Abstract
The transcription factor GLI3 is a member of the GLI family and has been shown to be regulated by canonical hedgehog (HH) signaling through smoothened (SMO). Little is known about SMO-independent regulation of GLI3. Here, we identify TLR signaling as a novel pathway regulating GLI3 expression. We show that GLI3 expression is induced by LPS/TLR4 in human monocyte cell lines and peripheral blood CD14+ cells. Further analysis identified TRIF, but not MyD88, signaling as the adapter used by TLR4 to regulate GLI3. Using pharmacological and genetic tools, we identified IRF3 as the transcription factor regulating GLI3 downstream of TRIF. Furthermore, using additional TLR ligands that signal through TRIF such as the TLR4 ligand, MPLA and the TLR3 ligand, Poly(I:C), we confirm the role of TRIF-IRF3 in the regulation of GLI3. We found that IRF3 directly binds to the GLI3 promoter region and this binding was increased upon stimulation of TRIF-IRF3 with Poly(I:C). Furthermore, using Irf3−/− MEFs, we found that Poly(I:C) stimulation no longer induced GLI3 expression. Finally, using macrophages from mice lacking Gli3 expression in myeloid cells (M-Gli3−/−), we found that in the absence of Gli3, LPS stimulated macrophages secrete less CCL2 and TNF-α compared with macrophages from wild-type (WT) mice. Taken together, these results identify a novel TLR-TRIF-IRF3 pathway that regulates the expression of GLI3 that regulates inflammatory cytokines and expands our understanding of the non-canonical signaling pathways involved in the regulation of GLI transcription factors.
Keywords: GLI3, inflammation, TLR
INTRODUCTION
Hedgehog (HH) signaling is well known for its role in embryonic development, cancer and inflammation [1–4]. At the center of HH signaling are the 2 receptors patched (PTCH1) and smoothened (SMO) along with GLI transcription factors [5]. In the absence of HH ligand, PTCH1 inhibits SMO. Upon ligand binding to PTCH1, the inhibition of SMO is removed and SMO transduces signal ultimately leading to the activation of GLI proteins [5]. Multiple studies have demonstrated that SMO-independent (non-canonical) pathways can regulate GLI proteins [6–10], leading to inflammation and cancer cell growth. Therefore, there is a need to understand the molecular pathways that regulate GLI proteins independent of SMO signaling.
There are 3 members of the GLI family of transcription factors (TFs): GLI1, GLI2 and GLI3. While GLI1 and GLI2 act primarily as transcriptional activators of SMO-dependent HH signaling, GLI3 is known for its transcriptional inhibitory effect where it negatively regulates HH signaling [11–13]. Despite the identification of several SMO-independent pathways that regulate GLI1 and GLI2 [9, 14], little is known about SMO-independent pathways that regulate GLI3. GLI3 is known to play a role in development-associated diseases such as Greig cephalopolysyndactyly (GCPS) and Pallister-Hall Syndrome (PHS) [15–18]. More recent studies suggest a role for GLI3 in the immune system [19].
Immune cells, such as monocytes, recognize pathogens through their pattern recognition receptors (PRR). Toll-like receptors (TLR) are PRR known to be strong inducers of inflammation and immune clearance [20, 21]. Of the TLRs, TLR4 is known to bind Lipopolysaccharide (LPS), which is expressed on the outer membrane of gram-negative bacteria. Upon LPS binding to TLR4, downstream signaling occurs through either MyD88 or TRIF adapter molecules [22]. Signaling through MyD88 activates NF-κB and MAPK, while signaling through TRIF activates IRF3 in addition to MAPK and NF-κB [23–25]. All other TLRs signal through MyD88 except TLR3, which signals through TRIF [26]. Using either adapter molecule, activation of these transcription factors leads to their nuclear translocation where they regulate the expression of inflammatory genes.
Here, we identify GLI3 as a downstream target of TLR signaling in monocytes. Stimulation of TLR4 with LPS induces an increase in GLI3 mRNA and protein expression independent of signaling through SMO. Further analysis identified TRIF as the adapter molecule used by TLR4 to modulate GLI3 expression. Using pharmacological inhibitors to target signaling molecules downstream of TRIF, we identify IRF3 as the transcription factor mediating TLR-TRIF-induced GLI3. This was further validated using the TLR4 ligand monophosphoryl lipid A (MPLA) and the TLR3 ligand polyinosinic:polycytidylic acid (Poly(I:C)), which activate TRIF signaling [27]. Both MPLA and Poly(I:C) increased GLI3 expression and overexpression of IRF3 increased GLI3 expression. We found that IRF3 directly binds the GLI3 promoter region upstream of exon 2 and this binding was increased upon activation of TRIF-IRF3 signaling. Using mouse embryonic fibroblasts (MEFs) lacking IRF3 expression (Irf3−/− MEFs), we show that activation of TRIF-IRF3 signaling was no longer able to increase GLI3. Finally, using macrophages from mice lacking Gli3 expression in myeloid cells (M-Gli3−/−), we found that LPS stimulation results in significantly reduced CCL2 and TNF-α levels compared with macrophages from WT mice. Taken together, this study identifies TLR-TRIF-IRF3 signaling as a novel pathway that regulates GLI3 expression and highlights a novel role for Gli3 in regulating inflammatory cytokines in response to LPS-TLR4 signaling.
RESULTS
LPS stimulation induces GLI3 expression
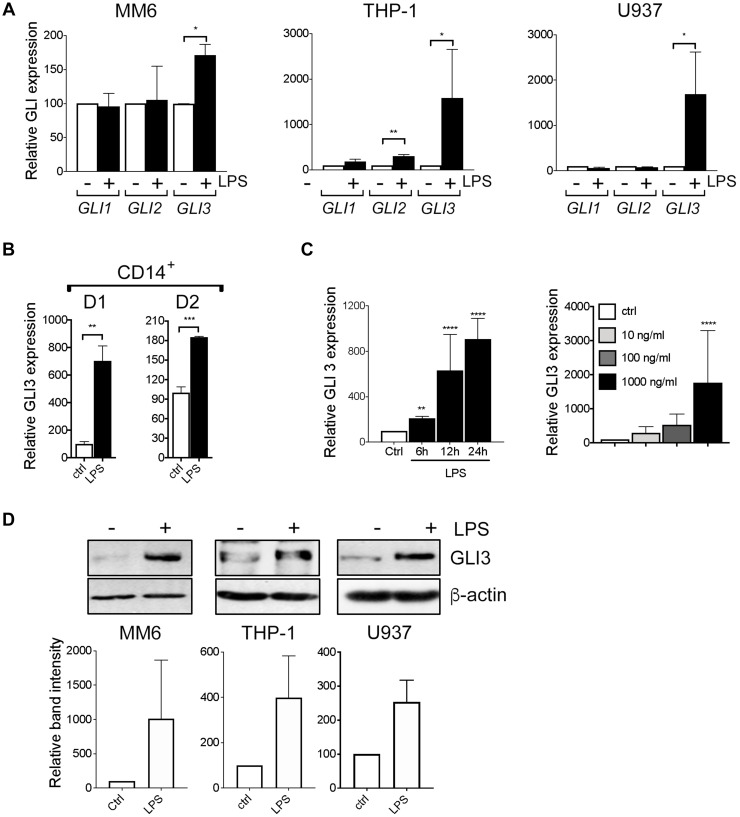
We stimulated human monocyte cell lines MM6, THP-1 and U937 with LPS and investigated the effects on GLI expression. To confirm that these monocyte cell lines are responsive to LPS stimulation, we examined the expression of LPS-induced cytokines (TNF-α, IL-1β and IFN-β) and found them to be induced in response to LPS (Supplementary Figure 1A). We found that LPS stimulation induces an increase in GLI3 mRNA expression in all 3 cells lines (Figure 1A). GLI1 expression was not altered in cell lines, while GLI2 expression was only induced in THP-1 cells. In addition, differentiation of MM6, THP-1 and U937 cells into macrophages using PMA stimulation also induced an increase in GLI3 expression (Supplementary Figure 1B), suggesting GLI3 is important for macrophage biological functions. Furthermore, we also confirm that GLI3 protein is mostly localized in the nuclear fraction (Supplementary Figure 2). Using CD14+ cells purified from peripheral blood mononuclear cells (PBMCs) of healthy donors, we confirm that LPS stimulation induces GLI3 expression in primary human monocytes in addition to monocyte cell lines (Figure 1B). The increase in GLI3 expression upon LPS stimulation occurred in a time and dose-dependent manner (Figure 1C). This increase in GLI3 mRNA expression also resulted in an increase in GLI3 protein expression in response to stimulation with LPS (Figure 1D). Taken together, these results suggest that the TLR4 signaling pathway can regulate GLI3 expression independent of HH signaling.
Figure 1. LPS induces GLI3 expression in a HH independent manner.
(A) Human monocyte cell lines (MM6, THP-1, U937) (2 × 106 cells/ml) were treated with/without 100 ng/ml LPS (0111; B4 E.coli) for 1 h followed by determination of GLI expression by qPCR. (B) CD14+ cells from PBMCs (2 × 106 cells/ml) were stimulated with 100 ng/ml LPS for 1 h followed by qPCR to determine GLI3 expression. (C) MM6 cells (2 × 106 cells/ml) were stimulated with 100 ng/ml LPS for the indicated times, or with the indicated doses of LPS for 1 h followed by determination of GLI3 expression by qPCR. (D) Human monocytes (5 × 106 cells/ml) were stimulated with LPS for 12 h followed by determination of GLI3 protein expression by immunoblotting. All experiments were repeated at least three times. Data are presented as average of at least three independent experiments ± SEM.
LPS/TLR4-induced GLI3 occurs through TRIF signaling
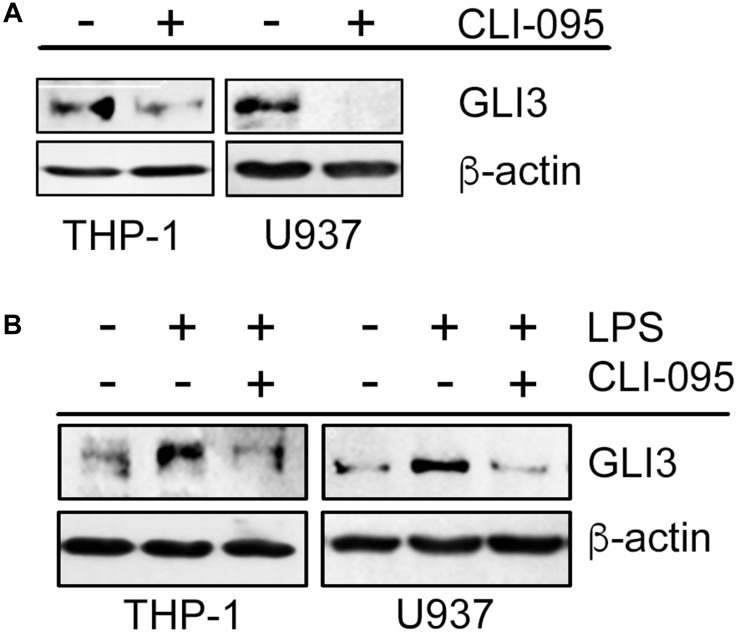
To elucidate the signaling mechanism downstream of TLR4 that results in increased GLI3 expression, we used the TLR4 signaling inhibitor (CLI-095), which inhibits TLR4 signaling by targeting the intracellular domain of TLR4. We found that monocyte cell lines treated with the TLR4 signaling inhibitor had reduced basal levels of GLI3 expression (Figure 2A). Furthermore, in the presence of the TLR4 signaling inhibitor, LPS was not able to induce GLI3 expression (Figure 2B) suggesting that an active TLR4 signaling pathway was required for LPS-induced GLI3.
Figure 2. Functional TLR4 is required for GLI3 expression.
(A) Monocytes (5 × 106 cells) were treated with 1 μg/ml TLR4 signaling inhibitor (CLI095) for 12 h followed by determination of GLI3 expression by western blot. (B) Monocytes (5 × 106 cells) were pretreated with CLI095 for 12 h followed by stimulation with 100 ng/ml LPS for an additional 12 h. GLI3 expression was determined by western blot. These experiments were repeated 3 times.
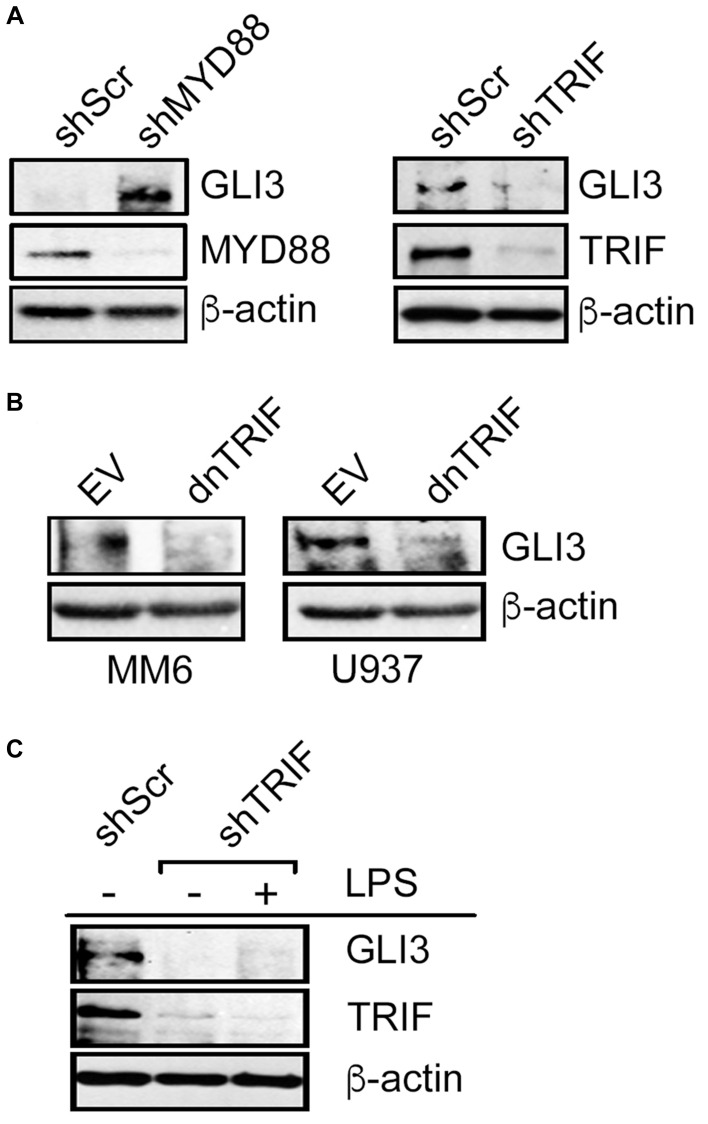
LPS-TLR4 signaling can utilize one of 2 adapter proteins, MyD88 or TRIF [28]. To determine the signaling mechanism downstream of TLR4 that regulates GLI3, we used RNAi targeting either MyD88 or TRIF to determine the effect on GLI3 expression. When monocytes were transfected with RNAi targeting MYD88, there was an induction in GLI3 expression (Figure 3A). In contrast, when cells were transfected with RNAi targeting TRIF, there was a reduction in GLI3 expression (Figure 3A) suggesting that signaling through TRIF (but not MYD88) is responsible for TLR4-induced GLI3 expression. To validate this, we utilized a dominant negative form of TRIF (dnTRIF) that lacks N- and C-terminus and harbors a proline to histidine mutation at amino acid 434 which is crucial for TLR-dependent signaling. We found that in cells expressing dnTRIF, there was a reduction in GLI3 protein expression (Figure 3B). Additionally, LPS stimulation was no longer able to induce GLI3 protein expression in cells transfected with shTRIF (Figure 3C). Taken together, these results suggest that LPS-TLR4-induced GLI3 occurs via signaling through the TRIF adaptor protein.
Figure 3. LPS-induced GLI3 is regulated by TRIF downstream of TLR4.
(A) U937 cells (10 × 106 cells) were transfected with 10 μg of either shMYD88, shTRIF or scrambled controls (shScr). After 2 days, cells were lysed and lysates were used to determine protein expression by western blot. (B) Monocytes (10 × 106 cells) were transfected with a dominant negative form of TRIF (dnTRIF) or empty vector (Ctrl) for 2 days followed by determination of protein expression by western blot. (C) MM6 cells (10 × 106 cells) were transfected with shTRIF or shScr and cultured for 30 h, followed by treatment with 100 ng/ml LPS. After an additional 12 h, cells were lysed and lysates were used for western blot to determine protein expression. These experiments were repeated at least 3 times with similar results.
TLR4-TRIF regulates GLI3 through the transcription factor IRF3
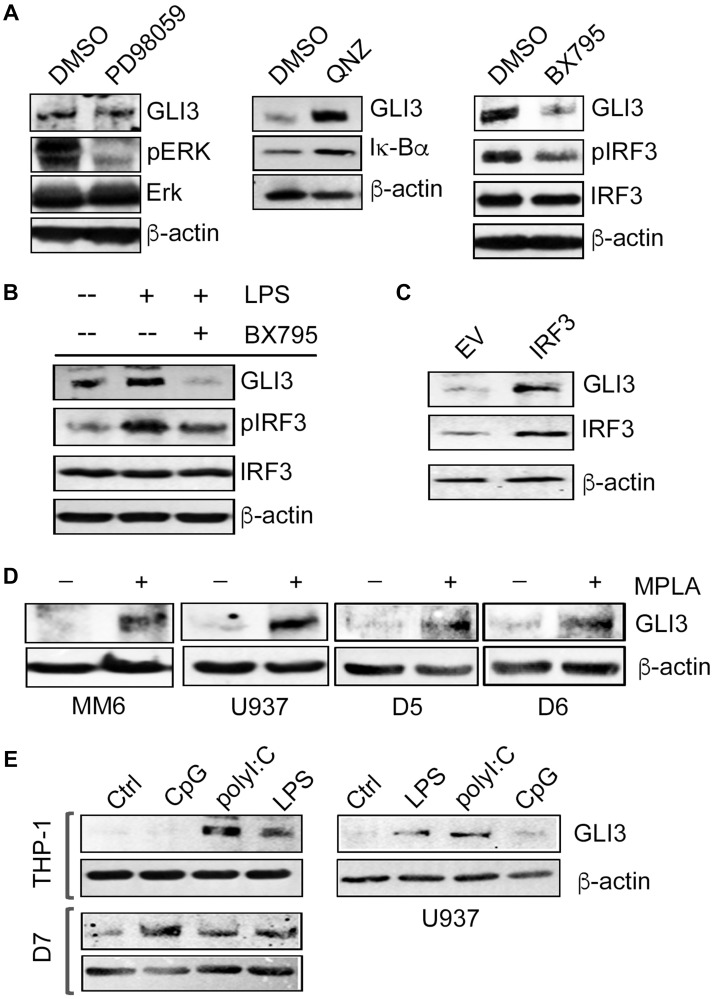
Following activation of TRIF, three different downstream signaling pathways can be initiated: NF-κB pathway, MAPK pathway and IRF3 pathway [28]. We used pharmacological inhibitors to target each of these pathways to identify the contribution of each in TLR4-TRIF-mediated regulation of GLI3. Pretreatment of monocytes with the inhibitor PD98059 (an inhibitor of MAPKK/Erk1/2) did not alter GLI3 expression. However, consistent with MYD88 knockdown experiments inducing increased GLI3 (Figure 3A), treatment with the inhibitor QNZ (an inhibitor of NF-κB activity) also increased GLI3 expression (Figure 4A). In contrast, western blot analysis showed that only inhibition of IRF3 signaling using the inhibitor BX795 (an inhibitor of the catalytic activity of TBK1) reduced basal levels of GLI3 (Figure 4A). Pretreatment of monocytes with BX795 also resulted in a loss of LPS-induced GLI3 (Figure 4B). To further validate the role of IRF3 as a regulator of GLI3, we overexpressed IRF3 in U-937 cells and found an increase in GLI3 expression (Figure 4C). Furthermore, we stimulated monocytes with monophosphoryl lipid A (MPLA), a TLR4 ligand that signals exclusively through TRIF and found an induction in GLI3 expression (Figure 4D). Finally, we compared GLI3 expression in response to LPS (TLR4 agonist which signals though MyD88 and TRIF), CpG (TLR9 agonist which signals through MyD88) and Poly(I:C) (TLR3 agonist which signals through TRIF). We found a robust increase in GLI3 expression in response to Poly(I:C) in monocyte cell lines and CD14+ cells from PBMCs, further supporting a role for TLR-TRIF-IRF3 signaling in the regulation of GLI3 (Figure 4E). Taken together, these data suggest that GLI3 is a novel target of TRIF-IRF3 signaling and can be regulated by TLR3 and TLR4.
Figure 4. GLI3 is regulated by IRF3 downstream of TRIF.
(A) THP-1 cells (5 × 106 cells) were treated with 50μM MEK/Erk inhibitor (PD98059) or DMSO control for 30 min, 500 nM NF-κB inhibitor (QNZ) or DMSO for 2 h, or 1 μM TBK1 inhibitor (BX795) or DMSO for 1 h. Cells were harvested, lysed and lysates were used to determine protein expression by western blot. (B) THP-1 cells (5 × 106 cells) were pretreated with 1 μM BX795 for 1 h, followed by stimulation with 100 ng/ml LPS for 12 h. Cells were lysed and lysates were used to determine protein expression by western blot. (C) U937 cells (5 × 106 cells) were transfected with an IRF3 expression construct or empty vector (EV) for 2 days followed by lysis and western blot. (D) Monocytes (5 × 106 cells) were treated with 1 μg/ml TLR4 agonist MPLA for 12 h followed by western blot to determine GLI3 expression. (E) Monocyte cell lines and primary donor CD14+ cells (D7) (5 × 106 cells) were treated with 10 μg/ml CpG, 10 μg/ml Poly(I:C), 100 ng/ml LPS or left untreated (Ctrl) for 12 h followed by western blot to determine GLI3 expression. Data shown are representative of at least three independent experimental replicates.
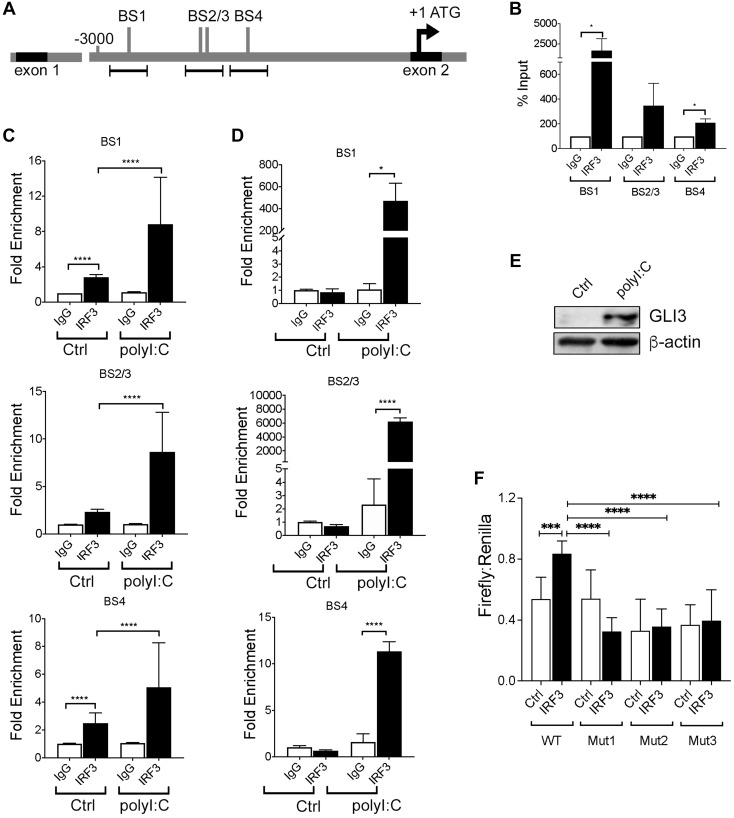
IRF3 directly binds the GLI3 promoter
Although several studies have characterized the promoter region of GLI1 and GLI2 [29, 30], less is known about the GLI3 promoter or it’s regulation. The GLI3 gene is composed of 15 exons with translation beginning in exon 2 [19]. Therefore, we examined approximately 3000 bp upstream of ATG (intron 1–2) of the GLI3 gene for candidate IRF3 binding sequences and identified 4 consensus sequences (AANNGAAA) that IRF3 may bind to regulate GLI3 expression (Figure 5A). Using MM6 cells (which have the highest copy number of GLI3 [data not shown]), we performed chromatin immunoprecipitation (ChIP) assay to determine IRF3 binding to the GLI3 promoter region and found IRF3 binding to all candidate sites (Figure 5B). Activation of TRIF signaling in MM6 cells using Poly(I:C) resulted in a significant increase in IRF3 binding to all candidate IRF3 binding sites (Figure 5C). Furthermore, using CD14+ cells purified from PBMCs that were treated with Poly(I:C), there was a significant increase in IRF3 binding to the GLI3 promoter in response to Poly(I:C) stimulation (Figure 5D); and stimulation of CD14+ cells from the same donor with Poly(I:C) increases GLI3 expression (Figure 5E). To confirm the specificity of IRF3 binding, we cloned a 1641 bp region upstream of translation start site of GLI3 into a luciferase plasmid and used it to examine the effect of IRF3 on luciferase reporter activity. Using this luciferase construct containing the GLI3 promoter region, we show that overexpression of IRF3 significantly increases luciferase activity (p = 0.0005), while deletion of IRF3 BS1-3 abolishes IRF3-induced GLI3 promoter activity (Figure 5F). Taken together, these results suggest that TLR-TRIF-mediated regulation of GLI3 occurs via direct binding of IRF3 to the GLI3 promoter and identifies IRF3 as a novel transcription factor that regulates GLI3 independent of HH signaling.
Figure 5. IRF3 regulates GLI3 by directly binding to IRF3 binding sites.
(A) Schematic diagram of ~ 3000 bp upstream of ATG located in exon 2 of GLI3. Several candidate IRF3 binding sites (BS) were identified. (B) Untreated MM6 cells (10 × 106) were lysed and a chromatin immunoprecipitation (ChIP) assay was performed followed by qPCR using three primer sets for the areas containing IRF3 binding sites upstream of ATG of GLI3. (C) MM6 cells (10 × 106) were treated with 10 μg/ml Poly(I:C) for 1h prior to ChIP and qPCR. (D) Primary human monocytes (D3) were treated with 10μg/ml Poly(I:C) for 1h prior to ChIP and qPCR. (E) Primary human monocytes (D3) were challenged with 10 μg/ml Poly(I:C) for 12h prior to western blot analysis. (F) U937 cells (4 × 106) were transfected with either IRF3 expression construct or empty vector along with pGL4.1_GLI3 promoter construct and pGL4.7 Renilla plasmid as described in the methods section. After 48 hr, cells were harvested and lysed and lysates were used to quantify luciferase activity. These experiments were performed at least 3 times with similar results.
IRF3 is required for TRIF-induced GLI3
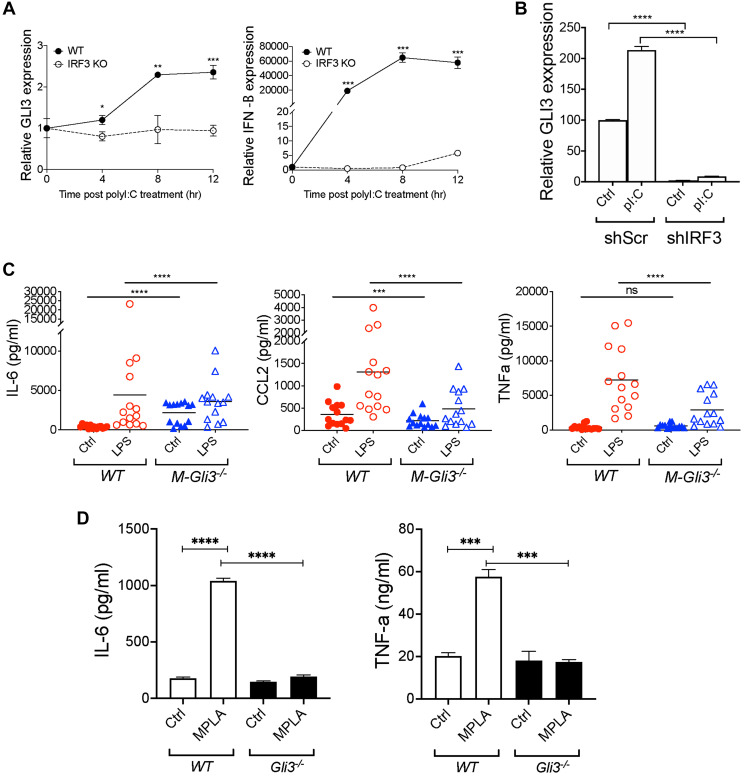
To confirm the role of IRF3 in TLR-TRIF-induced GLI3, we used mouse embryonic fibroblasts (MEFs) that lack IRF3 (Irf3−/−) or wild-type (WT) MEFs stimulated with Poly(I:C). Consistent with previous results in monocytes, in WT MEFs treated with Poly(I:C), there was a time-dependent increase in Gli3 expression (Figure 6A). This Poly(I:C)-induced Gli3 was lost in Irf3−/− MEFs. This finding was consistent with an increase in interferon beta (IFN-β) expression in the WT MEFs, but not in the Irf3−/− MEFs (Figure 6A). Additionally, we used RNAi to target IRF3 in human monocytes, and found that IRF3 knockdown reduces polyI:C-induced GLI3 expression (Figure 6B). Taken together, these results support the requirement for IRF3 in TLR-TRIF-induced GLI3.
Figure 6. Gli3 mediates TLR-induced inflammation.
(A) WT and Irf3−/− MEFs (1.5x105) were treated with transfected with 10 μg/mL Poly(I:C) for the indicated time followed by qPCR to determine GLI3 expression. (B) MM6 cells (4 × 106) were transfected with shScr or shIRF3. After 24 hr, cells were stimulated with 10 μg/mL poly(I:C) for an additional 24 hr. Cells were harvested and used for RNA isolation and qPCR to determine GLI3 expression. (C) IFA-elicited macrophages from M-Gli3−/− or WT mice (n = 14/group) were cultured in the presence or absence of 100 ng/ml LPS for 24 hours. Supernatants were used to quantify cytokine levels by ELISA. (D) WT and Gli3−/− MEFs (1 × 106 cells/ml) were plated in 0.5 ml volume in 12-well plates. Cells were treated with 1 μg/mL MPLA for 24 hr followed by ELISA to quantify cytokine secretion. MEF experiments were repeated twice with similar results.
Gli3 mediates TLR-TRIF-induced inflammation
We observed that GLI3 was induced in response to TLR stimulation, so we hypothesized that GLI3 regulates inflammatory gene expression in response to TLR signaling. Since Gli3−/− mice are embryonically lethal [31, 32], we generated mice with conditional Gli3 knockout in myeloid cells (M-Gli3−/−) by crossing Gli3fl/fl mice with LysM-cre mice. We verified the genotyping by PCR by confirming the expression of homozygous Gli3 floxed allele (Gli3fl/fl), expression of the cre allele (LysMcre+/−), and the resultant recombined Gli3 (Supplementary Figure 3A). We also confirmed GLI3 knockout in peritoneal macrophages by western blot (Supplementary Figure 3B). Additionally, Gli3 knockout in myeloid cells did not affect overall mice survival (Supplementary Figure 3C) or the expression of TLR4 (Supplementary Figure 3D) or downstream IRF3 or MYD88 (Supplementary Figure 3E). Using IFA-elicited peritoneal macrophages from M-Gli3−/− or WT mice, we treated cells with 100 ng/ml LPS or DPBS control for 24 h and harvested supernatants to quantify inflammatory cytokine levels by ELISA. IRF3 has been reported to regulate CCL2, IL-6 and TNF-α levels, strong regulators of inflammation [28]. As expected, LPS stimulation induced a significant increase in the secretion of CCL2, IL-6 and TNFα (Figure 6C). However, the induction of CCL2 and TNFα secretion was significantly reduced in macrophages from M-Gli3−/− mice compared with macrophages from WT mice (Figure 6C). This suggests that GLI3 is required for TLR-TRIF-induced cytokine secretion. Interestingly, only basal levels of CCL2 were significantly reduced in the absence of GLI3 suggesting GLI3 regulates both basal and TLR-TRIF-induced levels of CCL2. In contrast, basal TNFα levels were unaffected by GLI3 loss and IL-6 levels were significantly elevated in the absence of GLI3 suggesting GLI3 negatively regulates basal IL-6 levels and positively regulates TLR-TRIF induced IL-6 levels. To confirm that this difference in cytokine secretion is not due to a difference in macrophage numbers or IFA-induced macrophage maturation, we stained peritoneal macrophages harvested post-IFA administration with antibodies targeting CD11b and F4/40. We found similar numbers of CD11b+ F4/80+ macrophages from both strains of mice (Supplementary Figure 4). The role of TRIF-IRF3-GLI3 axis in regulating cytokines is further supported by data using Gli3−/− MEFs, where we found that the absence of GLI3 results in a significant reduction in MPLA-induced IL-6 and TNF-α secretion (Figure 6D). Because MyD88 knockdown resulted in an increase in GLI3 expression (Figure 3A), we examined the effect of CpG as a TLR9 ligand that signals through MYD88 on cytokine secretion. We found no difference in IL-6 or TNFα secretion in macrophages from M-Gli3−/− or WT mice stimulated with CpG (Supplementary Figure 5). Taken together, these results show that the TLR-TRIF-GLI3 axis modulates inflammation in macrophages and identifies a novel role for the transcription factor GLI3 in macrophages biological functions.
DISCUSSION
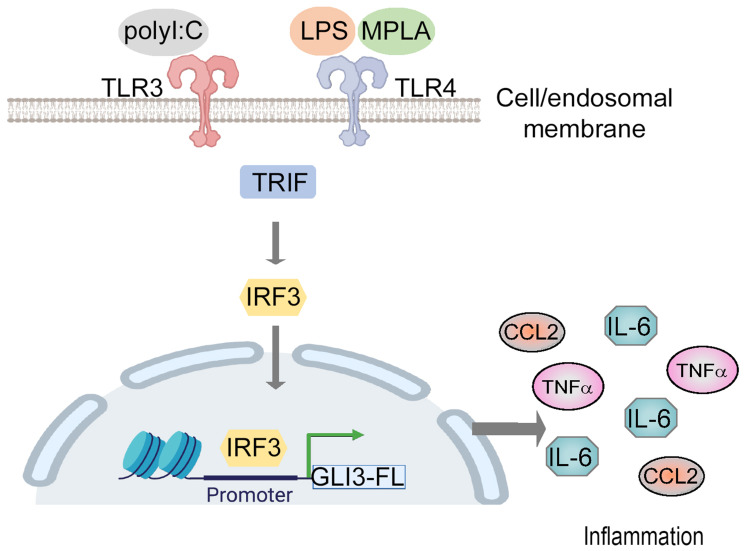
The regulation of GLI transcription factors by canonical HH signaling is well established [33]. Several studies have shown that the GLI2 family member is regulated in a HH-independent manner [9, 10, 34]. Additional research shows GLI3’s role in the p53 and AKT pathway [35, 36] suggesting GLI3’s potential to regulate pathways outside of HH signaling as well. Here, we show that GLI3 is a downstream target of TLR signaling where the TRIF-IRF3 axis directly regulates GLI3 expression (Figure 7). TLRs are important immune receptors which are crucial for the innate immune response to pathogens [28, 37]. TLRs are expressed on a variety of immune and non-immune cells including monocytes. We found a significant induction in GLI3 expression upon challenge of monocytes with LPS (Figure 1). This was found to be due to activation of TRIF and subsequently IRF3 (Figures 3–5). The role of TRIF in regulating GLI3 was validated using the TLR3 ligand Poly(I:C) (which signals through TRIF) and the TLR4 ligand MPLA (which activates TRIF but not MyD88) [28]. Therefore, we have identified GLI3 as a novel target of TLR-TRIF-IRF3 signaling in monocytes.
Figure 7. Gli3 mediates TLR-induced inflammation.
Model of TLR-TRIF-IRF3-dependent regulation of GLI3.
Due to its attributes as transcription factor, we hypothesized that TLR-induced Gli3 regulates inflammatory cellular responses. To investigate the role of Gli3 in regulating inflammatory gene expression, we generated M-Gli3−/− mice, which lack Gli3 expression in myeloid cells such as macrophages. Using IFA-elicited peritoneal macrophages from M-Gli3−/− and WT littermates, we identified a reduction in LPS-induced CCL2 and TNFα secretion in the absence of Gli3. This suggests a major regulatory role of Gli3 in inflammation.
Several studies have suggested a role for GLI3 in the immune system, spanning both innate and adaptive immunity [38, 39]. GLI3 was found to regulate B- and T-cell development as well as the expression of CD155 on NK cells [38, 40, 41]. More interestingly, in a cDNA microarray analysis, Gli3 was one of the upregulated genes when bone marrow derived macrophages and RAW264 cells were challenged with LPS [42]. Therefore, our findings in human monocytes are consistent with studies in mouse macrophages showing an induction of Gli3 in response to LPS stimulation. We characterize the signaling pathway downstream of LPS/TLR4 that regulates GLI3 (Figures 3–5) and identify IRF3 signaling to regulate GLI3. Using macrophages from mice with conditional deletion of Gli3 in myeloid cells (M-Gli3−/−), we show that Gli3 modulates LPS-induced IL-6, CCL2 and TNF-α levels (Figure 6C). Due to the potency of these inflammatory cytokines, a role of Gli3 in inflammation can be extrapolated. Since Gli3 knockout resulted in a reduction in CCL2 secretion by macrophages, Gli3 may play a role in immune cell trafficking and therefore in the initiation of the inflammatory response. High levels of CCL2 have also been shown to correlate with high cancer occurrence in several cancer types [37, 43, 44], suggesting Gli3 may play a role in cancer in addition to pathogen-induced inflammation. Indeed, a role for GLI3 in cancer progression has been suggested [19]. Additionally, elevated serum CCL2 levels were associated with multiple sclerosis (MS), rheumatoid arthritis (RA) and atherosclerosis [43, 45, 46]. TNF-α is another inflammatory cytokine that was reported to regulate RA, psoriasis and inflammatory bowl disease [47–49]. Since Gli3 knockout reduced LPS-induced TNF-a secretion, Gli3 may modulate the pathology of these diseases. In addition to CCL2 and TNF-α, we show that Gli3 regulates IL-6 secretion (Figure 6C). IL-6 plays a role in the initiation and progression of inflammation. Therefore, it is not surprising that IL-6 plays a role in a multitude of inflammatory diseases. High levels of IL-6 were reported in Castleman’s and Crohn’s disease as well as in RA patients [50, 51], suggesting GLI3 may play a role in these diseases. Additionally, increased expression of TLR3 and TLR4 was reported in RA patients and TLR4 was upregulated in Crohn’s disease [52, 53], suggesting GLI3 may regulate the pathology of Crohn’s disease and RA through modulation of IL-6.
Upon stimulation of TLR4, signaling can occur through either MYD88 or TRIF adapter proteins [28, 54]. We identified TRIF as the adaptor protein mediating TLR4 signaling to regulate GLI3 expression in both our cell line models and in primary CD14+ cells from PBMCs. Additionally, our data suggests a negative regulation of GLI3 by MYD88, since knockdown of MYD88 using RNAi results in an increase in GLI3 protein expression (Figure 3A). Downstream of MYD88, NF-κB and MAPK signaling can be activated [26]. While targeting Erk1/2 with pharmacological inhibitor did not change GLI3 levels, inhibition of NF-κB resulted in an increase in GLI3 protein expression (Figure 4A). While this does not preclude a potential role for p38 or JNK1/2, this suggests a potential role for MYD88 as a negative regulator of GLI3 by activating NF-κB to decrease GLI3 levels. MYD88 is known to be a protein regulating inflammatory cytokine production while TRIF-IRF3 signaling is known to regulate the production of interferons in addition to proinflammatory cytokines [55]. Siednienko et al. (2011) suggest a negative regulatory effect of MYD88 on interferon production since the absence of MYD88 increased TLR3-dependent phosphorylation of IRF3 [56]. Therefore, it can be extrapolated that MYD88 negatively regulates GLI3 by compromising IRF3 phosphorylation. However, it remains unclear what role NF-κB plays in this inhibitory effect and future studies focused on analyzing the role of NF-κB in MYD88-dependent inhibition of GLI3 would increase our understanding of this potential negative feedback signaling.
Several transcription factors have been shown to regulate the promoter of GLI1 and/or GLI2 [34, 57, 58]. However, our understanding of the regulation of the GLI3 promoter is significantly lacking. The human GLI3 gene is composed of 15 exons with transcription start signal (ATG) located in exon 2. Here we examined approximately 3000 bp sequences upstream of exon 1 and exon 2 and identify candidate IRF3 consensus sequences upstream of exon 2 (which contains the ATG start codon) (Figure 5). In our cell lines, IRF3 binds to the GLI3 promoter region and this binding is enhanced upon TLR-TRIF stimulation (Figure 5A, 5B). However, in resting CD14+ cells from PBMCs, IRF3 does not bind the GLI3 promoter unless TLR-TRIF signaling is activated (Figure 5D). Therefore, these studies identify a novel TF (IRF3) that regulates GLI3 by binding to the GLI3 promoter region.
In summary, we have identified TLR signaling as a novel HH-independent signaling pathway that regulates GLI3 and demonstrate that this regulation is dependent on TRIF-IRF3 signaling axis. Additionally, to our knowledge, this is the first report of the regulatory components of the TLR-GLI3 axis where IRF3 is identified as a novel transcription factor that can modulate the promoter region of GLI3. These results increase our understanding of the signaling molecules that can regulate GLI3 in a HH-independent manner. Furthermore, we show that GLI3 is required for TLR-induced regulation of CCL2 and TNFα, therefore identifying a novel role for GLI3 in mediating TLR-induced inflammation.
MATERIALS AND METHODS
Cell lines and primary cells
The cell line Mono-Mac-6 (MM6) was purchased from DSMZ and cultured in RPMI 1640 supplemented with 10% FBS, 1% 2 mM L-glutamine, non-essential amino acids, 1mM Sodium Pyruvate, and 10 μg/ml human insulin. THP-1 and U937 cell lines were purchased from ATCC. THP-1 cells were cultured in RPMI 1640 with 10% FBS supplemented with 0.05 mM β-Mercaptoethanol and U937 cells were grown in RPMI 1640 with 10% FBS. An antibiotic-antimycotic was added to all cell culture media. Wild-type (WT) and Irf3−/− MEFs were a generous gift from Benjamin R. tenOever, and were generated as previously described [59]. Gli3−/− MEFs were a generous gift from Dr. Martin E. Fernandez-Zapico (Mayo Clinic, Rochester, MN, USA). MEFs were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Corning, NY, USA) supplemented with 10% v/v fetal bovine serum (FBS) (HyClone) and 1% v/v penicillin-streptomycin (Corning). Poly(I:C) HMW stimulations (10 μg/mL) were performed with Lipofectamine 2000 according to the manufacturer recommendations using a ratio of 1 μg:0.2 μL (Poly(I:C):Lipofectamine 2000).
Primary human monocytes were purified from peripheral blood mononuclear cells (PBMCs) obtained from buffy coats purchased from the blood bank of New York (NYC, NY, USA) or the Oklahoma Blood Institute (Oklahoma City, OK, USA). Experiments using cells from different donors are numbered donor 1 – donor 6 (D1–D6). Monocytes were isolated by negative selection using the monocyte enrichment kit from STEMCELL Technologies (Cambridge, MA, USA). Cell purity was confirmed by flow cytometry using CD14+ staining and was found to be >90%. Primary cells (CD14+) were plated and treated immediately after purification as described.
Reagents
Lipopolysaccharide (LPS) (E.coli, Serotype 0111;B4) and Poly(I:C) were purchased from MilliporeSigma (St. Louis, MO, USA). CpG was purchased from Integrated DNA Technologies (Coralville, IO, USA). The TLR4 signaling inhibitor CLI-095) and MPLA were purchased from Invivogen (San Diego, CA, USA). The MAPK inhibitor (PD98059), NF-κB inhibitor (QNZ), TBK1 inhibitor (BX-795) and SMO inhibitor (Cyclopamine) were purchased from SelleckChem (Houston, TX, USA).
RNA isolation and Quantitative RT-PCR (qPCR)
For total RNA isolation, TRIsure reagent (Bioline, obtained through Thomas Scientific, Swedensboro, NJ, USA) was used following the manufacturer’s recommendations as previously published [10, 60, 61]. cDNA synthesis was performed using Moloney Murine Leukemia Virus Reverse Transcriptase (MML-V)(Promega Corporation, Madison, WI, USA). ViiA-7 instrument (Life Technologies, Grand Island, NY, USA) was used to perform qPCR analysis. Amplification results from GLI1, GLI2 and GLI3 were normalized to human GAPDH. Amplification results from IFN-β were normalized to murine β-Actin. The following qPCR primers were used human h-GAPDH, 5′-CTCGACTTCAACAGCGACA-3′ (forward) and 5′-GTAGCCAAATTCGTTGTCATACC-3′ (reverse). H-GLI1, 5′-TCACGCCTCGAAAACCTGAA-3′ (forward) and 5′-AGCTTACATACATACGGCTTCTCAT-3′ (reverse); h-GLI2, 5′-GGAGAAGCCATATGTGTG TGAG-3′ (forward) and 5′-CAGATGTAGGGTTTCTCG TTGG-3′ (reverse); h-GLI3, 5′-GGGACCAAATGGATGG AGCA-3′ (forward) and 5′-TGGACTGTGTGCCATTT CCT-3′ (reverse); m-IFN-β, 5′-CAGCTCCAAGAAAGGA CGAAC-3′ (forward) and 5′- GGCAGTGTAACTCTTCT GCAT-3′ (reverse); m-β-Actin, 5′- CGGTTCCGATGCCC TGAGGCTCTT-3′ (forward) and 5′-CGTCACACTTCAT GATGGAATTGA-3′ (reverse).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed as previously published [10, 61]. Briefly, 10 × 106 cells were cross-linked using 0.37% formaldehyde for 10 min at room temperature then lysed and sonicated for 20 minutes (30s on/30s off) to shear DNA to ~ 500 bp (QSonica, Q800R3). Samples were immunoprecipitated using anti-IRF3 antibody or isotype control (Biolegend, San Diego, CA, USA) using magnetic Protein A/G beads (ThermoFisher Scientific) at 4°C overnight. Samples were washed and reverse crosslinked overnight in 5 M NaCL at 65°C. After RNase A and Proteinase K digestion, DNA was purified using GeneJET PCR Purification Kit (ThermoFisher Scientific) and used for qPCR analysis using the following primers: Binding site 1 (BS1), 5′-GGCTAAGAATGGAGTGTTTGGA-3′ (forward) and 5′-CACCACTGGTTTAGCTACATACAT-3′ (reverse); Binding site 2 and 3 (BS2/3), 5′-CTGGGCAACAGAGTGAGAC-3′ (forward) and 5′-CACCCGGAACCTCAATGTTA-3′ (reverse); Binding site 4 (BS4), 5′-CTGCCCTTGGGACTCAC-3′ (forward) and 5′-CAAAGGGCACTGACAAAGTATT-3′ (reverse). To determine the effect of TLR-TRIF signaling on IRF3-mediated regulation of GLI3, 10 × 106 cells were cultured at a density of 5 × 106 cells/ml and treated with 10 μg/ml Poly(I:C) for 1h or left alone followed by fixation and ChIP analysis as described earlier. One-ten (1–10%) of starting chromatin was used as input and the relative basal binding was determined as % input (signals obtained from the ChIP are divided by signals from input [prior to immunoprecipitation which represents the amount of chromatin used in the ChIP]). Fold enrichment was used to calculate signal over background. This represents ChIP signals as fold increase relative to the isotype antibody control and 2−ΔΔCt was used to calculate fold enrichment.
Plasmid constructs and cell transfections
Short hairpin RNA targeting TRIF and MYD88 (shTRIF, shMYD88) were purchased from OriGene Technologies (Rockville, MD, USA). The plasmid expressing dominant negative TRIF (dnTRIF) was purchased from Invivogen (San Diego, CA, USA). Human V5-IRF3-pcDNA3 was a gift from Saumen Sarkar (Addgene plasmidn # 32713; http://n2t.net/addgene:32713; RRID:Addgene_32713) [62]. Transfections were performed by electroporation using BTX ECM 630 (Holliston, MA, USA) using the following parameters: MM6: 240v/25 ms and U937: 300v/10 ms. Five million cells were electroporated with 10 μg shTRIF, shMYD88 or scrambled control (shScr) or 5 μg dnTRIF or empty vector in OPTI-MEM followed by culture for 48 h.
Immunoblotting
Immunoblotting was performed as previously described [9, 10, 61, 63, 64]. Cell lines and primary monocytes (5 × 106 cells/ml) were treated as indicated and harvested in 100 μl RIPA buffer (ThermoFisher Scientific). Total protein concentration was determined using a BCA assay (ThermoFisher Scientific). For GLI3, 5% self-prepared SDS gels were used while for all other proteins 10% gels were used. Transfer to nitrocellulose membranes was performed using a Trans-Blot Turbo Transfer system (Biorad, Hercules, CA, USA). GLI3 antibody was purchased from Abcam (cat# ab123495), β-actin antibody was purchased from Millipore Sigma (cat# A3854), IRF3, phospho-IRF3, ERK, phospho-ERK, IκBα, TRIF and MYD88 antibodies were purchased from cell signaling (cat# 4302, cat# 4947, cat# 9102, cat# 9101, cat# 9242, cat# 4596, and cat# 4283 respectively). GLI3 antibody was validated by western blot using cells transfected with RNAi targeting GLI3 (Supplementary Figure 6).
Mice
Gli3fl/fl mice and lysozyme M (LysM)-cre mice were obtained from Jackson Labs (Bar Harbor, ME). Mice were bred and maintained at the UNH animal resource office (ARO). Mice were crossed to generate mice with conditional deletion of Gli3 in myeloid cells (M-Gli3−/−). Mice were genotyped at p6-p9 using toe clipping and were separated into M-Gli3−/− or WT groups. All experiments were approved by the UNH IACUC following guidelines of the National Institutes of Health. Mice were used for experiments when they reached 8–14 weeks of age.
Genotyping was performed using DNA extracted from mice toes using Phire Animal Tissue Direct PCR Kit (Fisher Scientific, F-140WH). The following PCR primer pairs were used: Gli3 flox, 5′-GATGAATGTGATCCAGGGC-3′ (forward) and 5′- GTCATATTGTGCCCAGTAGTAGC-3′ (reverse); Cre, 5′-GCGGTCTGGCAGTAAAAACTATC-3′ (forward) and 5′-GTGAAACAGCATTGCTGTCACTT-3′ (reverse); and recombined Gli3 (rGLI3), 5′-CTGGATGA ACCAAGCTTTCCATC-3′ (forward) and 5′-CAGTA GTAGCCTGGTTACAG-3′ (reverse). Samples were analyzed on 2% agarose gels.
Enzyme-linked immunosorbent assay (ELISA)
Mouse IL-6 ELISA (R&D Systems; DY406), mouse CCL2 ELISA (R&D Systems; DY479) and mouse TNFα ELISA (R&D Systems; DY410) were used to quantify cytokine secretion in cultured peritoneal macrophages. Peritoneal macrophages were isolated by injection of mice with 500 ml incomplete Freund’s Adjuvant (IFA) i.p. then after 3 days, mice were euthanized and macrophages were harvested via peritoneal lavage with IMDM containing 10% FBS as previously described [65]. Peritoneal cells were washed once and 1 × 106/ml were allowed to adhere to cell culture plates for 1 h. Cells were then gently washed with DPBS, and 1 ml of fresh media (IMDM + 10% FBS + 1% A/A) was added with either 100 ng/ml LPS (E.coli; Serotype 0111:B4; Sigma Aldrich) or control for 24 h. Supernatants were harvested and diluted 1:40 for then used in ELISA following manufacturer recommendations.
Cloning of GLI3 regulatory region and mutations of IRF3 binding sites
A 1641bp region of the GLI3 intron 1–2 regulatory region containing candidate IRF3 binding sites was PCR amplified with the following primers: 5′-ggtacctgagctcgctagcctcgagGTCGAAGGGAACAATTATAG-3′ and 5′-gaggccagatcttgatatcctcgagCCATCTCTTTTCTGAGATTATC-3′ from MM6 genomic DNA and cloned into the pGL4.10 luciferase plasmid digested with XhoI using the NEB HiFi DNA Assembly kit (NEB E2621S). The resulting plasmid pGL4-GLI3-WT was verified by sequencing. Three independent IRF3 binding sites within the GLI3 promoter (aaaagaaa, aaaagaaa, aaccgaaa) were individually deleted using the NEB Q5 Site-Directed Mutagenesis Kit following the manufacturer’s instructions (NEB E0554S). pGL4-GLI3-Mut1 (lacking binding site 1; BS1) was generated using the primers 5′-ATGTATGTAGCTAAACCAG-3′ and 5′-TATTCAGTGTTTCGTTGAAAAC-3′, pGL4-GLI3-Mut2 (lacking BS2) was generated with the primers 5′-CAAAACCGAAAAAACAAAAAC-3′ and 5′-GAGACAGAGTCTCACTCTG-3′, and pGL4-GLI3-Mut3 (lacking BS3) was generated with the primers 5′-AAACAAAAACAAACAAACAAAAAAAC-3′ and 5′-TTGTTTCTTTTGAGACAGAG-3′. The resulting plasmids were verified by sequencing.
Dual luciferase reporter assay
Cells (4 × 106) were transfected by electroporation with 4 mg luciferase reporter plasmid (pGL4.1 containing either WT, Mut1, Mut2 or Mut3), 10 ng Renilla plasmid (pGL4.7) and 4 mg either V5 IRF3 expression construct or empty vector control. Cells were plated in triplicate wells in 6-well plates containing 2 ml cell culture media. After 2 days, cells were harvested and lysed in 1× Passive lysis buffer and luminescence (relative light units) were measured using the Dual-Luciferase Assay System (Promega Corporation, Madison, WI, USA). Data is presented as the ratio of Firefly:Renilla readouts.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (San Diego, CA, USA). A two-tailed t test was used to determine statistical significance between two variables and a two-way ANOVA was used to compare more than 2 variables. Statistical significance is indicated on each figure as follows: *(p < 0.05), **(p < 0.01), ***(p < 0.001), and ****(p < 0.0001).
SUPPLEMENTARY MATERIALS
ACKNOWLEDGMENTS AND FUNDING
We thank Dr. Jerry Blazey, Vice President of the Division of Research and Innovation Partnerships at Northern Illinois University for providing the funds to establish the mouse model. S.F.E was supported by an NIH COBRE Center of Integrated Biomedical and Bioengineering Research (CIBBR, P20 GM113131) through an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences. Funding from NIH/NIAIN to R.R. grant numbers R01 AI134907, R01 AI166668 and R01 AI155466.
Abbreviations
- GLI
glioma associated protein
- HH
hedgehog
- SMO
smoothened
- PTCH1
patched 1
- GCPS
Greig cephalopolysyndactyly
- PHS
Pallister-Hall Syndrome
- TLR
toll-like receptor
- LPS
lipopolysaccharide
- MYD88
myeloid differentiation primary response gene 88
- TRIF
TIR containing adapter-inducing interferon-beta
- MAPK
mitogen-activated protein kinase
- IRF3
interferon regulatory factor 3
- MPLA
monophosphoryl lipid A
- MEF
mouse embryonic fibroblasts
- PBMCs
peripheral blood mononuclear cells
Editorial note
This paper has been accepted based in part on peer-review conducted by another journal and the authors’ response and revisions as well as expedited peer-review in Oncotarget.
Author contributions
S.J.M. performed experiments and wrote the paper; M.K., W.H., L.L.K. D.S.G., A.B., E.Y-G, A.H., and K.F. performed experiments; V.J. provided reagents and conceptual insight; R.R. provided reagents and professional conceptual insight; and S.F.E. conceptualized the study, wrote the paper and oversaw all aspects of the research.
Ethical statement
All experiments were approved by the UNH IACUC following guidelines of the National Institutes of Health. Mice were used for experiments when they reached 8–14 weeks of age.
CONFLICTS OF INTEREST
The authors have no conflict of interests to declare.
REFERENCES
- 1. McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003; 53:1–114. 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 2. Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010; 16:3130–40. 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merchant JL, Ding L. Hedgehog Signaling Links Chronic Inflammation to Gastric Cancer Precursor Lesions. Cell Mol Gastroenterol Hepatol. 2017; 3:201–10. 10.1016/j.jcmgh.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherman AE, Zavros Y. Role of Sonic Hedgehog signaling during progression from inflammation to cancer in the stomach. World J Gastrointest Pathophysiol. 2011; 2:103–8. 10.4291/wjgp.v2.i6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han W, Allam SA, Elsawa SF. GLI2-Mediated Inflammation in the Tumor Microenvironment. Adv Exp Med Biol. 2020; 1263:55–65. 10.1007/978-3-030-44518-8_5. [DOI] [PubMed] [Google Scholar]
- 6. Liu Z, Li T, Reinhold MI, Naski MC. MEK1-RSK2 contributes to Hedgehog signaling by stabilizing GLI2 transcription factor and inhibiting ubiquitination. Oncogene. 2014; 33:65–73. 10.1038/onc.2012.544. [DOI] [PubMed] [Google Scholar]
- 7. Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, Mohri D, Tanaka Y, Ijichi H, Tateishi K, Kanai F, Kawabe T, Omata M. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog. 2009; 48:703–12. 10.1002/mc.20516. [DOI] [PubMed] [Google Scholar]
- 8. Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz i Altaba A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007; 104:5895–900. 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elsawa SF, Almada LL, Ziesmer SC, Novak AJ, Witzig TE, Ansell SM, Fernandez-Zapico ME. GLI2 transcription factor mediates cytokine cross-talk in the tumor microenvironment. J Biol Chem. 2011; 286:21524–34. 10.1074/jbc.M111.234146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han W, Jackson DA, Matissek SJ, Misurelli JA, Neil MS, Sklavanitis B, Amarsaikhan N, Elsawa SF. Novel Molecular Mechanism of Regulation of CD40 Ligand by the Transcription Factor GLI2. J Immunol. 2017; 198:4481–89. 10.4049/jimmunol.1601490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM, Aberger F. Human GLI2 and GLI1 are part of a positive feedback mechanism in Basal Cell Carcinoma. Oncogene. 2002; 21:5529–39. 10.1038/sj.onc.1205748. [DOI] [PubMed] [Google Scholar]
- 12. Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999; 126:3915–24. 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 13. Zeng H, Jia J, Liu A. Coordinated translocation of mammalian Gli proteins and suppressor of fused to the primary cilium. PLoS One. 2010; 5:e15900. 10.1371/journal.pone.0015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou J, Zhu G, Huang J, Li L, Du Y, Gao Y, Wu D, Wang X, Hsieh JT, He D, Wu K. Non-canonical GLI1/2 activation by PI3K/AKT signaling in renal cell carcinoma: A novel potential therapeutic target. Cancer Lett. 2016; 370:313–23. 10.1016/j.canlet.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 15. Kang S, Graham JM Jr, Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997; 15:266–68. 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- 16. Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998; 20:54–57. 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 17. Wild A, Kalff-Suske M, Vortkamp A, Bornholdt D, König R, Grzeschik KH. Point mutations in human GLI3 cause Greig syndrome. Hum Mol Genet. 1997; 6:1979–84. 10.1093/hmg/6.11.1979. [DOI] [PubMed] [Google Scholar]
- 18. Wilson SL, Wilson JP, Wang C, Wang B, McConnell SK. Primary cilia and Gli3 activity regulate cerebral cortical size. Dev Neurobiol. 2012; 72:1196–212. 10.1002/dneu.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matissek SJ, Elsawa SF. GLI3: a mediator of genetic diseases, development and cancer. Cell Commun Signal. 2020; 18:54. 10.1186/s12964-020-00540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sabroe I, Parker LC, Dower SK, Whyte MK. The role of TLR activation in inflammation. J Pathol. 2008; 214:126–35. 10.1002/path.2264. [DOI] [PubMed] [Google Scholar]
- 21. Surendran N, Hiltbold EM, Heid B, Akira S, Standiford TJ, Sriranganathan N, Boyle SM, Zimmerman KL, Makris MR, Witonsky SG. Role of TLRs in Brucella mediated murine DC activation in vitro and clearance of pulmonary infection in vivo . Vaccine. 2012; 30:1502–12. 10.1016/j.vaccine.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 22. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008; 42:145–51. 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 23. Reimer T, Brcic M, Schweizer M, Jungi TW. poly(I:C) and LPS induce distinct IRF3 and NF-kappaB signaling during type-I IFN and TNF responses in human macrophages. J Leukoc Biol. 2008; 83:1249–57. 10.1189/jlb.0607412. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol. 2014; 47:136–47. 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]
- 25. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001; 410:37–40. 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 26. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–84. 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 27. Escoubet-Lozach L, Benner C, Kaikkonen MU, Lozach J, Heinz S, Spann NJ, Crotti A, Stender J, Ghisletti S, Reichart D, Cheng CS, Luna R, Ludka C, et al. Mechanisms establishing TLR4-responsive activation states of inflammatory response genes. PLoS Genet. 2011; 7:e1002401. 10.1371/journal.pgen.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boi SK, Elsawa SF. Epigenetic regulation of toll-like receptor signaling: Implications for cancer development. Med Epigenet. 2013; 1:19–30. 10.1159/000353684. [DOI] [Google Scholar]
- 29. Dennler S, André J, Verrecchia F, Mauviel A. Cloning of the human GLI2 Promoter: transcriptional activation by transforming growth factor-beta via SMAD3/beta-catenin cooperation. J Biol Chem. 2009; 284:31523–31. 10.1074/jbc.M109.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gurung B, Feng Z, Hua X. Menin directly represses Gli1 expression independent of canonical Hedgehog signaling. Mol Cancer Res. 2013; 11:1215–22. 10.1158/1541-7786.MCR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993; 3:241–46. 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- 32. Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997; 124:113–23. 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- 33. Ruiz i Altaba A. Gli proteins and Hedgehog signaling: development and cancer. Trends Genet. 1999; 15:418–25. 10.1016/s0168-9525(99)01840-5. [DOI] [PubMed] [Google Scholar]
- 34. Zheng X, Vittar NB, Gai X, Fernandez-Barrena MG, Moser CD, Hu C, Almada LL, McCleary-Wheeler AL, Elsawa SF, Vrabel AM, Shire AM, Comba A, Thorgeirsson SS, et al. The transcription factor GLI1 mediates TGFβ1 driven EMT in hepatocellular carcinoma via a SNAI1-dependent mechanism. PLoS One. 2012; 7:e49581. 10.1371/journal.pone.0049581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lo Ré AE, Fernández-Barrena MG, Almada LL, Mills LD, Elsawa SF, Lund G, Ropolo A, Molejon MI, Vaccaro MI, Fernandez-Zapico ME. Novel AKT1-GLI3-VMP1 pathway mediates KRAS oncogene-induced autophagy in cancer cells. J Biol Chem. 2012; 287:25325–34. 10.1074/jbc.M112.370809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang HN, Oh SC, Kim JS, Yoo YA. Abrogation of Gli3 expression suppresses the growth of colon cancer cells via activation of p53. Exp Cell Res. 2012; 318:539–49. 10.1016/j.yexcr.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 37. Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014; 515:130–33. 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 38. Hager-Theodorides AL, Dessens JT, Outram SV, Crompton T. The transcription factor Gli3 regulates differentiation of fetal CD4- CD8- double-negative thymocytes. Blood. 2005; 106:1296–304. 10.1182/blood-2005-03-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang T, Cui H, Wen M, Zuber J, Kogan SC, Wei G. TCEA1 regulates the proliferative potential of mouse myeloid cells. Exp Cell Res. 2018; 370:551–60. 10.1016/j.yexcr.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solanki A, Lau CI, Saldaña JI, Ross S, Crompton T. The transcription factor Gli3 promotes B cell development in fetal liver through repression of Shh. J Exp Med. 2017; 214:2041–58. 10.1084/jem.20160852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solecki DJ, Gromeier M, Mueller S, Bernhardt G, Wimmer E. Expression of the human poliovirus receptor/CD155 gene is activated by sonic hedgehog. J Biol Chem. 2002; 277:25697–702. 10.1074/jbc.M201378200. [DOI] [PubMed] [Google Scholar]
- 42. Ravasi T, Wells C, Forest A, Underhill DM, Wainwright BJ, Aderem A, Grimmond S, Hume DA. Generation of diversity in the innate immune system: macrophage heterogeneity arises from gene-autonomous transcriptional probability of individual inducible genes. J Immunol. 2002; 168:44–50. 10.4049/jimmunol.168.1.44. [DOI] [PubMed] [Google Scholar]
- 43. Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013; 19:3404–15. 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshidome H, Kohno H, Shida T, Kimura F, Shimizu H, Ohtsuka M, Nakatani Y, Miyazaki M. Significance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastases. Int J Oncol. 2009; 34:923–30. 10.3892/ijo_00000218. [DOI] [PubMed] [Google Scholar]
- 45. Kanzler I, Liehn EA, Koenen RR, Weber C. Anti-inflammatory therapeutic approaches to reduce acute atherosclerotic complications. Curr Pharm Biotechnol. 2012; 13:37–45. 10.2174/138920112798868557. [DOI] [PubMed] [Google Scholar]
- 46. Rantapää-Dahlqvist S, Boman K, Tarkowski A, Hallmans G. Up regulation of monocyte chemoattractant protein-1 expression in anti-citrulline antibody and immunoglobulin M rheumatoid factor positive subjects precedes onset of inflammatory response and development of overt rheumatoid arthritis. Ann Rheum Dis. 2007; 66:121–23. 10.1136/ard.2006.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caporali R, Pallavicini FB, Filippini M, Gorla R, Marchesoni A, Favalli EG, Sarzi-Puttini P, Atzeni F, Montecucco C. Treatment of rheumatoid arthritis with anti-TNF-alpha agents: a reappraisal. Autoimmun Rev. 2009; 8:274–80. 10.1016/j.autrev.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 48. Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000; 51:289–98. 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 49. Victor FC, Gottlieb AB. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol. 2002; 1:264–75. [PubMed] [Google Scholar]
- 50. Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988; 31:784–88. 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 51. Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010; 22:347–52. 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 52. Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000; 68:7010–17. 10.1128/IAI.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, Gay RE, Gay S, Kyburz D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008; 58:3684–92. 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 54. Lucas K, Maes M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013; 48:190–204. 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Piras V, Selvarajoo K. Beyond MyD88 and TRIF Pathways in Toll-Like Receptor Signaling. Front Immunol. 2014; 5:70. 10.3389/fimmu.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Siednienko J, Gajanayake T, Fitzgerald KA, Moynagh P, Miggin SM. Absence of MyD88 results in enhanced TLR3-dependent phosphorylation of IRF3 and increased IFN-β and RANTES production. J Immunol. 2011; 186:2514–22. 10.4049/jimmunol.1003093. [DOI] [PubMed] [Google Scholar]
- 57. Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, Endo Y, Rubin JS, Toretsky J, Uren A. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009; 284:9074–82. 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-β/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011; 71:5606–10. 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000; 13:539–48. 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 60. Jackson DA, Misurelli JA, Elsawa SF. GLI Family Zinc Finger 2. In: Choi S, ed. Encyclopedia of Signaling Molecules. Springer, Cham. 2018; 2077–88. 10.1007/978-3-319-67199-4_101917. [DOI] [Google Scholar]
- 61. Karbalivand M, Almada LL, Ansell SM, Fernandez-Zapico ME, Elsawa SF. MLL1 inhibition reduces IgM levels in Waldenstrom macroglobulinemia. Leuk Res. 2022; 116:106841. 10.1016/j.leukres.2022.106841. [DOI] [PubMed] [Google Scholar]
- 62. Zhu J, Smith K, Hsieh PN, Mburu YK, Chattopadhyay S, Sen GC, Sarkar SN. High-throughput screening for TLR3-IFN regulatory factor 3 signaling pathway modulators identifies several antipsychotic drugs as TLR inhibitors. J Immunol. 2010; 184:5768–76. 10.4049/jimmunol.0903559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jackson DA, Smith TD, Amarsaikhan N, Han W, Neil MS, Boi SK, Vrabel AM, Tolosa EJ, Almada LL, Fernandez-Zapico ME, Elsawa SF. Modulation of the IL-6 Receptor α Underlies GLI2-Mediated Regulation of Ig Secretion in Waldenström Macroglobulinemia Cells. J Immunol. 2015; 195:2908–16. 10.4049/jimmunol.1402974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matissek SJ, Han W, Karbalivand M, Sayed M, Reilly BM, Mallat S, Ghazal SM, Munshi M, Yang G, Treon SP, Walker SR, Elsawa SF. Epigenetic targeting of Waldenstrom macroglobulinemia cells with BET inhibitors synergizes with BCL2 or histone deacetylase inhibition. Epigenomics. 2021; 13:129–44. 10.2217/epi-2020-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elsawa SF, Bost KL. Murine gamma-herpesvirus-68-induced IL-12 contributes to the control of latent viral burden, but also contributes to viral-mediated leukocytosis. J Immunol. 2004; 172:516–24. 10.4049/jimmunol.172.1.516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.