calAD | ||||||
| Human Bone1 | Burial 1 | OxA-39692 | 390±19 BP | -17.4‰ | 1445 (81.5%) 1515calAD | |
| 1598 (13.9%) 1618calAD | ||||||
| Charcoal1 | 30–40 | Beta-542851 | 520±30BP | -28.1‰ | 1324 (10.5%) 1345calAD | |
| 1393 (84.9%) 1443calAD | ||||||
| Charcoal1 | 60–70 | OxA-33027 | 967±28 BP | -31.0‰ | 1018 (95.4%) 1155calAD | |
| Charcoal1 | 20–30 | Beta-542854 | 370±30BP | -23.3‰ | 1485 (95.4%) 1650calAD | |
| Charcoal1 | 50–60 | Beta-552222 | 350±30BP | -23.6‰ | 1458 (41.4%) 1531calAD | |
| 1538 (54.1%) 1635calAD | ||||||
| Pimental 2 (Koriabo and Tupi) | Charcoal1 | 40–50 | OxA-33028 | 248±26 BP | -26.9‰ | 1525 (7.5%) 1558calAD |
| 1631 (62.5%) 1675calAD | ||||||
| 1777 (22.3%) 1800calAD | ||||||
| 1941(3.1%) until now. | ||||||
| Charcoal1 | 20–30 | Beta-554247 | 680±30BP | -26.0‰ | 1324 (10.5%) 1345calAD | |
| 1393 (84.9%) 1443calAD | ||||||
| Palmeiras (Koriabo and Tupi) | Human Bone1 | Burial 1 | OxA-X-3050-27 | 371±26 BP | -15.6‰ | 1270 (60.4%) 1316calAD |
| 1355 (35.0%) 1390calAD | ||||||
| Human Bone1 | Burial 2 | OxA-X-3050-28 | 342±26 BP | -15.8‰ | 1470 (95.4%) 1637calAD | |
| Charcoal1 | 40–50 | Beta-552227 | 810±30BP | -24.1‰ | 1169 (95.4%) 1270calAD | |
| Pedra do Navio (Koriabo and Tupi) | Charcoal2 | 30–40 | Beta-542864 | 610±30 BP | -27.0‰ | 1295 (95.4%) 1404calAD |
| Charcoal2 | 60–70 | Beta-542863 | 630±30BP | -24.9‰ | 1287 (95.4%) 1399calAD | |
| Vila Rica 2 (Koriabo and Tupi) | Charcoal3 | 40–50 | Beta-554251 | 850±30BP | -24.0‰ | 1052 (5.2%) 1080calAD |
| 1152 (90.2%) 1260calAD | ||||||
| São José 1 (Arauquinóide, Koriabo and Tupi) | Charcoal1 | 40–50 | Beta-554248 | 2.240±30BP | -25.5‰ | 390 (25%) 345calBC |
| 323 (70.4%) 205calBC | ||||||
| Carrazedo (Koriabo) | Charcoal4 | - | - | - | - | 1.260–1.460cal AD |
| Charcoal4 | - | - | - | - | 750–870 cal AD |
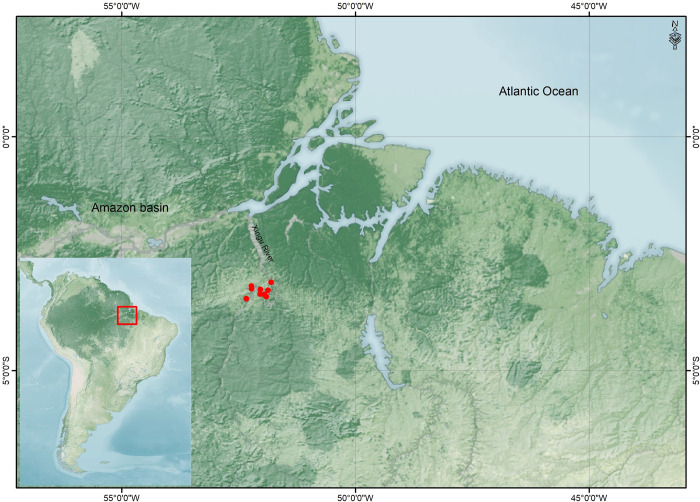
Fig 1. Map showing the location of the Amazon rainforest (darkest part), and archaeological sites of study in the Volta Grande do Rio Xingu (red points).
The map was created for this study by Renato Gonzalez (Geoprocessing Analyst for the Scientia Consultoria Científica, Brazil) using QGIS 3.2.4 https://qgis.org/pt_BR/site/ and the Natural Earth Database from https://visibleearth.nasa.gov/images/147190/explorer-base-map (Landsat images courtesy of NASA Goddard Space Flight Center and US Geological Survey).
Background
Environmental and cultural contexts of the Xingu River
The Xingu River has its source in transitional environments between Amazonian evergreen rainforests and the Brazilian Cerrado and flows south-north into the Amazon River. It is a clear water river [42] and covers more than 1,900 km from its source to its mouth. The transition between the middle and lower reaches of the river is characterized by large rapids with a vertical difference of 85 m in the space of 160 km–the so-called Volta Grande of the Xingu River (VGRX). In the lower portion of the river, the slope is more gradual, resulting in larger floodplains that are more prone to flooding as a product of backwater effects from the Amazon River [43, 44]. The climate of the region is considered a ‘humid tropical micro-climate’ [45], with the average temperature of the coldest month being above 18°C. The vegetation along the middle and lower reaches of the Xingu River is characterized by dense ombrophylous forest [46]. Recent studies on the fauna and flora of the middle and lower reaches of the Xingu River point to the presence of more than 440 species of birds, 259 mammals–including taxa regularly reported as food sources used by Indigenous populations such as deer (Mazama nemorivaga and Mazama americana), tapir (Tapirus terrestris), paca (Cuniculus paca) and agouti (Dasyprocta aguti), as well as primates, giant otters (Pteronura brasiliensis) and otters (Lutrinae)– 174 reptile species including alligators (Melanosuchus niger), tortoise (Chelonoidis sp.) and tracajá (Podocnemis Unifilis), and rich ichthyofauna populations (fish) frequently consumed by humans [47]. Within this ecological context, the rapids of the Volta Grande act as a geological barrier to certain taxa such as porpoises, manatees and important fish such as pirarucu (Arapaima gigas) and dourada (Salminus brasiliensis), which are not found in the portion above the waterfall [47]. Available tree species, such as Brazil Nut (Bertholletia excelsa), babassu (Attalea speciosa), açai-palm (Euterpe oleracea) and a wide variety of other fruit trees, are known to be favoured by Indigenous hunter-gatherers [19, 48].
Anthropological and ethnohistorical studies show that a diversity of Indigenous communities have lived, and still live, in the middle and lower reaches of the river [49, 50], speaking languages belonging to one of three groups–Tupiguarani, Aruak and Jê [51, 52]. Recent archaeological surveys [29, 38, 53] indicate that there was an increase in population density along the middle and lower Xingu River around 1,500 cal years BP, with large archaeological sites, increased densities of material culture and ADEs occurring at this time. The recovered ceramics from archaeological sites dated to this period indicate significant regional and temporal diversity, with pottery often associated with Koriabo, Arauquinoid (both related to Karib speakers), and Tupiguarani (related to Tupiguarani speakers) industries [54, 55]. Koriabo ceramics are traditionally found in the state of Amapá (Brazil’s State) [56] and Guianas [57], and their presence at the mouth of the Xingu River [28], middle Xingu River [38, 58], and lower Amazonas [59] has led to hypotheses of population movements from the Guianas into the Brazilian Amazon. The same is true of Arauquinoid ceramics, initially identified in Venezuela and French Guiana, but noticed in different archaeological sites along the Amazon River [60]. Tupiguarani ceramics are found in many archaeological sites in the interfluvial area between the Tocantins and Xingu rivers, displaying a well-established temporal sequence, beginning in the third century AD [61, 62].
Ethnohistorical studies of accounts of the earliest travelers into the region (17th-20th centuries) suggest that the majority of Indigenous groups living along the middle and lower Xingu River had subsistence strategies based on the cultivation of manioc, maize or sweet potato [51, 63–67]. Nevertheless, no archaeobotanical remains of these crops have been found at any of the excavated sites in the region, although no microbotanical analysis has yet been performed. Ethnohistorical accounts also indicate varying reliance on wild fauna, fish and plant resources [51, 63–68]. The preliminary results of faunal analyses indicate the presence of many faunal taxa at ADE sites, including tapir (Tapirus sp.), paca (Cuniculus paca), agouti (Dasyprocta sp.), capybara (Hydrochoerus hydrochaeris), Brazilian rabbit (Silvilagus brasiliensis), monkeys (Primatas), deer (Cervidae), turtles (Testudines), alligators (Alligatoridae), nine-banded armadillo (Dasypus novemcinctus), sloth (Bradypus sp.) and fish such as the Vampire Fish/Cachorra (Hydrolycus scomberoides). Some of these remains have cut marks and signs of fire exposure. In the context of wider Amazon Basin subsistence questions, of particular interest is the spread of maize (known to be present on the Upper Madeira River back to 6,500 cal. BP and at the mouth of the Amazon from 4,300 cal. BP) [8, 9, 69], as well as the types of subsistence strategies that supported growing populations, including the ‘Garden Cities’, found in upper Xingu River from 1,000 cal AD [35], and dietary variability between individuals associated with pottery of different cultural affiliations.
Isotope analysis in the tropics
Bioarchaeological studies that can directly assess the varied subsistence practices of past Xingu River populations are sorely needed. Stable isotope analysis has been used for paleodietary reconstruction in archaeology since the 1970s [70]. Stable carbon isotope (δ13C) variability in tropical terrestrial ecosystems is primarily driven by plants that utilize the two different dominant photosynthetic pathways, C3 and C4 [71]. In C3 plants, strong discrimination against 13C during CO2 fixation results in lower δ13C values in the vast majority of trees and shrubs which dominate tropical forest environments, as well as domesticates such as manioc, relative to wild C4 grasses or domesticates such as maize [72]. C3 δ13C values vary from c. -24 to -36‰ (global mean -26.5‰), while C4 values range from c. -9 to -17‰ (global mean -12‰) [71]. These distinctions are reflected in the tissues of consumers eating these plants, with small trophic level effects of 1–2‰ [73]. Within a C3-dominated context, further variation occurs due to the ‘canopy effect’ which results in C3 plants living under a dense canopy having lower δ13C than those living in more open environments [74].
δ15N in terrestrial ecosystems varies with trophic level, and δ15N trophic shifts of between +2–6‰ have been documented in terrestrial and aquatic systems [75, 76]. The long length of marine food chains, leads to distinctively high δ15N in marine organisms [77]. Freshwater organisms tend to also have high δ15N for the same reason. However, freshwater δ13C does not follow the same co-varying trend with δ15N towards higher measurements that is seen in marine food chains due to different sources of carbon in these environments, and highly variable δ13C has been observed in freshwater settings around the world [78]. These principles and distinctions make stable isotope analysis a highly powerful methodology for testing a number of important questions about past human diets. However, although thresholds of 100% C3 consumption, 100% C4 consumption, or ‘canopy consumption’ have been established, environmental factors [79], which can lead to both δ13C and δ15N variability as a result of soil dynamics and climatic effects (e.g. rainfall), mean that it is essential to generate baseline data from associated animal remains in archaeological sites.
δ13C and δ15N analysis of human bone collagen (δ13Cco) primarily determines the isotopic values of the protein input to the diet, with a much more minor contribution of lipid and carbohydrate sources [73]. This means that the δ13Cco and δ15N values of bone collagen will be heavily affected by foods that are high in protein, such as fish and meat [73]. By contrast, δ13C measurements of tooth enamel bioapatite (δ13Cap) reflect the ‘whole-diet’ during the period of enamel formation that will vary depending on species and tooth sampled [80]. These tissues will also represent different periods of diet depending on the element sampled. Femur bones have been argued to reflect the last 10 years of life. Meanwhile, tooth enamel will reflect different periods depending on the formation of the tooth sampled, with M3s representing the latest period of childhood (c. 9–11 years) [81].
Materials and methods
Archaeological sites
The human material analyzed comes from ten open-air archaeological sites located along the middle Xingu river (VGRX) and one along the lower Xingu river where it flows into the Amazon river (site descriptions are found in S1 File). The faunal remains come from seven archaeological sites located at VGRX and one from the lower Xingu River site (Table B in S1 File). With the exception of the Bela Vista and Carrazedo sites, the archaeological sites were excavated between 2011 and 2015 as part of the environmental licensing process for the Belo Monte hydroelectric dam, located in the middle Xingu River. The Bela Vista site was preliminarily studied by a team from the Museu Paraense Emílio Goeldi (MPEG) through a rescue excavation, when an urn was exhumed [43]. In 2018, its internal contents were excavated in laboratory [82]. The Carrazedo site has been excavated by the MPEG team since 2014 as part of an international project called OCA [28, 83]. With the exception of one site from the middle Xingu (Santo Antônio 1) [84], all of the studied sites presented ADE soils, and organic materials, such as faunal and human remains, found in association with these ADEs (see more descriptions in S1 File).
Existing radiocarbon archaeological assays from VGRX indicate a long and intensive occupation of the region by human societies for at least 10,000 years, initially represented by foraging groups [85]. Populations associated with simple ceramic forms are found from 4.000 cal. years BP, prior to a period of demographic and socio-cultural diversification around 1.500 cal. years BP, reflected in the presence of Arauquinoid, Koriabo, Tupiguarani ceramic styles and some influences from the Upper Xingu style [29, 58]. Occasionally, some of these styles are present in the same occupation layers of the same site and the same burial contexts [38, 58]. Human burial patterns are also diverse, both in their form and in terms of grave goods. Two or more burial practices may occur in the same place (see more descriptions in S1 File), as primary burials without funerary accompaniments, primary burials accompanied by Tupiguarani and Koriabo style vessels, and burials within urns. To make the chronology of occupation of these sites more robust, samples of charcoal (Palhal 2, Pimental 2, Palmeiras and São José 1 sites), human bones (Palhal 2 and Palmeiras sites) and fauna (Palhal 2 site) were sent for 14C analysis at the Beta Analytic Inc Laboratory and the Research Laboratory for Archeology and History of Art (RLAHA), University of Oxford (the method of analysis and details of samples analyzed at these laboratories can be found in Table A in the S1 File).
Samples
Research permissions were issued by the Instituto do Patrimônio Histórico e Artístico Nacional (Iphan, Brazil), through process n01492.000507/2018-79. We analyzed human bones and teeth from 26 single burials from the sites discussed above, representing all of the available human individuals for sampling. Of the 26 individuals investigated, 13 had both bone and teeth samples analyzed, nine had only bones, and four had only teeth (Table C in S1 File). For stable isotope analysis of bone collagen, femurs and tibias were preferably sought as representative of the longest period of life [86]. However, skull fragments (parietals or occipitals bones) were used where they were the only bone elements available. When present, the 2nd and 3rd molars were sampled for isotope analysis of human tooth enamel, as they represent the latest period of diet (c. 4–11 years of age) available from this biogenic material [87]. Where not available, we sampled the teeth that were present in a given burial context. Some of these will potentially be impacted by the ‘weaning effect’ which has been argued to alter enamel δ13C by, on average, 0.5‰ [88]. However, this is unlikely to impact broader interpretations of resource use (Fig A in S1 File).
In order to reconstruct an isotopic baseline to aid interpretation of the data from the human remains, we analyzed 176 faunal bones and 28 teeth from the archaeological sites mentioned above, representing the available faunal material with clear taxonomic identification and good preservation (Table D in S1 File). Seven of these sites are situated in the VGRX and one, from the Carrazedo site, at the mouth of the Xingu, meaning that these remains act as suitable comparison for the human remains sampled. Sampling was primarily based on the material preserved and it was not possible to follow strict selection criteria (e.g. minimum number of individuals per species or a particular bone from the same anatomical region of all individuals). Instead, the samples reflect what was randomly preserved at archaeological sites that can be directly related to contexts with human remains. The fauna sampled include fish (Osteichthyes) such as Vampire tetra fish (Hydrolycus scomberoides) and Pacu fish (Myleinae); small and medium herbivorous mammals, such as deer (Mazama sp.), Lowland paca (Cuniculus paca), Tapir (Tapirus sp.), Agouti (Dasyprocta sp.), Capybara (Hydrochoerus hydrochaeris), Collared peccary (Pecari tajacu), and White-lipped peccary (Tayassu pecari); omnivorous mammals such as the Nine-banded armadillo (Dasypus novemcinctus),; reptiles such as Tortoise (Chelonoidis), Turtle (Testudinata), Alligator (Alligatoridae); and birds (See S1 File for more information about fauna behaviour in the Amazon). The fauna were identified by Mariane Ferreira and Dr. Renato Kipnis (Scientia Consultoria Científica, Brazil) using the reference collections from Scientia Consultoria Científica and the University of São Paulo (Laboratório de Estudos Evolutivos Humanos do Instituto de Biociências -LEEH-IB- and Laboratório de Zooarqueologia e Bioarqueologia—LABZB). Broad identifications were often necessary due to the poor preservation of these specimens.
The human and faunal material from the Bela Vista and Carrazedo sites was sampled at MPEG in the year 2018. The human and faunal material from the Pedra do Navio, Gaioso 13, Vila Rica 2, Palmeiras, Palhal 2, Santo Antônio 1, São José 1, Santa Luzia 1, and Pimental 2 sites was sampled in the laboratory of Scientia Consultoria Científica, in Belém (PA), also in 2018.
Stable isotope analysis
Bone collagen was extracted from a range of skeletal elements following standard procedures [89]. Although humic acids have been shown to potentially represent a contamination problem in tropical conditions, no corresponding staining of the bone material was identified here. As a result, we followed a similar extraction protocol to that undertaken by Hermenegildo et al. [24] in the Amazon Basin in order to ensure that our data is comparable (cf. [90]). Future work is needed to explore the exact impact of humic acids on bone preservation in the Amazon Basin (see also [91]). In this study, we employ the usual standard protocol for evaluating collagen preservation and quality.
Approximately 1.5 grams of bone, cleaned using sand abrasion, was demineralized in 10 ml aliquots of 0.5M HCl at 4°C. Acid was changed every 48 hours until the bone was fully demineralized. Demineralization took 3–20 days depending on the sample. The sample was then rinsed three times in deionized water before being gelatinized in pH3 HCl at 70°C for 48 hours. The resulting solution was filtered using an EZEE filter, and frozen overnight. The samples were then lyophilized over a period of 24 hours or until completely dry. After calculating the collagen yield, 1mg of extracted collagen sample was measured into a tin capsule to be analyzed for δ13C and δ15N in duplicate at the Stable Isotope Research Laboratory of the Department of Archaeology, Max Planck Institute for the Science of Human History using the Thermo Fisher Elemental Analyzer coupled to a Thermo Fisher Delta V Advantage Isotope Ratio Mass Spectrometer via a ConFloIV system. Two-point calibrations were performed using measurements of international standard reference materials (USGS40 L-Glutamic Acid: δ13Craw = -26.4‰±0.1‰, v13Ctrue = -26.4‰±0.0‰, δ15Nraw = -4.4‰±0.1‰, δ15Ntrue = -4.5‰±0.1‰; IAEA N2 δ15Nraw = 20.2‰±0.1‰, δ15Ntrue = 20.3‰±0.2‰; IAEA C6 δ13Craw = -10.9‰±0.1‰, δ13Ctrue = -10.5‰±0.0‰) with each analytical run.
Teeth or tooth fragments were cleaned using sand abrasion to remove adhering external material. 8 mg of enamel powder was obtained using gentle abrasion with a diamond-tipped drill along the full length of the buccal surface or fragment in order to maximize the period of formation represented by the sample. Enamel powder was pre-treated using a protocol to remove any organic or secondary carbonate contaminates [92, 93]. This consisted of the application of 1% sodium hypochlorite for 60 minutes, followed by three rinses in ultra-pure H2O, with vortexing and centrifuging, after each rinse, before 0.1M acetic acid was added for 10 minutes, followed by another three rinses in ultra-pure H2O, vortexing and centrifuging [80, 93]. Samples were then frozen and freeze dried for 4 hours. Approximately 2.5 mg of the treated sample were weighed into borosilicate glass vials and capped. The vials were flush/filled with helium at 100ml/min for 10-min and 20ul of phosporic acid was added. Following reaction with 100% phosphoric acid, gases evolved from the samples were analyzed for δ13C and δ18O using a Thermo Gas Bench 2 connected to a Thermo Delta V Advantage Mass Spectrometer at Stable Isotope Research Laboratory of the Department of Archaeology, Max Planck Institute for the Science of Human History. Obtained δ13C and δ18O isotope values were calibrated against international standards (IAEA NBS 18: δ13C -5.014 ± 0.032 ‰, δ18O -23.2±0.1 ‰, IAEA 603: δ13C +2.46±0.01 ‰, δ18O -2.37±0.04 ‰, IAEA CO8: δ13C -5.764±0.032‰, δ18O -22.7±0.2 ‰, USGS44: δ13C = ~ -42.1 ‰) registered by the International Atomic Energy Agency. Replicate analyses of standards suggest that machine measurement error is c. ± 0.1‰ for δ13C and ± 0.2‰ for δ18O. Overall measurement precision was studied through the measurement of repeat extracts from a bovid tooth enamel standard (n = 20, ± 0.2‰ for δ13C and ± 0.4‰ for δ18O).
Statistical analysis
All isotopic datasets were tested for normality using the Shapiro Wilk test. Following this test, the significance of the variation of δ15N and δ18O between dietary groups within the faunal datasests from VGRX (i.e. omnivores, carnivores, herbivores and fish) was determined using comparative ANOVA tests. An ANOVA test was also applied to compare the values of δ15N found for the fauna from VGRX with the values obtained in previous studies for archaeological samples from Ucayali Basin, in Peruvian by taxa [40]. In both cases, where variance was found to be significant, this was combined with a post-hoc Tukey pair-wise comparison test to determine which taxa or dietary groups were significantly different from each other.
The significance of δ13Cco and δ13Cap variation between dietary groups of fauna at VGRX was determined using Kruskal-Wallis comparative tests, followed by Pairwise comparisons using Wilcoxon comparisons. The same approach was used to compare δ13Cap values between fauna taxa from VGRX and values obtained in previous studies for archaeological and modern samples from the Peruvian Amazon [40, 79], and to compare δ13Cco values between fauna taxa from VGRX and values obtained in previous studies for archaeological samples from Ucayali Basin, in Peru [40]. Kruskal-Wallis tests were also used to compare the δ13Cco and δ15N values of the VGRX human groups with other groups previously studied (Ucayali Basin, Orinoco Basin, Lower Amazonas Basin and São Luis). The complete statistical results tables can be found in the S1 File (Tables I-U in S1 File). All statistical analyses were conducted using the free program R software (R Core Team, 2013).
Results
Radiocarbon dating
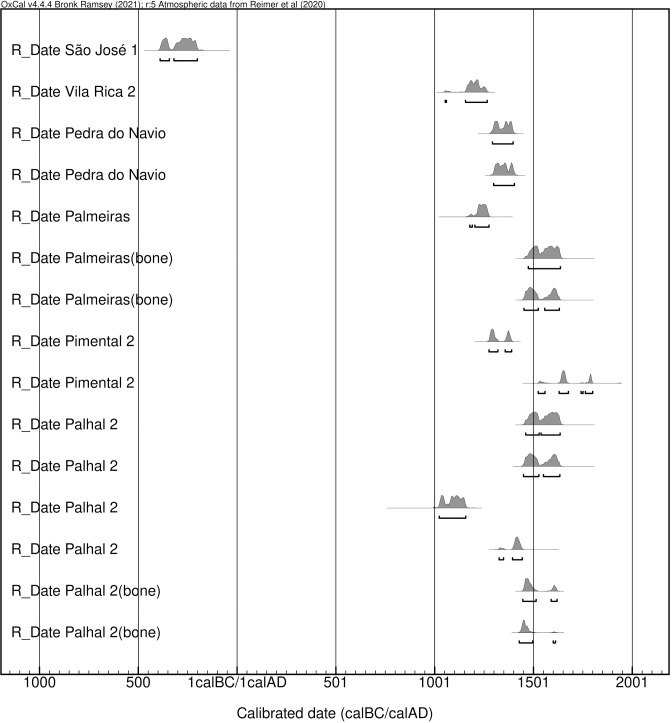
From the 11 sites studied in this research, previously published dates come from the Carrazedo, Vila Rica 2 and the Pedra do Navio sites. Carrazedo is located at the mouth of the Xingu River and has two dates that place the occupation of the site between 750–870 cal AD and 1260–1460 cal. AD [36]. Vila Rica 2, located at the VGRX, has one date that places the occupation of the site between 1052–1260 cal. AD [38], and Pedra do Navio, also located at VGRX, has two dates, placing the occupation of the site between 1295–1399 cal. AD [58]. Here, we present new 14C dates, using charcoal samples and human bone collagen, from a further four of the 11 sites studied in this research. These dates have not yet been published and provide a chronological picture of occupations at VGRX over the past two millennia (Table 1). The radiocarbon dates show a general focus of site occupations between 1,050 cal AD and 1,650 cal AD, with one displaying greater antiquity, 300 cal BC, possibly reflecting the beginning of the occupation of that archaeological site (Table 1, Fig 2) (See in S1 File more information). The São José 1 site, from which the oldest date comes, is also the only site sampled with Arauquinoid type ceramics, with which the four exhumed individuals are possibly associated.
Fig 2. Dates from archaeological sites from VGRX.
Most were occupied in the last millennium. Calibrations performed online using OxCal 4.4.4.
δ13Cco and δ15N from collagen
The poor preservation of organic materials within tropical forests is well-attested (e.g. [96]), and this study has faced some issues in this regard. For the analysis of bone collagen, of the 22 human individuals from which small portions of bone were analyzed, only four adults from two archaeological sites had δ13Cco and δ15N values from collagen with acceptable C/N ratios (between 2.9 and 3.6) and collagen yields (>1%) [97, 98] (Table 2) (Table V in S1 File). The δ13Cco values of the two individuals from the Palmeiras site are very close to each other with values of -16.3‰ and -15.7‰ (dated to 371±26 BP and 342±26 BP, respectively). These values are also similar to the δ13Cco value obtained from an individual from Palhal 2 site (-16.0‰), though higher than that of a second individual from the same site (-18.2‰) (dated 390±-196 BP and 424±24 BP, respectively). The δ15N values for the four individuals varied by up to 1.5‰, being slightly higher in individuals from the Palhal 2 site (13.2‰ and 12.4‰) than in the Palmeiras site (11.3‰ and 11.5‰).
Table 2. Human stable isotopes results from collagen.
| Site | Burial | Lab Cod | δ13Cco ‰VPDB | %C | δ15N ‰AIR | %N | Ratio C/N | Sex |
|---|---|---|---|---|---|---|---|---|
| Palmeiras 1 | Burial 1 | PAM-001 | -16.3 | 25.0 | 11.3 | 8.9 | 3.30 | Male |
| Palmeiras 1 | Burial 2 | PAM-002 | -15.7 | 44.6 | 11.5 | 17.6 | 3.50 | Male |
| Palhal 2 | Burial 1 | PAL-001 | -18.2 | 36.5 | 13.2 | 13.4 | 3.20 | Male |
| Palhal 2 | Burial 2 | PAL-002 | -16.0 | 44.8 | 12.4 | 18.1 | 2.90 | Male |
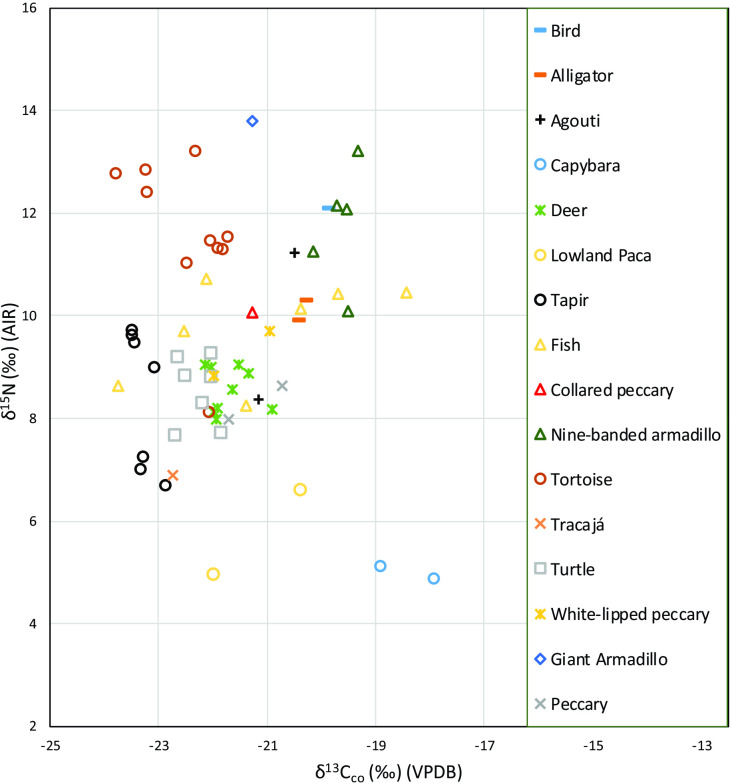
Low preservation was also observed for the collagen of the faunal bones analyzed, and, of the 176 samples analyzed, only 61 presented satisfactory C/N ratios and collagen yields (Table E in S1 File). The analyzed terrestrial fauna are composed of a wide variety of herbivorous taxa (capybara, paca, tapir, agouti, peccary, and deer) and represent the largest dataset of pre-industrial δ13Cco isotopic characterization for an Amazonian faunal community to date (Figs 3 and 4). The average δ13Cco and δ15N values for herbivores are -21.7‰±1.3‰ and 8.2‰±1.6‰, respectively (n = 27, range = -23.5‰ to -17.9‰ for δ13Cco and 4.9‰ to 11.2‰ for δ15N). Among the sampled omnivores (tortoises, turtles, tracajá, and armadillos) the average values of δ13Cco and δ15N are -21.8‰±1.3‰ and 10.6‰±2.1‰, respectively (n = 24, range = -23.8‰ to -19.3‰ for δ13Cco and 6.9‰ to 13.8‰ for δ15N). Among carnivores (alligator) the mean values of δ13Cco and δ15N are -20.3‰±0.1‰ and 10.1‰±0.3‰, respectively (n = 2, range = -20.4‰ to -20.3‰ for δ13Cco and 9.9‰ to 10.3‰ for δ15N). These values fall very close to those obtained for a non-identified bird, which presented a δ13Cco of -19.7‰ and δ15N of 12.1‰.
Fig 3. Fauna bulk bone collagen δ13C and δ15N results for VGRX.
Fig 4. Fauna and human bulk bone collagen δ13C and δ15N results grouped by diet for VGRX.
Freshwater fish resources, rich in species diversity and widely consumed by past and present Amazonian populations, were identified in the archaeological record to the level of class. The seven specimens analyzed from the archaeological contexts had average δ13Cco and δ15N values of -21.2‰±1.8‰ and 9.8‰±1.0‰, respectively (n = 7, range = -23.8‰ to -18.5‰ for δ13Cco and 8.3‰ to 10.7‰ for δ15N). This large variation in the values of δ13Cco is evident among individuals of the same species and for different species and highlights the isotopic unpredictability of freshwater ecosystems, especially those as ecologically diverse as those of the Amazon Basin [78]. While different dietary sources also contribute to δ13Cco, variation in the values of δ15N may be related to trophic level [77] as well as the size and age of the fish sampled. Using Kruskal-Wallis statistical testing, we found no significant differences between the δ13Cco values of herbivores, carnivores, omnivores and fish in the VGRX sample (chi-squared = 3.2098, df = 3, p-value > 0.05) (Table I in S1 File). ANOVA and post-hoc Tukey pair-wise comparison showed that for δ15N, herbivores were statistically different from fish (p<0.003) and omnivorous taxa (p = 0.000), but did not statistically differ from carnivorous taxa (represented by only two alligators) (p = 0.089). There are no statistically significant δ15N differences between fish, omnivores, and carnivores (Table K in S1 File).
δ13Cca and δ18O from enamel bioapatite
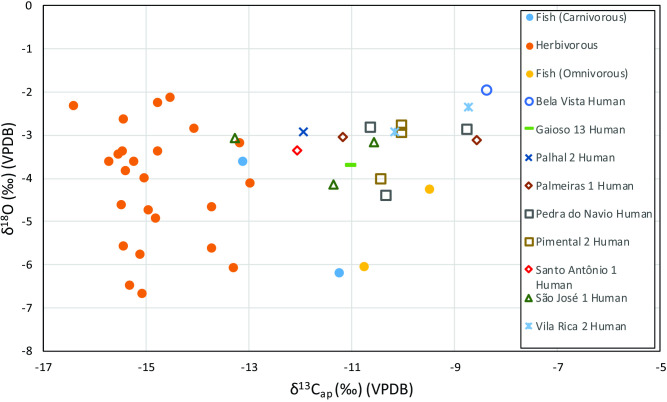
Tooth enamel bioapatite differs from bone collagen and dentine in that it is more resistant to post-mortem replacement and diagenetic degradation [99, 100]. For the analysis of tooth enamel stable isotope measurements, all of the 17 human subjects, from nine different archaeological sites, who had small portions of teeth analyzed, presented results (Table 3) (Table F in S1 File). When observing the results of human δ13Cap from VGRX a relatively large range of values is observed, including between individuals from the same site. The average human value of δ13Cap was -10.4‰±1.3‰ (range = -13.3‰ and -8.4‰). δ18O values, meanwhile, presented an average of -3.2‰±0.6‰ and a range of -4.4‰ to -2.0‰.
Table 3. δ13Cap and δ18O values for enamel from prehistoric human from VGRX, archeological sites in the Xingu River Basin, Brazil.
| Archaeological site | Burial | Lab Cod | Tooth | δ13C (‰) (VPDB) | Std. dev. | δ18O (‰) (VPDB) | Std. dev. | Sex |
|---|---|---|---|---|---|---|---|---|
| Bela Vista | Urn 1 | BEL-001 | M | -8.4 | 0.3 | -2.0 | 0.2 | Not identified |
| Gaioso 13 | Structure 1 | GAI-001 | M | -11.0 | 0.1 | -3.7 | 0.1 | Not identified |
| Palhal 2 | Burial 2 | PAL-002 | 3RLM | -11.9 | 0.1 | -2.9 | 0.1 | Male |
| Palmeiras 1 | Burial 1 | PAM-001 | 3RLM | -11.2 | 0.2 | -3.0 | 0.1 | Male |
| Palmeiras 1 | Burial 2 | PAM-002 | 2LUM | -8.7 | 0.2 | -3.1 | 0.1 | Male |
| Pedra do Navio | Urn 1 | PED-001 | 1LLPM | -10.3 | 0.1 | -4.4 | 0.1 | Not identified |
| Pedra do Navio | Burial 1 (structure 4) | PED-002 | 2LLPM | -8.8 | 0.2 | -2.9 | 0.1 | Not identified |
| Pedra do Navio | Burial 2 (structure 6) | PED-003 | 1LRPM | -10.6 | 0.2 | -2.8 | 0.1 | Female |
| Pimental 2 | Burial 2 | PIM-002 | M | -10.0 | 0.2 | -2.8 | 0.1 | Female |
| Pimental 2 | Burial 4 | PIM-004 | 1LLM | -10.0 | 0.2 | -3.0 | 0.1 | Not identified |
| Pimental 2 | Urn 2 | PIM-007 | M | -10.4 | 0.2 | -4.0 | 0.1 | Not identified |
| Santo Antônio 1 | Urn 2 | SAN-001 | M | -12.1 | 0.2 | -3.4 | 0.2 | Not identified |
| São José 1 | Burial 2 | SÃO-002 | 1RUM | -11.4 | 0.1 | -4.1 | 0.1 | Male |
| São José 1 | Burial 3 | SÃO-003 | M | -13.3 | 0.1 | -3.1 | 0.1 | Not identified |
| São José 1 | Burial 4 | SÃO-004 | PM | -10.6 | 0.2 | -3.2 | 0.1 | Female |
| Vila Rica 2 | Structure 28 | VIL-001 | 2LM | -10.2 | 0.6 | -2.9 | 0.2 | Not identified |
| Vila Rica 2 | Structure 29 | VIL-002 | LPM | -8.8 | 0.2 | -2.6 | 0.1 | Not identified |
The individual with the lowest δ13Cap came from the São José 1 site (δ13Cap -13.3 ‰), the site with the oldest radiocarbon date (2,240 ± 30 BP). The other two individuals from the São José 1 site, had δ13Cap values of -11.4 ‰ and -10.6 ‰, that are similar to those seen in other sites such as Gaioso 13 (-11.0 ‰), Palhal 2 (-11.9 ‰, bone from the same individual was dated to 390±19 BP), Pimental 2 (-10.0 ‰, -10.0 ‰ and -10.4 ‰, the site was dated to 248 ± 26 BP and 680 ± 30 BP), one individual from Palmeiras 1 (-1.2 ‰, bone from the same individual dated to 371±26 BP), two individuals from Pedra do Navio (-10.3 ‰ and -10.6 ‰, site dated by radiocarbon to 610 ± 30 BP and 630 ± 30 BP), and an individual from Vila Rica 2 (-10.2 ‰, site dated by radiocarbon on charcoal to 850 ± 30 BP). The highest human δ13Cap value belonged to the individual from the Bela Vista site (δ13Cap -8.4 ‰). Although this archaeological site has yet to yield an absolute date, its material culture is similar to that of other sites in the region dated to the last millennium. Other humans with similar values come from the Palmeiras site (-8.6 ‰, bone from the same individual dated to 342±26 BP) and one individual from Pedra do Navio (-8.8 ‰).
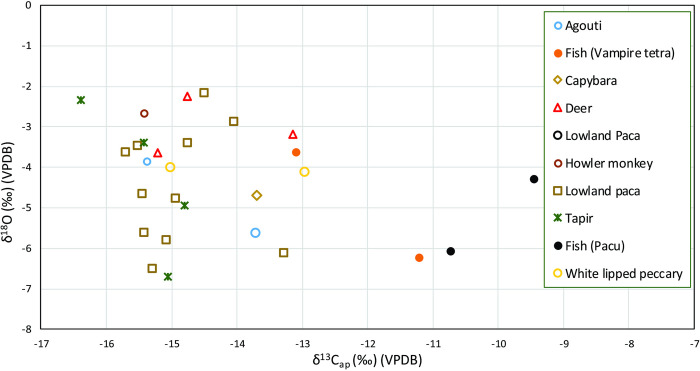
The tooth enamel samples from terrestrial and aquatic fauna comprise 28 individuals that can be grouped into herbivorous and fish taxa [101] (Table G in S1 File) (Figs 5 and 6). The herbivorous average values of δ13Cap and δ18O are -14.8‰±0.9‰ and -4.2‰±1.4‰, respectively (n = 24, range = -16.4‰ to -13.0‰, and -6.7‰ and -2.2‰ for δ18O). Two types of fish had their dental enamel analyzed, one with an omnivorous diet (Myleinae, n = 2) and one with a carnivorous diet (Hydrolycus scomberoides, n = 2). The omnivorous specimens had average δ13Cap and δ18O values of -10.1‰±0.9‰ and -5.2‰±1.3‰, respectively (n = 2, range = -10.7‰ to -9.5‰ for δ13Cap and -6.1‰ and -4.3‰ for δ18O). The carnivorous specimens had average δ13Cap and δ18O values of -12.2‰±1.3‰ and -4.9‰±1.8‰, respectively (n = 2, range = -13.1‰ to -11.2‰ for δ13Cap and -6.2‰ and -3.6‰ for δ18O). We found no significant differences between the δ18O values by dietary group in the VGRX sample (Tables M and N in S1 File). A Kruskal-Wallis test confirms that there is a difference in δ13Cap by diet (chi-squared = 9.6218, df = 2, p-value < 0.05) but a Wilcoxon Pairwise comparison fails to draw out any group as significantly different from each other, probably due to small sample sizes for some of the groups (Table L in S1 File).
Fig 5. Faunal bulk tooth enamel δ13C and δ18O for VGRX.
Fig 6. Faunal and human bulk tooth enamel δ13C and δ18O grouped by diet for VGRX.
Discussion
Amazonian baselines
Within tropical rainforests, plants that grow close to the ground, under a closed forest canopy, are expected to be heavily depleted in 13C due to low light and recycled CO2 [72, 74]. Previous research in the Amazon Basin has noted that the leaves from plants growing in the lower forest strata have δ13C values of -34.3‰ in laterite forest and -35.2‰ in podsol forests, while canopy leaves have an average of -28.7‰ in the laterite forest and -30.5‰ in the podsol forest [102]. This ‘canopy effect’ is expected to be reflected in the tissues of animals that feed in these environments [103]. However, recent studies of living fauna in the Peruvian Amazon carried out by Tejada and colleagues [79] have questioned the degree to which rainforest animals will reflect such negative sub-canopy values and display a median δ13Cdiet value of -27.4 ‰ for mammalian herbivores. The average of δ13Cap of herbivorous fauna of archaeological origin sampled by Roosevelt in Peru [40] (δ13Cap –13.3 ‰ ± 1.4 ‰ in archaeological Ucayali- excluding capybaras, δ13Cap –12.5 ‰ ± 2.2 ‰ in archaeological Ucayali–including capybaras) is even higher than the average values obtained by Tejada and colleagues for modern fauna [79] (δ13Cap -15.6 ‰ ± 1.6 for the modern Peruvian study- excluding capybaras, δ13Cap -15.0 ‰ ± 3.1 to the modern Peruvian Amazon–including capybaras. See Tables W and X in S1 File). The modern Peruvian values were already corrected back to 1750 by Tejada et al. [79] and it is those values we use here for comparison. Our values of herbivorous fauna (δ13Cap -14.9 ‰ ± 0.8 ‰ in VGRX—disregarding capybaras; δ13Cap -14.8 ‰ ± 0.9 ‰ in VGRX—including capybaras), is higher than the ones reported for the modern Peruvian samples (Kruskal-Wallis chi-squared = 16.425, df = 2, p-value < 0.05 including Capybara, Kruskal-Wallis chi-squared = 27.276, df = 2, p-value < 0.05 excluding Capybara) (Tables O and P in S1 File). Nevertheless, despite this variability, values still fall below the -14.0‰ threshold predicted for fauna living under a dense canopy. The only exception is the archaeological sample from Ucayali, average δ13Cap that shows values below the -14.0‰ threshold. The data suggests that tropical forest fauna can still be easily distinguished from fauna living in more open areas or consuming C4 resources.
As noted above, stable carbon isotope values of δ13Cap from Peruvian and VGRX mammalian herbivores were compared, both including capybara specimens and excluding the capybara specimens. This is due to the fact that, in the modern Peruvian sample, the only C4 consuming herbivore identified was the capybara (Hydrochoerus hydrochaeris) (n = 6, δ13Cap -3.1 ‰ ± 3.4 ‰ corrected Suess Effect for 1750). The modern analyzed individuals have higher values than the single specimen of the same genus analysed from VGRX (δ13Cap -13.7 ‰) and the two specimens analysed by Roosevelt [40] from Ucayali archaeological site in Peruvian (n = 2, δ13Cap -8.6 ± 0.1 ‰, using bone apatite; n = 1, δ13Cco -15.9 ‰). Nevertheless, in both the modern and archaeological Peruvian studies, the values confirm that the water-land interface is used intensely by capybaras which is unsurprising given their known food preferences for semiaquatic grasses [104, 105]. Although the VGRX capybara has a lower δ13C value when compared to the values of capybara in the Peruvian Amazon, both for collagen (n = 2, δ13Cco -18.4 ‰ ± 0.7 ‰) and for bioapatite, these values are still the highest of the VGRX fauna measured here, confirming the preference of these animals for more open aquatic environments in VGRX too but, different from the Peruvian case, they seem to retain a more dominant reliance on C3 resources in VRGX. All other terrestrial herbivores measured in the modern and archaeological studies, including our own, show a clear preference for C3 consumption with a strong influence of the canopy effect.
In terms of δ15N in VGRX, one of the two agouti (Dasyprocta sp.) analyzed from VGRX (Table E in S1 File) yielded a higher value than expected for herbivorous taxa (11.2‰), suggesting the possible omnivorous potential of this species [106]. It is usually assumed that herbivores will have values of δ15N that reflect the local vegetation, and existing data shows that variations within the population of a given species can easily exceed 1‰ [107]. This variation was not only detected among the population of the same species in VRGX (Table E in S1 File), but it can be even greater when compared to species from other regions of the Amazon, such as the Ucayali Basin, for example (archaeological samples) [40]. The values of δ15N in VGRX are higher than in the Ucayali Basin for peccary (Tayassuidae, mean of 8.3 ± 0.5 ‰ for VGRX and 5.7 ± 0.1 ‰ for Ucayali Basin), for capybara (Hydrochoerus hydrochaeris, mean of 5.0 ± 0.2 ‰ for VGRX and 3.6 ‰ for Ucayali Basin) and for deer (Cervidae, mean of 8.6 ± 0.4 ‰ for VGRX and 5.7 ± 0.4 ‰ for Ucayali Basin). An ANOVA test showed there are δ15N differences between species from VGRX and Ucayali (F(8,24) = 8.469, p<0.05), although a post-Hoc Tukey Pairwise comparison failed to find significant differences (Tables R and S in S1 File). This work further highlights the importance of developing a robust baseline for human δ15N and δ13C interpretation, as variation can occur even within a given biome and within a given species.
Overall, our data show a clear reliance on C3 resources across the terrestrial fauna studied. Furthermore, a distinction in δ15N is noted between terrestrial herbivores and omnivorous and aquatic taxa. However, our data also highlight the potential confounding isotopic effects of complex freshwater systems [108]. This is particularly difficult in the Amazon where some fish are noted for their consumption of fruits and seeds of tree species [109, 110]. The range of fish δ13Cco (-23.8 to -18.5 ‰) and δ13Cap (-13.1 to -9.5 ‰) covers a major portion of expected C3 and mixed C3/C4 space, making it difficult to distinguish reliance on terrestrial resources versus aquatic resources. Indeed, the average values of δ13Cco of fish measured here are not significantly different from the average values of δ13Cco of terrestrial herbivores (p >0.05), terrestrial omnivores (p >0.05) and alligators (p >0.05) (Table I in S1 File), confirming the predilection of at least some of these fish for the consumption of fruits and seeds of C3 species [109, 110]. However, it is clear that terrestrial herbivores and omnivores experiencing the canopy effect broadly show lower δ13C than most aquatic samples, particularly for δ13Cap. Similarly, while it is difficult to distinguish terrestrial omnivores from aquatic resources based on δ15N, herbivores emerge as being distinct from both of these groups.
Diets on the Xingu River between 390 cal. BC and 1,675 cal. years AD
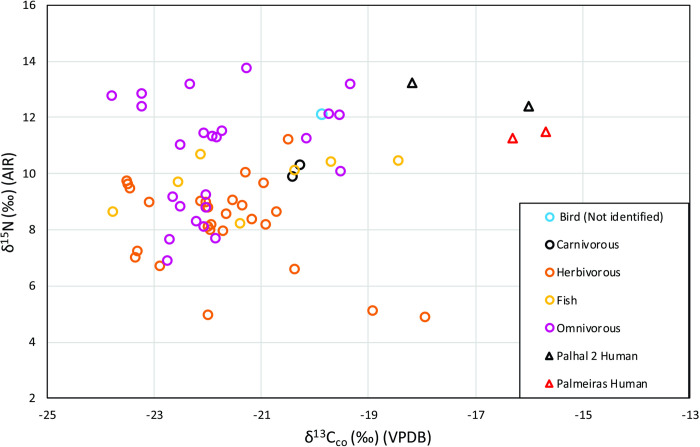
Despite the fact that freshwater δ15N and δ13C cover a large range of isotopic space, we are still able to observe some notable distinctions in the human isotopic data. The values of stable isotopes of human δ13Cco from the four VGRX individuals with preserved collagen are higher (average of -16.5 ± 1.1 ‰) than the values obtained for terrestrial herbivores (-21.7 ± 1.4 ‰), carnivores (-20.3 ± 0.1 ‰), omnivores (-21.8 ± 1.3‰) and fish (-21.2 ± 1.8 ‰), suggesting the addition of an additional food source into their diets compared to local fauna. When comparing the human values of δ13Cap to those of the faunal baseline, a large variation in individual human values is visible. However, the human dataset is also higher (-10.4 ± 1.3 ‰) than the δ13Cap of herbivorous fauna (-14.8 ± 0.9 ‰), but similar to carnivorous fish (-12.2 ± 1.3 ‰) and omnivorous fish (-10.1 ± 0.9 ‰). The human δ15N collagen values are also higher (12.1 ± 0.9 ‰) than those of fauna (8.2 ± 1.6 ‰ for herbivorous terrestrial fauna, 10.1 ± 0.3 ‰ for carnivorous terrestrial fauna, 10.6 ± 2.1 ‰ for omnivorous terrestrial fauna and 9.8 ± 1.0 ‰ for fish) (Fig 4). Overall, then, there seems to be a clear spectrum of variation in human diets ranging from a reliance on closed canopy terrestrial plants, animals and different types of aquatic resources to a clear input of δ13C resources that are not documented in the faunal baseline and are likely representative of additional C4 resource use such as maize (e.g. for individuals BEL-001, PAM-002, PED-002 and VIL-002).
When considering possible differences in economies between Terra Firme and Várzea sites [3], it does not appear that the economies of the groups that inhabited the Vila Rica 2 site (the only terra firma context ultimately available due to the lack of preservation of organic material in the other excavated sites) differ isotopically from the groups who inhabited the riverside (average of -9.5 ‰ ± 1.0 ‰, n = 2 to Vila Rica 2, average of -9.9 ‰ ± 1.0 ‰, n = 3 to Pedra do Navio, for example, all from the last millennium). The characteristics of the material culture of both are similar and the isotopic values would suggest limited differences in economic strategy, although it should be noted that low sample sizes currently hinder a statistically-valid comparison between the two types of sites and more research testing this suggestion is needed. Furthermore, we have seen that terrestrial fauna and freshwater resources can be difficult to distinguish from each other, meaning that more subtle economic variations may be hard to discern isotopically. The same can be said for C3 wild and domesticated (e.g. manioc) plant resources, which will have similar isotopic values and thus be effectively indistinguishable. With regards to isotopic differences between different cultural contexts among the VGRX sites some intragroup variation is clear. São José 1 is the only site with Arauquinoid ceramic-styles. The lowest human δ13Cap value (-13.3 ‰) comes from this site, perhaps suggesting greatest reliance on local terrestrial fauna from forest biomes (mean of δ13Cap herbivores of -14.9 ‰ and omnivorous of -14.0 ‰). Nevertheless, within the same site, variation of δ13Cap could be up to 2.7 ‰ in São José 1 and 2.6 ‰ in Palmeiras. Low variation in δ18O values among all individuals does not support long distance migration being a significant phenomenon in these sites and is rather consistent with resident intragroup dietary differences, although human δ18O is notoriously difficult to interpret in isolation.
Differences in δ13Cap values between individuals at a given site also cannot be explained in relation to the treatment given to the dead (as a possible way of assessing social stratification or differentiated social positions). The four individuals with higher δ13Cap values from VGRX come from four different archaeological sites (Bela Vista, Palmeiras 1, Vila Rica 2, and Pedra do Navio) and were deposited in four different forms of burial. The individual with higher δ13Cap values (-8.4 ‰) was a probably child and was buried inside of an urn (Bela Vista site). The individual from Burial 2/Palmeiras (δ13Cap -8.7 ‰) was an adult man and was found as a primary burial, without visible goods around the body. The individual from Structure 29/Vila Rica 2 (δ13Cap -8.7 ‰), buried with a vessel overturned over the head, could not be sexed or aged due to poor preservation. The individual from Burial 1/Pedra do Navio (δ13Cap—8.8 ‰) was an adult and was buried in primary form, with at least three items of pottery as grave goods. The three individuals with lower δ13Cap values come from three archaeological sites and were in two different burial forms: an adult, primary burial without visible goods (Burial 3/São José 1 site, δ13Cap− 13.3 ‰), a child buried with vessel overturned on the head (Structure 2/Santo Antônio 1 site, δ13Cap− 12.1 ‰), and an adult, man, primary burial without visible goods (Burial 2/Palhal 2 site, δ13Cap− 11.9 ‰) (Fig A in S1 File). When looking for differentiation between the sexes, there are few individuals that can be confidently identified as male or female (n = 7). However, it should be noticed that the three females have δ13Cap values closer to each other (-10.6‰, -10.0‰ and -10.6‰, from Pedra do Navio, Pimental 2 and São José 1 archaeological site respectively), while males have more dispersed values, from the lowest (-11.9‰ from Palhal 2) to the highest (-8.6‰, from Palmeiras 1) (Fig B in S1 File), suggesting that there could be some possible dietary variation. Unfortunately, the number of individuals whose sex has not been identified is quite high (n = 10), meaning there may be bias in the sample size, as well as the fact that comparisons are being performed between different sites and different biomes. To confidently identify sex-based differences in diet and economy a more detailed investigation of sexed skeletons at a given site and context would be required.
Comparison with other Amazon datasets
Ethnographic and ethno-historical reports from the time of European invasion document the existence of dense populations living in a complex system of social organization along the Amazon River [111, 112]. Several archaeological sites have already been excavated in this region confirming, alongside palaeoecological work, contemporary occupation and cultural and social relationships between the Terra firme and Várzea occupations [113], the production of ADE, and the cultivation of squash (Cucurbita sp.), maize (Zea mays) and cassava (Manihot esculenta) [9, 113]. For the time being, there are no human stable isotopic studies of individuals exhumed from these sites (of the so-called Tapajós culture) to compare with the ethno-historical data and determine the intensity of maize consumption and its relationship with the social structure of these groups. However, previous studies carried out on individuals from the Corazal phase in Venezuela (Orinoco Basin), suggested a change in the dietary patterns of populations between 800 years BC and 400 years AD, with the increasing incorporation of maize as a staple food (Table H in S1 File). This change has been linked to the development of larger settlements to support denser populations [22]. A similar transition in dietary patterns was also noticed in the western region of the Amazon, in Peru (Ucayali Basin) [40].
Nevertheless, while maize appears to be well-established in the diets of populations of the north and west of the Amazon by the first millennium AD [22, 40], in other regions its dietary significance appears to be more subtle. At the mouth of the Amazon river, isotopic analysis of human remains from two important cultures (Maracá and Marajó) yielded δ13Cco values which suggest that maize, despite being found in the archaeological sediments in the lower Amazon river dating to around 4.300 cal BP [8, 9], was not a significant foodstuff for Maracá and Marajó populations, who probably focused on the exploitation of forest and riverine resources [24]. At the mouth of the Amazon River, δ13Cco analysis of human remains from Marajó sites are slightly higher than the values obtained for the Maracá, though the data has again been interpreted as being indicative of a mixed diet, with variability being related to differential access to certain food groups [40]. In both cases, the available faunal and freshwater baselines are currently limited, making more detailed discussions of dietary variability challenging. Meanwhile, one the eastern edge of the Amazon, the values of δ13Cco found in individuals from different archaeological cultures from São Luis Island also suggest the superficial consumption of maize, along with other C3 items and mammal hunting, reinforcing the secondary role of this crop for the populations that lived in the eastern Amazon in the Late Holocene [41].
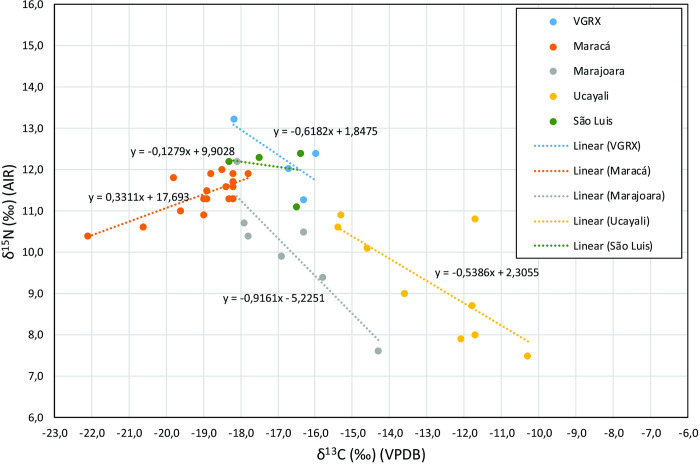
When the δ13Cco values from the VGRX samples are compared with those available from the Orinoco basin, it appears that the four individuals from VGRX present δ13Cco values intermediate to the oldest and most recent phases, suggesting a mixed diet that includes both C3 and C4 resources. However, VGRX values (average of -16.5 ± 1.1) are lower than values found among the Ucayali individuals (-14.7 ± 0.8 to Early Ucayali, -11.5 ± 0.7 to Late Ucayali), suggesting less reliance on maize. Indeed, comparing the values of δ13Cco of VGRX with the populations from the mouth of the Amazon and São Luis, it is clear that they are very close to each other (average of -19.0 ± 1.1 to Maracá, -16.7 ± 1.4 to Marajó, -17.0 ± 0.9 to São Luis). A Kruskal-Wallis test showed there are δ13Cco differences between them (chi-squared = 39.954, df = 7, p-value < 0.05) and Pairwise comparisons using Wilcoxon showed that Maracá is different from Marajó (p = 0.009) and Late Ucayali (p = 0.026). However, the small sample size means further pairwise comparison differences could not be observed (Table T in S1 File). When comparing δ15N between the published studies, our VGRX data have the highest values (average of 12.1 ± 0.9), very close to those of the São Luis value (12.1 ± 0.6). Again, a Kruskal-Wallis test showed the existence of differences between human groups (chi-squared = 25.358, df = 5, p-value < 0.05) though pairwise comparisons using Wilcoxon tests showed only Maracá (average of 11.4 ± 0.5) to be significantly different from Late Ucayali (average of 8.6 ± 1.3) (p = 0.025) (Table U in S1 File). Interestingly, a linear regression analysis shows a strong negative correlation between δ13Cco and δ15N for VGRX, as at Ucayali and Marajó, which implies that higher δ13Cco is related to lower trophic foods, perhaps in the form of maize (Fig 7).
Fig 7. δ13Cco and δ15N values of archaeological human bones from VGRX, Marajó, Maracá, São Luis and Ucayali.
Overall, then, the human and faunal data from VGRX, as well as its comparison with data from elsewhere in the Amazon Basin, indicate the varying incorporation of maize into individual diets even within a given region. This agrees with the descriptions of travellers and researchers from the 16th to the 19th centuries, who note the widespread cultivation of maize by Indigenous populations [51, 65, 67, 111, 112]. Nevertheless, maize was not the primary basis of diets and economies at VGRX and, as at Amazon mouth, a combination of hunting, fishing, vegetable harvesting and horticulture provided a rich diet and a more regular supply of food, ensuring that these groups were not dependent on a single foodstuff, therefore, decreasing the risk of exposure of communities to problems obtaining any particular given food source. The data presented is also consistent with an emerging consensus that there was no single adaptive pattern for pre-colonial Amazonian populations and proposes that diversified economic strategies, based on the management of wild and cultivated plants combined with the exploitation of aquatic and terrestrial fauna resources, could have developed over centuries and sustained long-term successful, often-large human populations [114]. Archaeological insights into a diversity of landscape management strategies, including the construction of raised fields, dikes, canals, wells, ponds, sidewalks, roads and hills for housing and burial [10, 21, 23, 35, 115, 116] support this, with more isotopic studies required to gain further insights into cultural, social, and ecological distinctions in diet and economies across this vast area.
Conclusions
There is growing archaeological evidence that the long-term history of occupation of Amazonia culminated in large population aggregates by 1,000 AD [117], as suggested by the clear increase in the size and number of archaeological sites as well as the widespread discovery of Anthropogenic Dark Earth sites. This fluorescence is sometimes associated with the arrival of maize into subsistence systems across the floodplain societies of the Greater Amazon; and previous analyses of dental pathologies, associated with microbotanical data suggest that grain crops may have become quite important between 500 AD and European conquest [23]. Isotopic analyses of human remains can provide privileged direct insights into the incorporation of maize into Amazonian diets and, in the northern and western Amazon, a correlation has been documented between population increase and maize consumption. Nevertheless, our data for VGRX, as well as other studies in Marajo and Maracá [24], and on São Luis island [41], indicate a far more limited consumption of maize, despite it being present in these regions as early as 4,300 cal. BP. Overall, the findings point to significant variability in the diets of Amazonian populations, something perhaps unsurprising given the vast cultural, ecological, and geographical variation of the Amazon Basin, an area roughly the size of Europe. In particular, management of wild trees, cultivation of tubers, the hunting and the capture of wild animals, and even corralling, of freshwater resources seem to have provided varied approaches to the maintenance of significant populations and settlement networks at the time of European invasion, calling into question the necessity of conventional ideas of ‘agriculture’ to sustain such phenomena. Further multidisciplinary research in different contexts is now essential to determine the degree to which pre-contact Amazonian diets varied across cultural and social contexts, as well as between ecological settings. In this way, understandings of economic resilience and land-use variability across different portions of the Amazon Basin can be better developed and factored into the ongoing debate about the impacts of pre-colonial societies on the tropical forests of this increasingly threatened biodiversity hotspot [118].
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the Scientia Consultoria Científica and Museu Paraense Emílio Goeldi for making the analyzed archaeological material available, the researchers from Scientia Colsultoria Científica and from the Stable Isotope Research Laboratory of the Department of Archaeology, Max Planck Institute for the Science of Human History in Jena/Germany for discussions in relation to this work; Fulvio Vinicius Arnt for reading the draft of this work and coments; the Programa de Pós Graduação em Antropologia da Universidade Federal do Pará, and the CAPES and the Max Planck Society for support. We would also like to thank the reviewers for their constructive comments that have helped improve the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001", Programa de Doutorado Sanduíche no Exterior -88881.190306/2018-01. https://www.gov.br/capes/pt-br. We would also like to thank the Max Planck Society for funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Koch A, Brierleya C, Maslina MM, Lewis SL. Earth system impacts of the European arrival and Great Dying in theAmericas after 1492. Quaternary Science Reviews, 207. 2019: p. 13–36. [Google Scholar]
- 2.Meggers BJ. Environmental limitation on the development of culture. American Anthropology. 1954; 56(5): p. 801–824. [Google Scholar]
- 3.Meggers BJ. Amazônia: a ilusão de um paraíso. Rio de Janeiro: Civilização Brasileira; 1977. [Google Scholar]
- 4.Bailey RC, Head G, Jenike M, Owen B. Hunting and Gathering in Tropical Rain Forest: Is it Possible? American Anthropology. 1989; 91(1): p. 59–82. [Google Scholar]
- 5.Clement CR, Cristo-Araújo M, d’Eeckenbrugge GC, Pereira AA, Picanço-Rodrigues D. Origin and Domestication of Native Amazonian Crops. Diversity 2010. 2010: p. 72–106. [Google Scholar]
- 6.Clement CR, Rodrigues DP, Alves-Pereira A, Mühlen GS, Cristo-Araújo M, Moreira PA, et al. Crop domestication in the upper Madeira River basin. Bol. Mus. Para. Emílio Goeldi. Ciênc. hum. 2016: p. 193–205. [Google Scholar]
- 7.Hilbert L, Neves EG, Pugliese F, Whitney BS, Shock M, Veasey E, et al. Evidence for mid-Holocene rice domestication in the Americas. Nature Ecology & Evolution, 1. 2017: p. 1693–1698. doi: 10.1038/s41559-017-0322-4 [DOI] [PubMed] [Google Scholar]
- 8.Kistler L, Maezumi SY, Souza JGd, Przelomska NAS, Costa FM, Smith O, et al. Multiproxy evidence highlights a complex evolutionary legacy of maize in South America. Science 362. 2018. December 14: p. 1309–1313. doi: 10.1126/science.aav0207 [DOI] [PubMed] [Google Scholar]
- 9.Maezumi SY, Alves D, Robinson M, Souza JG, Levis C, Barnett RL, et al. The legacy of 4,500 years of polyculture agroforestry in the eastern Amazon. Nature Plants, 4. 2018: p. 540–547. doi: 10.1038/s41477-018-0205-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prestes-Carneiro G, Béarez P, Shock MP, Prümers H, Betancourt CJ. Pre-Hispanic fishing practices in interfluvial Amazonia: Zooarchaeological evidence from managed landscapes on the Llanos de Mojos savanna. PLOS ONE. 10.1371/journal.pone.0214638. 2019. May 15: p. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro MBN, Jerozolimski A, Robert P, Salles NV, Kaiapó B, Pimentel TP, et al. Anthropogenic Landscape in Southeastern Amazonia: Contemporary Impacts of Low-Intensity Harvesting and Dispersal of Brazil Nuts by the Kayapo´ Indigenous People. PLoS ONE. 2014. doi: 10.1371/journal.pone.0102187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyo-Kalin M. Slash-burn-and-churn: Landscape history and crop cultivation in pre-Columbian. Quaternary International, 249. 2012: p. 4–8. [Google Scholar]
- 13.Levis C, Costa FRC, Bongers F, Peña-Claros M, Clement CR, Junqueira AB. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science. 2017. March: p. 925–931. [DOI] [PubMed] [Google Scholar]
- 14.ter Steege H, Salomão RP, Magnusson WE, Pitman NCA, Guevara JE, Molino JF, et al. Hyperdominance in the Amazonian Tree Flora. Science. 2013. October. doi: 10.1126/science.1243092 [DOI] [PubMed] [Google Scholar]
- 15.Palace MW, McMichael CNH, Braswell BH, Hagen SC, Bush MB, Neves E, et al. Ancient Amazonian populations left lasting impacts on forest structure. Ecosphere, 8 (12). 2017: p. 1–19.29552374 [Google Scholar]
- 16.Heckenberger MJ, Kuikuro A, Kuikuro UT, Russell JC, Schmidt M, Fausto C, et al. Amazonia 1492: Pristine Forest or Cultural Parkland? Science,301. 2003: p. 1710–1714. doi: 10.1126/science.1086112 [DOI] [PubMed] [Google Scholar]
- 17.Rostain S. Pre-Columbian Earthworks in Coastal Amazonia. Diversity, 2. 2010: p. 331–352. [Google Scholar]
- 18.Erickson CL. Amazonia: the historical ecology of a domesticated lansdcape. In Silverman H, Isbell WH. The Haandbook of South American Archaeology. New York: Springer; 2008. p. 157–183. [Google Scholar]
- 19.Clement CR, Denevan WM, Heckenberger MJ, Junqueira AB, Neves EG, Teixeira WG, et al. The domestication of Amazonia before European conquest. Proc. R. Soc. London Ser. B 282, 20150813. 2015. doi: 10.1098/rspb.2015.0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fausto C, Neves EG. Was there ever a Neolithic in the Neotropics? Plant familiarisation and biodiversity in the Amazon. Antiquity. 2018: p. 1604–1618. [Google Scholar]
- 21.Schaan D. Long-Term Human Induced Impacts on Marajó Island Landscapes, Amazon Estuary. Diversity. 2010; 2: p. 182–206. [Google Scholar]
- 22.van der Merwe NJ, Roosvelt AC, Vogel JC. Isotopic evidence for prehistoric subsistence change at Parmana, Venezuela. Nature. 1981: p. 536–538. [Google Scholar]
- 23.Roosevelt AC. The Rise and Fall of the Amazon Chiefdoms. L’Homme, 33 (126–128). La remontée de l’Amazone. 1993: p. 255–283. [Google Scholar]
- 24.Hermenegildo T, Guapindaia VLC, Neves EG. New evidence for subsistence strategies of late pre-colonial societies of the mouth of the Amazon based on carbon and nitrogen isotopic data. Quaternary International 448. 2017. Agosto 20: p. 139–149. [Google Scholar]
- 25.Cascon LM. Alimentação na floresta tropical: Um estudo de caso no sítio Hatahara, Amazônia Central, com base em Microvestígios Botânicos. Dissertação de mestrado ed. Rio de Janeiro: Museu Nacional, Universidade Federal do Rio de Janeiro—UFRJ; 2010. [Google Scholar]
- 26.Lathrap DW. The "Hunting" Economies of the Tropical Forest Zone of South America: An Attemp at Historical Perspective. In Lee RB, DeVore I, editors. Man the Hunter. Chicago: Aldine Press; 1968. p. 23–29. [Google Scholar]
- 27.Perota C. Adaptação agrícola no baixo Xingu. In Meggers BJ. Prehistoria Sudamericana: Nuevas Perspectivas. Washington: Taraxacum; 1992. p. 219–229. [Google Scholar]
- 28.Lima HP, Fernandes GCB. Cerâmicas arqueológicas da Foz do Rio Xingu: uma primeira caracterização. In Barreto C, Lima HP, Betancourt CJ. Cerâmicas arqueológicas da Amazônia: Rumo a uma nova síntese. Belém: Iphan, Ministério da Cultura; 2016. p. 224–236. [Google Scholar]
- 29.Müller LM, Kipnis R, Caldarelli SB, Santos MCMM. Considerações iniciais sobre a cerâmica arqueológica da Volta grande do Xingu. In Barreto C, Lima HP, Betancourt CJ. Cerâmicas arqueológicas da Amazônia: Rumo a uma nova síntese. Belém: Iphan; Ministério da Cultura; 2016. p. 196–209. [Google Scholar]
- 30.Costa BLS, Rapp Py-Daniel A, Gomes J, Neves EG. Urnas funerárias no Lago Amaña, Médio Solimões, Amazonas: contextos, gestos e processos de conservação. Amazônica. 2012; 4(1): p. 60–91. [Google Scholar]
- 31.Py Daniel A. Arqueologia da morte no sítio Hatahara durante a fase paredão. Dissertação de mestrado em arqueologia. São Paulo: MAE/USP; 2009. [Google Scholar]
- 32.Py-Daniel AR. Os constextos funerários na arqueologia da calha do rio Amazonas. Tese de doutorado em arqueologia São Paulo: Museu de Arqueologia e Etnologia; Universidade de São Paulo; 2015. [Google Scholar]
- 33.Costa ÁGM. Contextos e práticas funerárias no Baixo Tapajós: um estudo dos sepultamentos em urna no sítio Paraná de Araué-Pá. Dissertação de mestrado em arqueologia. São Cristóvão: Universidade Federal De Sergipe; 2015. [Google Scholar]
- 34.Lima HP, Neves EG. Cerâmicas da Tradição Borda Incisa/Barrancóide na Amazônia Central. Museu de Arqueologia e Etnologia. 2015; 21: p. 205–2030. [Google Scholar]
- 35.Heckenberger MJ, Russell JC, Fausto C, Toney JR, Schmidt MJ, Pereira E, et al. Pre-Columbian Urbanism, Anthropogenic Landscapes, and the Future of the Amazon. Science. 2008. a August 29; 321: p. 1214–1217. doi: 10.1126/science.1159769 [DOI] [PubMed] [Google Scholar]
- 36.Fernandes GCB, Lima HP, Ribeiro AB. Cerâmicas Koriabo e problematizações iniciais sobre a arqueologia na Foz do Rio Xingu. Habitus. 2018: p. 403–424. [Google Scholar]
- 37.Garcia LLW. Cerâmicas e histórias indígenas no médio-baixo Xingu. In Barreto C, Lima HP, Betancourt CJ. Cerâmicas arqueológicas da Amazônia: rumo à uma nova síntese. Belém: Iphan: Ministério da Cultura; 2016. p. 183–195. [Google Scholar]
- 38.Castro AM, Muller LM, Heinen ILS, Kipnis R. The Koriabo pottery in the Volta Grande do Rio Xingu: the cases of the Sabiá 2 and Vila Rica 2 sites. In Barreto C, Lima H, Rostain S, Hofman C. Koriabo: From the Caribbean Sea to the Amazon River. Belém: Museu Paraense Emílio Goeldi; 2021. p. 203–225. [Google Scholar]
- 39.Costa FJA, Caldarelli SB. Programa de Estudos Arqueológicos na área do reservatório de Kararaô (PA). Relatório de Viabilidade. Belém: Museu Paraense Emílio Goeldi; 1988. [Google Scholar]
- 40.Roosevelt A. Resource Management in Amazonia before the Conquest: Beyond Ethnographic Projection. Advances in Economic Botany. 1989. June 27: p. 30–62. [Google Scholar]
- 41.Colonese AC, Winter R, Brandi R, Fossile T, Fernandes R, Soncin S, et al. Stable isotope evidence for dietary diversification in the pre-Columbian Amazon. Scientific Reports. 2020. doi: 10.1038/s41598-020-73540-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Instituto Brasileiro de Geografia e Estatística. Geografia do Brasil—Região Norte. Vol I. Rio de Janeiro: 1977. [Google Scholar]
- 43.Pereira ES. Levantamento arqueológico na área da UHE-Belo Monte, rio Xingu (PA). In ELETROBRÁS/ELETRONORTE, editor. CHE Belo Monte–Estudo de Impacto Ambiental. Meio Sócio-Econômico. Brasília; 2002. [Google Scholar]
- 44.Neves EG, Petersen JB, Bartone RN, Silva CA. Historical and socio-cultural origins of amazonian dark earths. In Lehmann J, Kern DC, Glaser B, Woods WI, editors. Amazon dark earths: origin, properties, management. New York, Boston, Dordrecht, London, Moscow: Kluwer Academic Publishers; 2004. p. 29–50. [Google Scholar]
- 45.Köeppen W. Climatologia: con un estudio de los climas de la tierra. México: Fondo de Cultura Econômica.; 1948. [Google Scholar]
- 46.Instituto Brasileiro de Geografia e Estatística. Mapa de vegetação do Brasil. Instituto Brasileiro de Geografia e Estatística; 2004. [Google Scholar]
- 47.Leme. RIMA- Relatório de Impacto Ambiental do Aproveitamento Hidrelétrico de Belo Monte. São Paulo:; 2009.
- 48.Posey DA. A Preliminary Report on Diversified Management of Tropical Forest by the Kayapó Indians of the Brazilian Amazon. Advances in Economic Botany. Ethnobotany in the Neotropics. 1984; 1: p. 112–126. [Google Scholar]
- 49.Faria ESS. Viagem Etno-Histórica e arqueológica ao Médio Xingu: memória e história indígena na Amazônia. Tese de doutorado em Antropologia. Belém: PPGA-UFPA; 2016. [Google Scholar]
- 50.Sneathlage E. A travessia entre Xingu e Tapajós Manaus: Governo do Estado do Amazonas; Secretaria de Estado da Cultura, Turismo e Desporto; 2002. [1912]. [Google Scholar]
- 51.Nimuendaju C. Tribes of the lower and middle Xingu River. In Handbook of South American Indians. Washington: Smithsonian Institution; 1948. p. 213–242. [Google Scholar]
- 52.Nimuendaju C. Mapa etno-histórico Rio de Janeiro: IBGE; 1981. [1944]. [Google Scholar]
- 53.Marques AdJM. A variabilidade artefatual cerâmica nas ocupações Bela Vista na Volta Grande do Xingu/PA Belém: Universidade Federal do Pará; 2020. [Google Scholar]
- 54.Scientia Consultoria Científica. Arqueologia Preventiva nas Áreas de Intervenção da Usina Hidrelétrica de Belo Monte, Rio Xingu, PA. Relatório Parcial 13. São Paulo: Scientia Consultoria Científica; 2018. [Google Scholar]
- 55.Scientia Consultoria Científica. Arqueologia Preventiva nas Áreas de Intervenção da Usina Hidrelétrica de Belo Monte, Rio Xingu, PA. Relatório Parcial 14. São Paulo: Scientia Consultoria Científica; 2019. [Google Scholar]
- 56.Cabral MP. Juntando cacos: Uma reflexão sobre a classificação da Fase Koriabo no Amapá. Revista Amazônica. 2011; 3: p. 88–106. [Google Scholar]
- 57.Van Den Bel M. A Koriabo site on the Lower Maroni River: results of the preventive archaeological excavation at Crique Sparouine, French Guiana. In Pereira ES, Guapindaia V. Arqueologia Amazônica. Belém: MPEG/IPHAN; 2010. p. 61–93. [Google Scholar]
- 58.Castro AM. Um regime de opulência: grupos ceramistas na Volta Grande do Rio Xingu Belém: Universidade Federal do Pará; 2020. [Google Scholar]
- 59.Barreto C, Nascimento HF, Pereira E. Lugares persistentes e identidades distribuídas no Baixo Amazonas: complexos cerâmicos pré-coloniais de Monte Alegre- Pará. Revista de Arqueologia. 2016; 29(1): p. 55–85. [Google Scholar]
- 60.Rostain S, Versteeg A. The Arauquinoid tradition in the Guianas. In Delpech A, Hofman C. Late Ceramic Age Societies in the Eastern Caribbean. London: Archaeopress; 2004. [Google Scholar]
- 61.Almeida FO, Neves EG. Evidencias arqueológicas para a origem dos Tupi-guarani no leste da Amazônia. Mana. 2015; 21(3): p. 499–525. [Google Scholar]
- 62.Almeida FO. Arqueologia dos Tupi-Guarani no Baixo Amazonas. In Barreto C, Lima HP, Betancourt CJ. Cerâmicas Arqueológicas da Amazônia: Rumo a uma nova síntese. Belém: Iphan: Ministério da Cultura; 2016. p. 171–182. [Google Scholar]
- 63.Domingues-Lopes RC. Artefatos Xikrin: documentos e testemunhos de um grupo indígena. Anais da VIII Reunião Regional de Antropólogos do Norte e Nordeste–ABANNE. 2003; 1: p. 1–24. [Google Scholar]
- 64.Horton D. The Mundurucu. In Steward JH, editor. Handbook of South American Indians, Vol.3. Washington DC; 1948. [Google Scholar]
- 65.Neme M. Dados para a história dos índios caiapó. Anais do Museu Paulista. 1969; XXIII: p. 101–147. [Google Scholar]
- 66.Turner T. Os Mebengokre Kayapó: história e mudança social. In Cunha MCd, editor. História dos Índios no Brasil. São Paulo: Companhia das Letras; 1992. [Google Scholar]
- 67.Ieipari Teixeira-Pinto M. Sacrifício e vida social entre os índios Arara. São Paulo: Hucitec; 1997. [Google Scholar]
- 68.Ramalho JP. Pesquisas antropológicas nos índios do Brasil. A cefalometria nos Kayapó e Karajá. Rio de Janeiro: s.n.; 1971. [Google Scholar]
- 69.Brugger SO, Gobet E, Leeuwen JFNv, Ledru MP, Colombaroli D, Knaap WOvd, et al. Long-term man–environment interactions in the Bolivian Amazon: 8000 years of vegetation dynamics. Quaternary Science Reviews. 2016;(132): p. 114–128. [Google Scholar]
- 70.Vogel JC, Merwe NJvd. Isotopic evidence for early maize cultivation in New York State. American Antiquity, 42. 1977: p. 238–42. [Google Scholar]
- 71.Smith BN, Epstein S. Two categories of 13C/12C ratios for higher plants. Plant Physiology, 47. 1971: p. 380–384. doi: 10.1104/pp.47.3.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology, 40. 1989: p. 503–537. [Google Scholar]
- 73.Ambrose SH, Norr L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In Lambert JB, Grupe G. Prehistoric Human Bone. Archaeology at the Molecular Level. Berlin: Springer-Verlag; 1993. p. 1–37. [Google Scholar]
- 74.van der Merwe NJ, Medina E. The canopy effect, carbon isotope ratios and foodwebs in amazonia. Journal of Archaeological Science. 1991; 18(3): p. 249–259. [Google Scholar]
- 75.DeNiro M, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta. 1981; 45(3): p. 341–351. [Google Scholar]
- 76.Sealy JC, van der Merwe NJ, A. LeeThorp J, L. Lanham J. Nitrogen isotopic ecology in southern Africa: implications for environmental and dietary tracing. Geochimica et Cosmochimica Acta. 1987; 51(10): p. 2707–2717. [Google Scholar]
- 77.Schoeninger MJ, DeNiro MJ. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochimica et Cosmochimica Acta. 1984; 48(4): p. 625–639. [Google Scholar]
- 78.Dufour E, Bocherens H, Mariotti A. Palaeodietary Implications of Isotopic Variability in Eurasian Lacustrine Fish. Journal of Archaeological Science. 1999; 26: p. 617–627. [Google Scholar]
- 79.Tejada JV, Flynna JJ, Antoine PO, Pacheco V, Salas-Gismondi R, Cerling TE. Comparative isotope ecology of western Amazonian rainforest mammals. PNAS. 2020. October 20: p. 26263–26272. doi: 10.1073/pnas.2007440117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Passey BH, Robinson TF, Ayliffe LK, Cerling TE, Sponheimer M, Dearing MD, et al. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. Journal of Archaeological Science. 2005; 32(10): p. 1459–1470. [Google Scholar]
- 81.Reid DJ, Dean MC. Variation in modern human enamel formation times. Journal of Human Evolution. 2006: p. 329–346. doi: 10.1016/j.jhevol.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 82.Müller LM. Relatório de escavação da estrutura do sítio Bela Vista (PA-AL-26). Belém: MPEG; 2018. [Google Scholar]
- 83.Ribeiro ATB, Lima HP, Marques FLT, Schmidt MJ, McDaniel KS. Results from pilot archaeological fieldwork at the Carrazedo site, lower Xingu river, Amazonia. Latin American Antiquity. 2016: p. 318–339. [Google Scholar]
- 84.Scientia Consultoria Científica. Arqueologia Preventiva nas Áreas de Intervenção da Usina Hidrelétrica de Belo Monte, Rio Xingu, PA. Relatório Parcial 7. São Paulo: Scientia Consultoria Científica; 2014. a. [Google Scholar]
- 85.Kipnis R, Caldarelli SB. Caçadores-coletores do Holoceno Inicial no Médio Xingu. Especiaria- Caderno de Ciências Humanas. 2018. [Google Scholar]
- 86.Hedges REM, Stevens RE, Pearson JA. Carbon and nitrogen stable isotope compositions of animal and human bone. In Benson D, Whittle A, editors. Building Memories: the Neolithic Cotswold Long Barrow at Ascott-under-Wychwood. Oxfordshire: Oxford; 2007. p. 239–246. [Google Scholar]
- 87.Bentley RA. Strontium isotopes from the Earth to the archaeological skeleton: A Review. Journal of Archaeological Method and Theory. 2006; 13(3): p. 135–187. [Google Scholar]
- 88.Wright LE, Schwarcz HP. Stable carbon and oxygen isotopes in human tooth enamel: Identifying breastfeeding and weaning in prehistory. J Phys Anthropol. 1998: p. 1–18. doi: [DOI] [PubMed] [Google Scholar]
- 89.Richards MP, Hedges REM. Stable Isotope Evidence for Similarities in the Types of MarineFoods Used by Late Mesolithic Humans at Sites Along theAtlantic Coast of Europe. Journal of Archaeological Science 26. 1999: p. 717–722. [Google Scholar]
- 90.Pestle WJ, Crowley BE, Weirauch MT. Quantifying Inter-Laboratory Variability in Stable Isotope Analysis of Ancient Skeletal Remains. Plos One. 2014; 9(7). doi: 10.1371/journal.pone.0102844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szpak P, Metcalfe JZ, Macdonald RA. Best practices for calibrating and reporting stable isotope measurements in archaeology. Journal of Archaeological Science: Reports. 2017;(13): p. 609–616. [Google Scholar]
- 92.Roberts P, Fernandes R, Craig OE, Larsen T, Lucquin A, Swift J, et al. Calling all archaeologists: guidelines for terminology, methodology, data handling, and reporting when undertaking and reviewing stable isotope applications in archaeology. Rapid Commun Mass Spectrom. 2018; 32: p. 361–372. doi: 10.1002/rcm.8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sponheimer M, Lee-Thorp JA. Oxygen Isotopes in Enamel Carbonate and their Ecological Significance. Journal of Archaeological Science. 1999; 26: p. 723–728. [Google Scholar]
- 94.Bronk Ramsey C. Bayesian analysis of radiocarbon dates. Radiocarbon. 2009;(51): p. 337–360. [Google Scholar]
- 95.Reimer PJ, Bard E, Bayliss A, Beck JW, Blackwell PG, Ramsey CB, et al. INTCAL13 and MARINE13 Radiocarbon age calibration curves 0–50,000 years CAL BP. Radiocarbon. 2013: p. 1869–1887. [Google Scholar]
- 96.Tappen M. Bone Weathering in the Tropical Rain Forest. Journal of Archaeologycal Science. 1994: p. 667–673.8171316 [Google Scholar]
- 97.DeNiro MJ. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to paleodietary reconstruction. Nature 317. 1985: p. 806–809. [Google Scholar]
- 98.Ambrose SH. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeological Science (4). 1990: p. 431–451. [Google Scholar]
- 99.Ja Lee-Thorp, Sealy JC Merwe NJvd. Stable Carbon Isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. Journal of Archaeological Science. 1989; 16: p. 585–599. [Google Scholar]
- 100.Lee-Thorp JA. On isotopes and old bones. Archaeometry. 2008; 50(6): p. 925–950. [Google Scholar]
- 101.Robinson JG, Redford KH. Body size, diet, and population density of neotropical forest mamals. The American Naturalist. 1986: p. 665–680. [Google Scholar]
- 102.Medina E, Minchin P. Stratification of δ13C values of leaves in Amazonian rain forests. Oecologia (Berl.). 1980: p. 377–378. doi: 10.1007/BF00540209 [DOI] [PubMed] [Google Scholar]
- 103.Cerling TE, Hart JA, Hart TB. Stable isotope ecology in the Ituri Forest. Oecologia. 2004: p. 5–12. doi: 10.1007/s00442-003-1375-4 [DOI] [PubMed] [Google Scholar]
- 104.Herrera EA, Salas V, Congdon ER, Corriale MJ, Tang-Martinez Z. Capybara social structure and dispersal patterns: variations on a theme. Journal of Mammalogy. 2011: p. 12–20. [Google Scholar]
- 105.Corriale MJ, Herrera EA. Patterns of habitat use and selection by the capybara (Hydrochoerus hydrochaeris): a landscape-scale analysis. Ecological Research. 2014: p. 191–201. [Google Scholar]
- 106.Jones KR, Lall KR, Garcia GW. Omnivorous Behaviour of the Agouti (Dasyprocta leporina): A Neotropical Rodent with the Potential for Domestication. Hindawi Scientifica. 2019; 2019: p. 1–5. doi: 10.1155/2019/3759783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hedges REM, Reynard LM. Nitrogen isotopes and the trophic level of humans in archaeology. Journal of Archaeological Science. 2007; 34: p. 1240–1251. [Google Scholar]
- 108.Bleasdale M, Wotzka HP, Eichhorn B, Mercader J, Zech J, Soto M, et al. Isotopic and microbotanical insights into Iron Age agricultural reliance in the Central African rainforest. Communications Biology. 2020. doi: 10.1038/s42003-020-01324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benedito-Cecilio E, Araujo-Lima CARM, Forsberg BR, Bittencourt MM, Martinelli LC. Carbon sources of Amazonian fisheries. Fish. Manag. Ecol. 2000: p. 305–315. [Google Scholar]
- 110.Mortillaro JM, Pouilly M, Wach M, Freitas CEC, Abril G, Meziane T. Trophic opportunism of central Amazon floodplain fish. Freshwater Biology. 2015. May 7: p. 1659–1670. [Google Scholar]
- 111.Heriarte M. Descripção do Estado do Maranhão, Pará, Corupá e Rio das Amazonas Vienna D’Austria: Imprensa do filho de Carlos Gerold; 1874. [Google Scholar]
- 112.Carvajal G, Rojas A, Acuña C. Descobrimento do Rio das Amazonas. São Paulo ed. São Paulo, Rio de Janeiro, Recife, Porto Alegre: Cia. Editora Nacional; 1941. [Google Scholar]
- 113.Troufflard J, Alves DT. Uma abordagem interdisciplinar do sítio arqueológico Cedro, baixo Amazonas. Bol. Mus. Para. Emílio Goeldi. Cienc. Hum. 2019: p. 553–580. [Google Scholar]
- 114.Capriles JM, Lombardo H, Maley B, Zuna C, Veit H, Kennett DJ. Persistent Early to Middle Holocene tropical foraging in southwestern Amazonia. Science Advances. 2019: p. 1–10. doi: 10.1126/sciadv.aav5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Souza JGd, Mateos JA, Madella M. Archaeological expansions in tropical South America during the late Holocene: Assessing the role of demic diffusion. PLoS ONE. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whitney BS, Dickau R, Mayle FE, Walker JH, Soto D, Iriarte J. Pre-Columbian raised-field agriculture and land use in the Bolivian Amazon. The Holocene. 2014: p. 231–241. [Google Scholar]
- 117.Moraes CP, Neves EG. O Ano 1000: Adensamento populacional, interação e conflito na Amazônia Central. Amazônica. 2012: p. 122–148. [Google Scholar]
- 118.Piperno DR, McMichael CH, Pitman NCA, Andino JEG, Paredes MR, Heijink BM, et al. A 5,000-year vegetation and fire history for tierra firme forests in the Medio Putumayo-Algodón watersheds, northeastern Peru. PNAS. 2021. doi: 10.1073/pnas.2022213118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.