Abstract
Objectives
We aimed to estimate the risk of HCQ retinopathy and its risk factors among incident users in the community.
Methods
Using the Rochester Epidemiology Project, a record-linkage system, a cohort of incident users of HCQ was identified from 27 counties in the American upper Midwest. HCQ retinopathy was defined based on characteristic paracentral automated 10-2 visual field (10-2 AVF) defects and parafoveal retinal photoreceptor layer changes on spectral domain optical coherence tomography. Cumulative incidence rates were estimated adjusting for competing risk of death. Risk factors for HCQ retinopathy were examined using Cox models.
Results
The study included 634 incident HCQ users (mean age at initial HCQ use was 53.7 years, 79% females, 91% white). Most common indications for HCQ were RA (57%) and SLE (19%). The average follow-up length was 7.6 years. Eleven patients developed HCQ retinopathy (91% females, 91% white). The majority used HCQ for RA (91%). The cumulative incidence rate at year 5 was 0%, which increased to 3.9% (95% CI 2.0, 7.4) by 10 years. Taking an HCQ dose ≥5 mg/kg was associated with a hazard ratio (HR) of 3.59 (95% CI 1.09, 11.84) compared with lower doses. There was a 48% increase [HR 1.48 (95% CI 1.03, 2.14)] in the risk of HCQ retinopathy for each 100 g of HCQ cumulative dose.
Conclusion
The risk of HCQ retinopathy at 10 years of use is lower compared with previous prevalence-based estimations. A dose ≥5 mg/kg was associated with higher HCQ retinopathy risk.
Keywords: HCQ, retinopathy, epidemiology, incidence risk
Rheumatology key messages.
The risk of HCQ retinopathy has been largely estimated using prevalent measures and referral populations.
In this community-based study, the incident risk of HCQ retinopathy was relatively low.
Taking an HCQ dose >5 mg/kg was associated with a higher retinopathy risk compared with lower doses.
Introduction
HCQ is used in the treatment of a wide variety of rheumatic diseases. Particularly in SLE, HCQ is considered the cornerstone of treatment, as it has been shown to improve survival and decrease disease activity, the risk of flares and organ damage [1, 2]. In general, HCQ is a safe and affordable medication compared with alternative therapies. However, HCQ-related retinopathy is a complication that has received significant attention in recent years and is potentially a source of concern for many patients.
The exact mechanism of HCQ retinopathy remains unclear. It may be the result of binding of HCQ to melanin in the retinal pigment epithelium layer. This binding is thought to ultimately damage the overlying photoreceptors, which results in vision changes [3, 4]. Early retinal toxicity is often asymptomatic and may be initially identified by clinical testing as paracentral retinal photoreceptor interdigitation zone and ellipsoid zone changes on spectral domain optical coherence tomography (SD-OCT) and/or paracentral automated 10-2 visual field (AVF) defects. As toxicity progresses, a ring of parafoveal retinal pigment epithelium depigmentation sparing the central fovea and corresponding outer retinal atrophy may be noted. This is known as ‘bull’s eye’ maculopathy [3, 4]. Symptomatically this can manifest as paracentral scotomata, reading difficulties, decreased visual acuity, reduced colour and/or night vision, flashing lights and eventually central scotomata and increasing visual field defects [4].
Recommendations for HCQ retinopathy screening differ by country. The American Academy of Ophthalmology (AAO) has recommended that patients who are using HCQ undergo a fundus examination within the first year of use. If maculopathy is present, these patients should undergo SD-OCT and 10-2 AVF testing. Annual screening with SD-OCT and 10-2 AVF testing are recommended after 5 years of use or earlier if major risk factors are present, which include an HCQ dose >5 mg/kg actual body weight, presence of renal disease, presence of macular disease and concomitant use of tamoxifen [4]. In contrast, the Royal College of Ophthalmologists of the United Kingdom does not recommend baseline testing, but rather recommends monitoring annually after 5 years of therapy or after 1 year of therapy if the previously discussed major risk factors are present. Visual field testing is not recommended as a first-line monitoring test; rather, screening is recommended through SD-OCT and widefield fundus autofluorescence [5].
The frequency of HCQ retinopathy in a community population has not been well elucidated. Recent advances in screening modalities, including 10-2 AVF testing, SD-OCT and multifocal electroretinography, have likely led to an increase in the reported prevalence of HCQ retinopathy due to the increase in screening sensitivity [6]. A study in the early 1980s of 900 patients taking HCQ at ≤6.5 mg/kg actual body weight/day and having regular eye exams reported no cases of HCQ retinopathy over 7 years of use [7]. A decade ago, a study of 3995 patients with either RA or SLE using HCQ estimated a prevalence of HCQ retinopathy based on self-reports and confirmation of bull’s eye maculopathy via medical records review at 5 years to be 0.3%, which rose towards 2% at 10–15 years of continuous usage [8]. More recently, a study of 2361 patients who had used HCQ continuously for at least 5 years and who received AVF and SD-OCT testing reported a retinal toxicity prevalence of 7.5% [mean duration of HCQ use was 12.2 years (s.d. 5.1)]. Approximately 2% of patients receiving 4–5 mg/kg actual body weight had retinal toxicity within the first 10 years of use, but the prevalence increased to 20% among those who had taken HCQ for ≥20 years [9]. Based on this study, the AAO in 2016 recommended a dose of ≤5 mg/kg actual body weight/day to reduce the risk of HCQ retinopathy [4]. However, the reliance on prevalence estimates (the proportion of individuals who have HCQ retinopathy at a specific point in time) to assess the risk of HCQ retinopathy will lead to overestimation compared with incidence estimates (new cases of HCQ retinopathy over a period of time) because HCQ retinopathy is permanent and non-fatal, therefore the prevalence of HCQ retinopathy is higher than its incidence at any point in time [10]. It is also important to note that risk estimates based on prevalence studies do not take into consideration the competing risk of death, which might lead to biased risk estimates of HCQ retinopathy. In addition, incidence studies have focussed on disease-specific populations at referral centres [11]. Using a large population-based cohort of HCQ users, we aimed to estimate the incidence of HCQ retinopathy and identify associated risk factors.
Methods
Through the resources of the Rochester Epidemiology Project (REP), a record-linkage system, we used the population of 27 counties in southwest Minnesota and southeast Wisconsin for investigation of the epidemiology of HCQ retinopathy, as comprehensive medical records for residents seeking medical care were available. The REP allows ready access to medical records from healthcare providers (including ophthalmologists and optometrists) for the local population, including from the Mayo Clinic (Rochester, MN, USA) and its affiliated hospitals, the Olmsted Medical Center (Olmsted County, MN, USA) and its affiliated hospitals, local nursing homes, etc. This system ensures comprehensive identification of HCQ users among the residents of the region [12]. The demographics, distribution of morbidity and death rates in the REP region are similar to those in the upper Midwest. The characteristics and strengths of the REP, as well as its generalizability, have been described elsewhere [13–15]. The study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center. Participants were not required to provide written consent, as this was a retrospective chart review study.
Prescription data were available starting in 2003 in Olmsted County and since 2010 for the rest of the 26 counties of the REP area. Patients residing in Olmsted County who used HCQ in 2003 or after and those residing in the other 26 counties who used HCQ in 2010 or after were included.
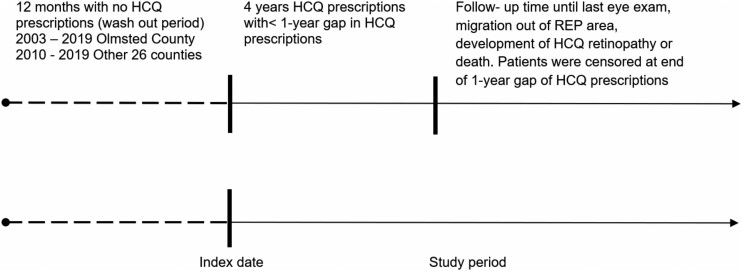
To identify incident HCQ users, we excluded residents with <1 year of medical history before their first HCQ prescription. HCQ users had to have at least 4 years of continuous HCQ use, defined as having <1 year gaps between prescription stops and starts during those 4 years, and at least one eye exam (either OCT, VF or both) to be included. The incident date of HCQ prescription was defined as the index date (Fig. 1). HCQ users with a ≥1 year gap after the first 4 years were censored when the gap reached 1 year. HCQ users were followed until HCQ retinopathy incidence, migration out of the region, their last OCT and/or VF or death. The medical records of these patients, including OCT and/or VF data, were manually reviewed in detail for the development of HCQ retinopathy. Patients using HCQ regardless of the indication were included in this study.
Fig. 1.
Schematic drawing of the study timeline
HCQ retinopathy was defined based on characteristic paracentral visual field defects and/or parafoveal retinal photoreceptor layer changes on OCT. Variables extracted from the REP database and medical records review included race/ethnicity, sex, indication for HCQ, duration of HCQ use (in years), age at HCQ use, weight (in kg), BMI, daily HCQ dose (in mg and in mg/kg), concurrent use of tamoxifen or anastrozole, the ophthalmological screening modalities and intervals (OCT, VF or both) and age at diagnosis. The cumulative HCQ dose (in mg/day and mg/kg/day) were computed from prescription start dates, supply days and dosages. The presence or absence of kidney disease was ascertained based on two diagnostic codes at least 30 days apart [International Classification of Diseases, Ninth Revision: 580.x, 584.x, 585.x, 586, v42.0, v45.1x, v56.x; or International Classification of Diseases, Tenth Revision: N00.x, N01.x, N17.x, N18.x, N19, T85.691x, T85.71Xx, Z48.22, Z49.x).
Descriptive statistics (percentages, means, etc.) were used to summarize the data. The cumulative incidence rates of HCQ retinopathy after 4 years of HCQ use were estimated by adjusting for the competing risk of death. Risk factors for HCQ retinopathy were estimated using Cox proportional hazards models. For all comparisons, a P-value <0.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
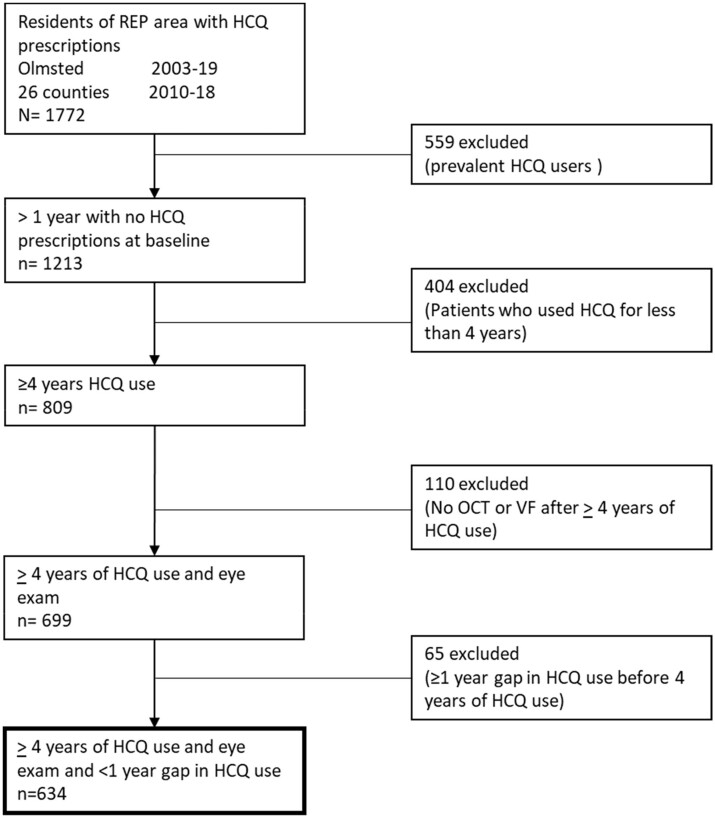
Of the 1772 HCQ users who were residents of the REP area, 634 met the inclusion criteria (Fig. 2). A total of 559 users were excluded because they had received HCQ before 2003 in Olmsted County or before 2010 in the rest of the 26 counties; 404 were excluded because they used HCQ for <4 years; 110 were excluded because they only had a baseline eye exam without any follow-ups (76 patients used HCQ for 4 years but <5 years and thus additional testing per the AAO recommendations was not performed; 22 patients had missing follow-up OCT and/or VF data; 2 patients refused continued testing; 1 patient had eye exams without the required VFs and/or OCTs; 9 patients did not have the OCTs and/or VFs exams completed due to technical difficulties/intolerance and 65 patients were excluded because they had a gap of ≥1 year of HCQ prescriptions in the first 4 years. Among these 65 patients, none have had visual symptoms or changes that led to discontinuation of therapy. A total of 70% used HCQ for RA, 20% used HCQ for SLE or connective tissue disease and 10% used HCQ for dermatological causes. A total of 55% did not benefit from HCQ and had to be switched to different therapies, while 35% experienced side effects, including gastrointestinal symptoms, headaches, rashes and alopecia. Finally, 10% used HCQ intermittently but not >4 years for their dermatological disease.
Fig. 2.
A flowchart of the initial cohort followed by numbers and reasons for exclusions
Table 1 summarizes the demographic and clinical characteristics of the study population. The average age at the time of initial HCQ prescription was 53.7 years (s.d. 15.5), with 79% being female and 91% being white. The most common indications for HCQ were RA (57%) and SLE (19%). The average follow-up was 7.6 years [s.d. 2.7; median 7.1; IQR 5.3 (Q1 2.5 ‒ Q3 7.8)]. The average HCQ dose was 356.9 mg/day (range 100.0–800.0), while the mean daily dose per actual body weight was 4.5 mg/kg (range 1.3–8.9). The average cumulative HCQ dose was 472 g (range 24–725). None of the HCQ users in this study were concurrently taking tamoxifen and/or anastrozole. At their last screening, all patients had both SD-OCT and 10-2 AVF done, except for one patient who only had SD-OCT done.
Table 1.
Study population and case demographic and clinical characteristics
| Characteristics | Study population (N = 634) | Retinopathy cases (n = 11) |
|---|---|---|
| Age at HCQ initiation, mean (s.d.), years | 53.7 (15.5) | 58.1 (11.5) |
| Sex, female, n (%) | 502 (79) | 10 (91) |
| Race/ethnicity, n (%) | ||
| White | 580 (91) | 10 (91) |
| Asian | 9 (1) | – |
| Other/mixed | 7 (1) | 1 (9) |
| Black | 10 (2) | – |
| American Indian | 3 (0.5) | – |
| Hispanic | 24 (4) | – |
| Indication for HCQ, n (%) | ||
| RAa | 363 (57) | 10 (91) |
| SLE | 120 (19) | – |
| CTDb | 61 (10) | – |
| Dermatologic | 35 (6) | – |
| Sjogren’s | 33 (5) | 1 (9) |
| Otherc | 20 (3) | – |
| BMI, kg/m2, mean (s.d.) | 29.8 (7.2) | 28.6 (5.0) |
| Presence of CKD, n (%) | 66 (10) | 3 (27) |
| HCQ dose, mg/day, mean (s.d.) | 356.9 (91.3) | 400 (0) |
| Range | 100–800 | 400–400 |
| HCQ dose, mg/kg/dayd, mean (s.d.) | 4.5 (1.4) | 5.7 (1.1) |
| Range | 1.3–8.9 | 3.8–6.7 |
| Cumulative HCQ dose, g, mean (s.d.) | 472 (131) | 540 (38) |
| Range | 24–725 | 463–585 |
| Length of follow up, years, mean (s.d.), median (Q1–Q3) (IQR) | 7.6 (2.7) | 8.1 (2.0) |
| 7.1 (2.5–7.8) (5.3) | 8.3 (3.3–9.2) (5.9) |
Seronegative, seropositive, RA/SLE overlap.
MCTD, UCTD, DM, APS, scleroderma.
Inflammatory OA, CPPD, palindromic rheumatism, Blau syndrome.
Actual body weight (in kg).
Of the 634 users, 11 developed HCQ retinopathy. Of these, 91% were females and 91% were white. The majority used HCQ for RA (91%). All the patients were on a daily dose of 400 mg, however, the mean daily dose per actual body weight was 5.7 mg/kg/day (range 3.8–6.7), with a cumulative mean dose of 540 g (range 463–585). Ten patients (91%) had signs of parafoveal disruption of the photoreceptor ellipsoid zone, parafoveal thinning of the outer nuclear layer and/or retinal pigment epithelium damage on the SD-OCT. Ten patients (91%) had paracentral defects on AVF (AVF data were missing for one patient), while one patient (9%) had paracentral defects on AVF without clear changes on SD-OCT.
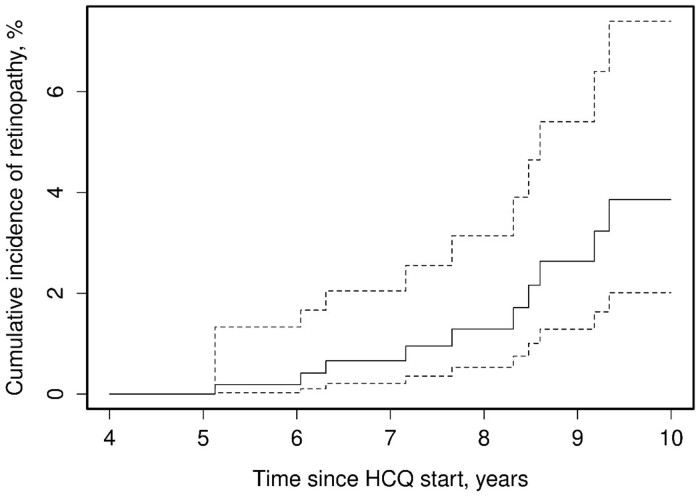
Fig. 3 depicts the cumulative incidence rates of HCQ retinopathy at years 5–10 of use. After 5 years of use, the rate was 0%, which progressively increased to 3.9% (95% CI 2.0, 7.4) at 10 years. The age at onset of therapy, female sex, BMI, daily HCQ dose and presence of chronic kidney disease did not significantly increase the risk of retinopathy (Table 2). However, those taking ≥5 mg/kg of HCQ had a hazard ratio (HR) of 3.59 (95% CI 1.09, 11.84) compared with those who took <5 mg/kg. For each 100 g of HCQ cumulative dose, the risk of HCQ retinopathy increased by 48% [HR 1.48 (95% CI 1.03, 2.14)]. Similarly, per each milligram of actual body weight the risk for developing HCQ retinopathy increased by 44% [HR 1.44 (95% CI 1.02, 2.02)]. Of note, due to the infrastructure of the REP, missing data were minimal. For example, missing BMI data were noted for 20 patients, and these were excluded from the Cox proportional hazards models that included BMI.
Fig. 3.
Cumulative incidence rates of retinopathy after HCQ initiation
Solid line depicts the cumulative incidence rate estimate (risk in %) of HCQ retinopathy per year. Dotted lines depict upper and lower limits of the 95% CI estimates of the cumulative incidence rate/risk of HCQ retinopathy per year.
Table 2.
Risk factors for retinopathy—unadjusted univariable models
| Risk factor | HR (95% CI) |
|---|---|
| Age at HCQ start (HR per 10 year increase) | 1.36 (0.88, 2.11) |
| Female | 3.42 (0.43, 26.89) |
| BMI (kg/m2) | 0.99 (0.91, 1.08) |
| Daily HCQ dose per 100 mg | 1.51 (0.88, 2.59) |
| Cumulative HCQ dose per 100 g | 1.48 (1.03, 2.14) |
| Daily dose (mg/kg)a | 1.44 (1.02, 2.02) |
| Daily dose ≥5 mg/kga (vs <5) | 3.59 (1.09, 11.84) |
| CKD | 1.89 (0.50, 7.16) |
Actual body weight.
Discussion
In this study of 634 incident HCQ users in the community, 11 developed HCQ retinopathy. The cumulative incidence was <5% at 10 years of use. The risk of retinopathy increased significantly with HCQ use duration and dose.
Most prior studies reported prevalent estimates of HCQ retinopathy. Studies based on prevalence estimates might overestimate the risk of HCQ retinopathy for two reasons. First, given that this condition is permanent and nonfatal, the prevalence of HCQ retinopathy will be higher than its incidence, resulting in risk overestimation. Second, prevalence-based studies are not able to adjust for the competing risk of death, as they include survivors only. Given that patients with SLE and other systemic autoimmune diseases (where HCQ is prescribed) have a higher risk of death than the general population, this would mean that the population at risk will be smaller in prevalence estimates compared with incidence estimates [10]. In our study, the mean dose of HCQ per actual body weight was 4.5 mg/kg, however, the incidence (risk) of HCQ retinopathy at 10 years was 3.9%. A prior prevalence study of 2361 HCQ users among Kaiser Permanente patients reported an overall prevalence of 7.5%, the mean dose of HCQ per actual body weight was 4.06 mg/kg and the mean follow-up was 12.2 years (overall prevalence estimate at 10 years was not reported) [9]. Although there are inherent differences between incidence and prevalence measures, the cumulative overall risk based on the prevalence of HCQ retinopathy reported by Melles et al. [9] seems higher than the estimate reported in our study. In another prevalence study [8], the risk of bull’s eye maculopathy, a fundoscopic finding, was estimated to be 0.3% at 5 years that increased towards 2% after 10 years of HCQ use. In this study, however, sensitive screening techniques like OCTs were not utilized. It is thought that the advent of more sensitive screening techniques such as OCT may increase the ability to detect HCQ retinopathy [6].
Compared with other incident estimates, Petri et al. [11] studied 537 incident users of HCQ among SLE patients at a tertiary referral centre. They reported an HCQ retinopathy risk of 4.3% (1% by year 5, 1.8% by year 10, 3.3% by year 15 and up to 8% after 20 years). A higher HCQ blood level predicted the risk of HCQ retinopathy. The risk at 5 years was higher than our reported risk. However, the risk at 10 years was lower. It is worth noting that this study used a referral population, unlike our community-based population. Additionally, only SLE patients were included. These differences could have accounted for the differences in risk estimates, however, is reassuring that the risk at 10 years was relatively low. The risk of HCQ retinopathy increased over time as reported in our study.
Our study identified a daily HCQ dose >5.0 mg/kg as a risk factor for developing HCQ retinopathy. This is in addition to the duration of use, as was discussed above. This was noted by Melles et al. [9] as well. However, chronic kidney disease was not seen to be associated with an increased risk of HCQ retinopathy in our study. Perhaps this could be due to the smaller number of cases in our study. Unlike the study findings reported by Melles et al. [9], none of our patients concurrently used tamoxifen citrate.
Although the discussion of the use of HCQ centres around SLE, in our study population, the majority of HCQ users and most patients who developed HCQ retinopathy used it for RA. In SLE, HCQ is the cornerstone of treatment; it has been shown to improve survival and decrease disease activity, risk of flares and organ damage [1, 2]. However, this has not been shown in RA and the use of methotrexate has been suggested to be the preferred first-line therapy as mentioned by the 2015 ACR guidelines on RA management [16]. In RA cases where HCQ is being used, clinicians should consider tapering HCQ after 5 years of use or transitioning to other effective therapies.
Some of the strengths of our study include a study cohort that included many HCQ users who were taking HCQ for multiple different indications. Our study design allowed us to estimate the cumulative incidence of HCQ retinopathy as opposed to the previous prevalence estimates. We also controlled for immortal bias by censoring at the last exam for those users who did not have the continued required eye exams and adjusted for the competing risk of death.
There were some limitations to our study. Being a retrospective study was one. The usual limitations regarding completeness of medical records documentation apply. Ideally, prospective studies will ultimately provide better estimates. However, prohibitive costs make it unlikely for a prospective study to be sufficiently powered and prolonged to demonstrate the retinal safety of HCQ. Another limitation was the reliance on prescription data. Although a user might have been given an HCQ prescription, there was no guarantee that they were taking the medication. A required 4 year use period of HCQ without a gap of >1 year was used to include users who were more likely to be compliant with their medication. Moreover, prescription data may be missing and/or incomplete and dosing may not be accurate. HCQ blood levels were not readily available to include in our study. Another limitation was the population of our study was predominantly white (∼90%), which may limit the generalizability of study results to other racial/ethnic groups. However, this is an inherent limitation of the population covered by the records-linkage system of the REP, which covers a well-defined geographic area in the upper Midwest. Due to the retrospective nature of this review, the exact screening modalities for HCQ retinopathy were not standardized, as they changed throughout the study period to reflect the evolving AAO recommendations in 2002, 2011 and 2016. The use of SD-OCT along with 10-2 AVF was recommended in 2011 and onward [17]. In Olmsted County, we followed patients prior to the 2011 guidelines, which recommended the addition of SD-OCT. In the other counties, patients were followed after the 2011 guidelines. It is possible that some of the 22 patients with missing OCT and/or VF data who were excluded were in that time frame prior to the 2011 guidelines (Fig. 2). Additionally, it is possible that the OCT and/or VF were performed at ophthalmology and/or optometry practices outside the REP region. In these instances, we were only able to review the OCT and/or VF data that were transmitted into the medical records in the REP region. Finally, it is possible that HCQ retinopathy may occur in the first 4 years of HCQ therapy. However, none of the patients who stopped HCQ prior to completion of 4 years did so due to visual symptoms.
Based on the estimates provided by Melles et al. [9], the AAO revised HCQ dosing recommendations in 2016 [4]. The recommendations include a maximum HCQ dose of 5 mg/kg of actual body weight/day. This has been criticized since then [18, 19]. Reasons include a lack of efficacy data for the 5 mg/kg actual body weight/day dose of HCQ in controlling autoimmune diseases, lack of input from medical specialists who prescribe this medication, lack of input from patients who take HCQ and the reliance on prevalence estimates, as previously described [18, 19]. Our study aimed to estimate the cumulative incidence of HCQ retinopathy in the community, which should be a better reflection of the true risk. This study provides information that the risk of HCQ retinopathy may be lower than previous estimates using novel screening technologies (OCT, VF) and incident measures. The duration of HCQ use and higher HCQ doses were risk factors for developing HCQ retinopathy. This should provide an argument that HCQ remains a safe medication to use, especially as it is highly effective in SLE, and perhaps the risk of HCQ retinopathy may have been overestimated in the past.
Acknowledgements
The research team thanks Barbara Abbott for her valuable help accessing the REP data.
Funding: This study was made possible using the resources of the REP, which is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG034676 and grant number UL1 TR002377 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Jesse Y Dabit, Division of Rheumatology, Mayo Clinic, Rochester, MN.
Mehmet Hocaoglu, Division of Rheumatology, Mayo Clinic, Rochester, MN; Department of Internal Medicine, University of Maryland Medical Center Midtown Campus, Baltimore, MD.
Kevin G Moder, Division of Rheumatology, Mayo Clinic, Rochester, MN.
Andrew J Barkmeier, Department of Ophthalmology.
Wendy M Smith, Department of Ophthalmology.
Thomas J O’Byrne, Department of Quantitative Health Sciences.
Cynthia S Crowson, Division of Rheumatology, Mayo Clinic, Rochester, MN; Department of Quantitative Health Sciences.
Alí Duarte-García, Division of Rheumatology, Mayo Clinic, Rochester, MN; Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN, USA.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA.. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. [DOI] [PubMed] [Google Scholar]
- 2. Fanouriakis A, Kostopoulou M, Alunno A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 3. Yusuf IH, Sharma S, Luqmani R, Downes SM.. Hydroxychloroquine retinopathy. Eye (Lond) 2017;31:828–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 2016;123:1386–94. [DOI] [PubMed] [Google Scholar]
- 5.Royal College of Ophthalmologists. Hydroxychloroquine and chloroquine retinopathy: recommendations on monitoring. London: Royal College of Ophthalmologists, 2020.
- 6. Browning DJ, Lee C.. Relative sensitivity and specificity of 10-2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathy. Clin Ophthalmol 2014;8:1389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med 1983;75:40–5. [DOI] [PubMed] [Google Scholar]
- 8. Wolfe F, Marmor MF.. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62:775–84. [DOI] [PubMed] [Google Scholar]
- 9. Melles RB, Marmor MF.. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014;132:1453–60. [DOI] [PubMed] [Google Scholar]
- 10. Jorge A, Ung C, Young LH, Melles RB, Choi HK.. Hydroxychloroquine retinopathy—implications of research advances for rheumatology care. Nat Rev Rheumatol 2018;14:693–703. [DOI] [PubMed] [Google Scholar]
- 11. Petri M, Elkhalifa M, Li J, Magder LS, Goldman DW.. Hydroxychloroquine blood levels predict hydroxychloroquine retinopathy. Arthritis Rheumatol 2020;72:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA.. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rocca WA, Grossardt BR, Brue SM. et al. Data resource profile: expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol 2018;47:368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. St Sauver JL, Grossardt BR, Leibson CL. et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. St Sauver JL, Grossardt BR, Yawn BP. et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 17. Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011;118:415–22. [DOI] [PubMed] [Google Scholar]
- 18. Browning DJ, Yokogawa N, Greenberg PB, Perlman E.. Rethinking the hydroxychloroquine dosing and retinopathy screening guidelines. Am J Ophthalmol 2020;219:101–6. [DOI] [PubMed] [Google Scholar]
- 19. Schwartzman S, Samson CM.. Are the current recommendations for chloroquine and hydroxychloroquine screening appropriate? Rheum Dis Clin North Am 2019;45:359–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.