Abstract
Objectives
To evaluate the inhibition of progression of structural joint damage through week 48 in patients with moderately to severely active RA receiving upadacitinib as monotherapy or in combination with MTX.
Methods
Radiographic progression was assessed in two phase 3 randomized controlled trials. MTX-naïve patients were randomized to upadacitinib 15 or 30 mg once daily or MTX monotherapy (SELECT-EARLY, n = 945), while MTX inadequate responders (IRs) were randomized to upadacitinib 15 mg once daily or adalimumab 40 mg every other week or placebo added to background MTX (SELECT-COMPARE, n = 1629). The mean changes from baseline in modified total Sharp score (mTSS), joint space narrowing and erosion scores were determined. Data were analysed both by linear extrapolation for missing data imputation and treatment switching and as observed.
Results
In patients naïve or with limited exposure to MTX (SELECT-EARLY), mean changes from baseline to week 48 in mTSS were 0.03 for upadacitinib 15 mg, 0.14 for upadacitinib 30 mg and 1.00 for MTX based on linear extrapolation (P < 0.001 for both upadacitinib doses vs MTX). Among patients with an inadequate response to MTX (SELECT-COMPARE), the mean change from baseline in mTSS was significantly reduced in the upadacitinib 15 mg plus MTX group vs placebo plus MTX (0.28 vs 1.73; P < 0.001). The mean change from baseline in the adalimumab plus MTX group was 0.39.
Conclusion
Upadacitinib monotherapy or in combination with background MTX was effective in inhibiting the progression of structural joint damage through week 48 in MTX-naïve and MTX-IR patients with RA.
Trial registration
ClinicalTrials.gov (https://clinicaltrials.gov), NCT02706873 and NCT02629159
Keywords: radiology, rheumatoid arthritis, biological therapies, DMARDs, outcome measures
Rheumatology key messages.
Upadacitinib monotherapy or combined with background methotrexate inhibited structural joint damage progression over 1 year.
Consistent results were observed between upadacitinib doses and across different analyses (linear extrapolation and as observed).
Results were consistent between patients receiving continuous therapy and those switched to upadacitinib following rescue.
Introduction
RA is a chronic inflammatory disease associated with progressive joint destruction, impaired physical function and work disability [1, 2]. Long-term inhibition of structural joint damage is one of the key treatment goals in the management of RA [3]. Biologic DMARDs, such as TNF inhibitors, have demonstrated sustained inhibition of radiographic progression with long-term treatment when administered as monotherapy or in combination with MTX in patients with early and established RA [4, 5]. In recent years, several novel targeted synthetic DMARDs have been approved for the treatment of RA, with demonstrated effects on structural joint progression.
Upadacitinib, an oral, selective and reversible inhibitor of Janus kinase (JAK) 1 [6], was recently approved in many countries for the treatment of RA [7, 8]. In randomized controlled trials, upadacitinib significantly reduced the progression of structural joint damage at 6 months as monotherapy in MTX-naïve patients with active RA (SELECT-EARLY study, NCT02706873) and in combination with MTX in patients with active RA who had an inadequate response to MTX (SELECT-COMPARE study, NCT02629159) [9–11]. The aim of this analysis was to evaluate the progression of structural (radiographic) joint damage over 1 year in patients enrolled in these two upadacitinib RA studies.
Methods
Study designs and patients
The primary results of the randomized, double-blind, phase 3 upadacitinib RA clinical trials, SELECT-EARLY and SELECT-COMPARE, have been previously published [9, 10]. Briefly, both trials enrolled patients with active RA (six or more swollen and six or more tender joints) and elevated high-sensitivity CRP (hs-CRP) ≥5 mg/l (upper limit of normal 2.87 mg/l). Enrolled patients were at increased risk of radiographic progression, with one or more erosion on hand and feet radiographs or positivity to both anti-CCP antibodies and RF in SELECT-EARLY [10] and the presence of at least one of the following features at baseline in SELECT-COMPARE: three or more erosions on hand and feet radiographs or one or more erosion and anti-CCP or RF positivity [9].
In SELECT-EARLY, patients who were MTX-naïve or had received three or fewer weekly lifetime doses of MTX and had completed a 4-week washout period before receiving the first dose of study drug were randomized to blinded upadacitinib 15 mg or 30 mg once daily (QD) monotherapy or MTX weekly monotherapy starting at 10 mg/week (7.5 mg/week for patients in China and Japan) and titrated up to a maximum of 20 mg/week (15 mg/week for patients in Japan) through week 8, as tolerated. The MTX dose increment was 5 mg/4 weeks with a minimum of 15 mg/week as the final dose if intolerance of 20 mg/week was documented. Optimization of background therapy (NSAIDs, low-potency analgesics, low-dose glucocorticoids but not DMARDs) was permitted for patients who did not achieve at least 20% improvement from baseline in both tender and swollen joint counts at two consecutive visits starting at week 12. Blinded addition of MTX or upadacitinib (i.e. combination study drug rescue) was required starting at week 26 for patients not meeting clinical remission by the Clinical Disease Activity Index (CDAI; defined as CDAI ≤2.8) and who did not achieve at least 20% improvement in both swollen and tender joint counts.
In SELECT-COMPARE, patients with an inadequate response to ≥3 months of MTX [stable dose 15–25 mg/week or ≥10 mg/week in patients who could not tolerate ≥15 mg/week for ≥4 weeks (MTX-IR)] were randomized to blinded placebo, upadacitinib 15 mg QD or adalimumab 40 mg every other week in addition to stable background MTX. Blinded rescue treatment from placebo and adalimumab to upadacitinib and upadacitinib to adalimumab occurred at weeks 14, 18 or 22 for patients who did not achieve at least 20% improvement from baseline in both tender and swollen joints (‘non-responders’). All patients initially randomized to placebo were switched to upadacitinib by week 26. At week 26, all remaining patients not meeting low disease activity (CDAI score ≤10) receiving adalimumab were rescued to upadacitinib, while those receiving upadacitinib were rescued to adalimumab in a blinded fashion.
All patients provided written informed consent and both studies were conducted in accordance with International Council for Harmonisation guidelines, applicable regulations and guidelines governing clinical study conduct and the ethical principles of the Declaration of Helsinki. Each study and all study-related documents were approved by independent ethics committees or institutional review boards (Supplementary Tables S1 and S2, available at Rheumatology online).
Assessment of radiographic progression
Bilateral radiographs of hands and feet acquired using side-verifying positioning frames were read in a dedicated session up to week 24/26 (reading session 1) and at the end of 1 year (reading session 2) from the baseline, week 24 (6 months) and week 48 (1 year) visits in SELECT-EARLY and baseline, week 14 (for non-responders only), week 26 (6 months) and week 48 (1 year) in SELECT-COMPARE. Radiographs from all visits for a patient were read simultaneously but in random order at the central imaging laboratory by two independent primary radiologists blinded to visit dates, treatment assignments and any clinical information and by a third independent assessor in cases requiring adjudication. The mean changes from baseline in van der Heijde modified total Sharp score (mTSS) [12, 13], joint-space narrowing score and erosion score at 6 months (week 24/26 radiographs) and 1 year (week 48 radiographs) were determined in both studies. In addition, the proportion of patients with no radiographic progression (change in mTSS ≤0) at 6 months and 1 year were determined in both studies.
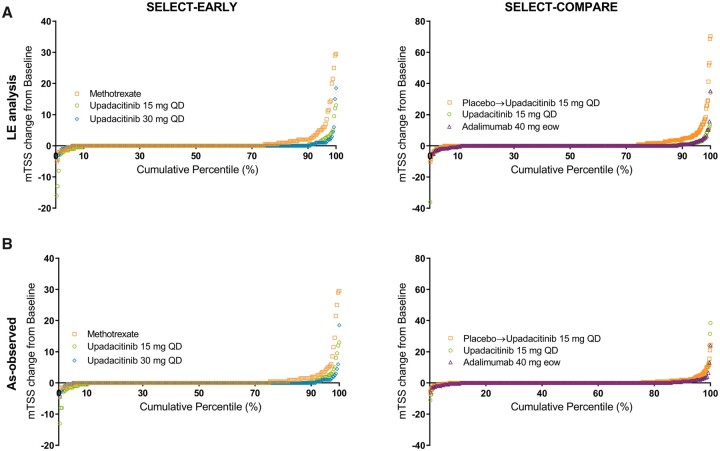
Cumulative probability plots were generated to depict the mean change in mTSS at week 48 on a patient level. Interreader agreement was assessed by the intraclass correlation coefficient for mTSS, joint-space narrowing and erosion scores.
To further assess whether there is a difference among patients with highly aggressive disease, the top 10% of radiographic progressors in each treatment arm were assessed in both studies using linear extrapolation (LE) analysis.
Statistical analyses
One-year radiographic outcomes were prespecified per the statistical analysis plan, which was written and signed prior to data analysis. Missing data and data after combination study drug rescue (SELECT-EARLY) or treatment switching (SELECT-COMPARE) were imputed by LE. A sensitivity analysis was conducted using last observation carried forward (LOCF) for missing data and data after rescue/treatment switching. Analyses were also conducted based on as-observed data without imputation. Of note, in SELECT-COMPARE, all patients initially randomized to placebo were switched to upadacitinib by week 26 at the latest. Consequently, all week 48 data for these patients were extrapolated in LE analysis.
A subgroup analysis for non-switchers (defined as patients who continued randomized monotherapy with upadacitinib 15 or 30 mg or MTX in SELECT-EARLY or those on upadacitinib 15 mg or adalimumab who did not switch therapy in SELECT-COMPARE) was also conducted. Patients who were rescued to combination therapy in SELECT-EARLY were excluded from the subgroup analysis to show data for upadacitinib or MTX monotherapy only and patients who switched study drug in SELECT-COMPARE were excluded to show data for continuous upadacitinib and continuous adalimumab only.
The mean change from baseline in mTSS and joint-space narrowing and erosion scores were summarized by point estimate and 95% CI for each randomized treatment arm. Comparisons between treatment arms were performed using the analysis of covariance model with treatment and main stratification factor [geographic region (SELECT-EARLY) or prior biologic DMARD (SELECT-COMPARE)] as fixed factors and the corresponding baseline value as the covariate.
The proportions of patients with no progression were summarized and comparisons were performed using the Cochran–Mantel–Haenszel test, adjusting for stratification factor. The proportion of patients with progression from baseline in mTSS less than or equal to the smallest detectable change (SDC) were also summarized [SDC in mTSS at 6 months and 1 year was defined as follows: ≤1.5 and ≤1.8 (SELECT-EARLY) or ≤1.7 and ≤1.8 (SELECT-COMPARE), respectively]. The SDC in mTSS was calculated for each study from the variability in baseline to 6 months or to 1 year changes in scores assigned by the two blinded readers [14].
The number needed to treat for preventing one additional patient from experiencing progression in erosion (defined as the change from baseline to 1 year in erosion score >0) for upadacitinib 15 mg and 30 mg compared with MTX was also evaluated in SELECT-EARLY.
Results
Overall, 945 MTX-naïve patients from SELECT-EARLY (MTX, n = 314; upadacitinib 15 mg, n = 317; upadacitinib 30 mg, n = 314) and 1629 MTX-IR patients from SELECT-COMPARE (placebo, n = 651; upadacitinib 15 mg, n = 651; adalimumab, n = 327) were included in this analysis. Baseline characteristics from both studies have been published previously [9, 10]. Briefly, the mean time since RA diagnosis ranged from 2.6 to 2.9 years (median 0.5–0.6 years) in SELECT-EARLY and 8.1–8.3 years (median 5.5–5.8 years) in SELECT-COMPARE. In SELECT-EARLY, <10% (range 6.1–9.5%) of patients had received MTX prior to study start (three or fewer lifetime weekly doses); the mean MTX dose at week 24 in the MTX group was 19.2 mg. The mean MTX dose at baseline in SELECT-COMPARE ranged from 16.8 to 17.1 mg/week across the treatment arms. The majority of patients were RF and/or anti-CCP positive (>80% in both studies) and mean hs-CRP ranged from 18 to 23 mg/l.
In SELECT EARLY, no data were extrapolated at week 24 because no patient had applicable data for LE; data for ∼12–22% of patients were extrapolated at week 48 across the three treatment groups in the LE analysis (Supplementary Table S3, available at Rheumatology online). In SELECT-COMPARE, data for 38%, 14% and 21% of patients were extrapolated in the placebo, upadacitinib and adalimumab groups, respectively, at week 26 in the LE analysis, whereas data for all patients receiving placebo and about half of patients receiving upadacitinib (44%) and adalimumab (57%) were extrapolated at week 48 (Supplementary Table S3, available at Rheumatology online).
Radiographic progression
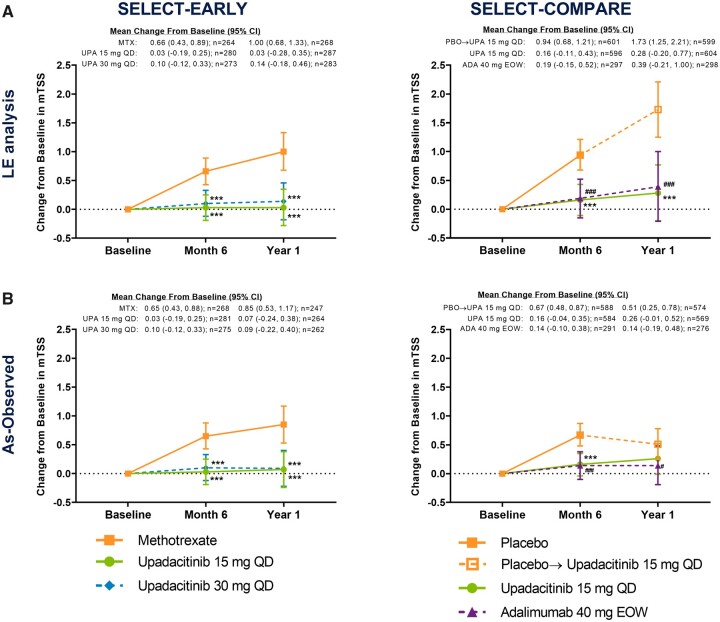
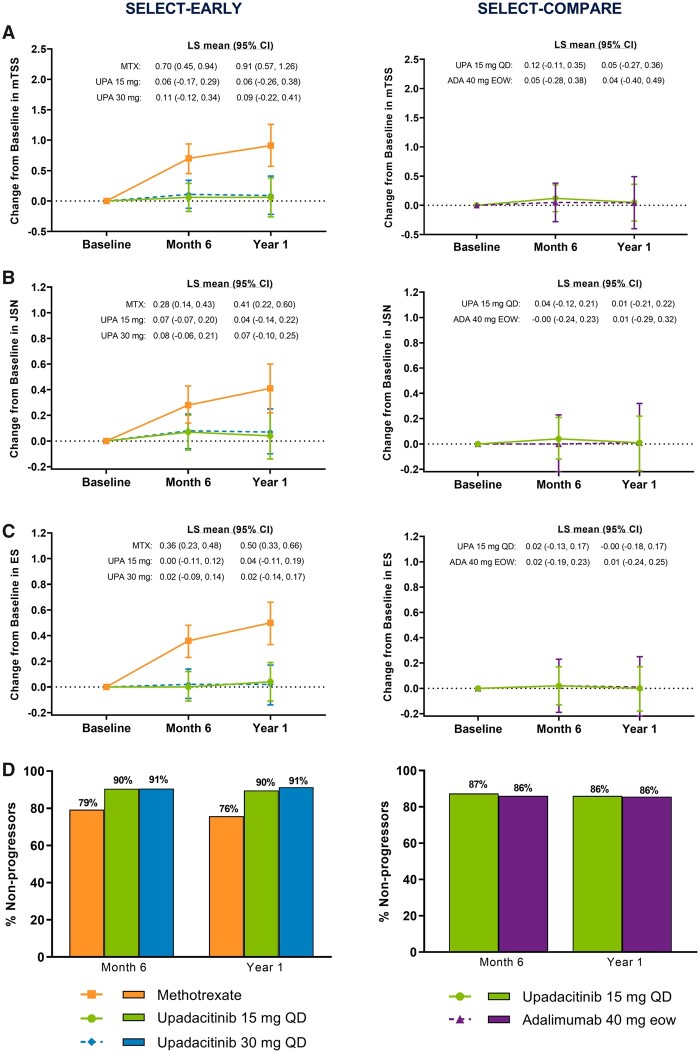
Upadacitinib treatment was associated with inhibition of progression of structural joint damage over 1 year in SELECT-EARLY. The mean changes from baseline in mTSS were 0.03 for upadacitinib 15 mg, 0.10 for upadacitinib 30 mg and 0.66 for MTX at 6 months and 0.03, 0.14 and 1.00, respectively, at 1 year based on LE analysis [treatment difference at 6 months: upadacitinib 15 mg vs MTX −0.63 (95% CI −0.94, −0.32) and upadacitinib 30 mg vs MTX −0.56 (−0.87, −0.24); at 1 year: upadacitinib 15 mg vs MTX −0.97 (95% CI −1.42, −0.53) and upadacitinib 30 mg vs MTX −0.87 (−1.31, −0.42); all P < 0.001; Fig. 1A]. Results were similar in the LOCF analysis, with P < 0.001 for all treatment comparisons (Supplementary Fig. S1, available at Rheumatology online). Similar and statistically significant results were also demonstrated in the as-observed analysis (Fig. 1B).
Fig. 1.
Change from baseline in mTSS in the (A) LE and (B) as-observed analyses over 1 year
In SELECT-COMPARE, at week 26 (6-month visit), patients receiving placebo were switched to upadacitinib. The month 6 analysis was conducted at week 24 in SELECT-EARLY and week 26 in SELECT-COMPARE; the year 1 analysis was conducted at week 48 in both studies. eow, every other week; PBO, placebo; UPA, upadacitinib. ***P < 0.001 upadacitinib vs MTX or placebo. ###P < 0.001 and #P < 0.05 adalimumab vs placebo.
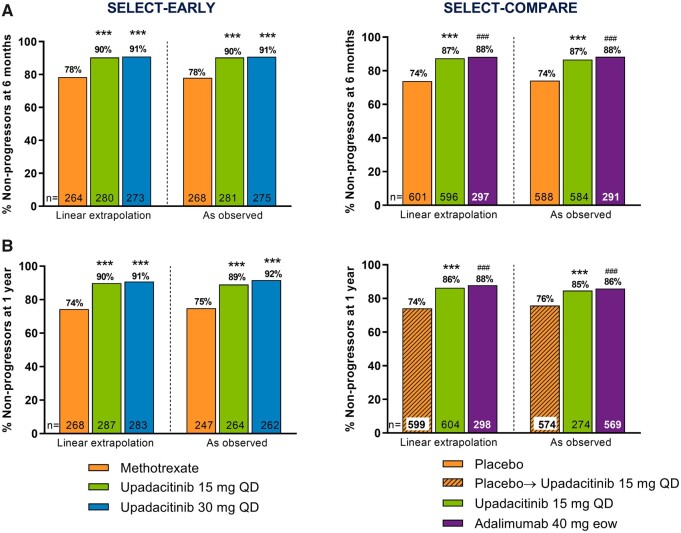
Significantly higher proportions of patients receiving upadacitinib 15 mg and 30 mg monotherapy did not experience radiographic progression (change from baseline in mTSS ≤0) compared with MTX monotherapy at 6 months and 1 year in the LE and as-observed analyses (all P < 0.001; Fig. 2). The cumulative probability of change from baseline to year 1 in mTSS was in accordance with these data (Fig. 3) and significantly more patients receiving upadacitinib showed radiographic progression less than or equal to SDC in mTSS at 6 months and 1 year vs MTX (Table 1).
Fig. 2.
Percentage of non-progressors at (A) 6 months and (B) 1 year
LE and as-observed analyses. Non-progressor defined as a patient with a change from baseline in mTSS ≤0. In SELECT-COMPARE, at week 26 (6-month visit), patients receiving placebo were switched to upadacitinib. The month 6 analysis was conducted at week 24 in SELECT-EARLY and week 26 in SELECT-COMPARE; the year 1 analysis was conducted at week 48 in both studies. eow, every other week. ***P < 0.001 upadacitinib vs MTX or placebo; ###P < 0.001 adalimumab vs placebo.
Fig. 3.
Cumulative probability plots of mean change in mTSS in the (A) LE and (B) as-observed analyses
The 1-year analysis. In SELECT-COMPARE, at week 26 (6-month visit), patients receiving placebo were switched to upadacitinib. eow: every other week.
Table 1.
The proportion of patients with progression less than or equal to the smallest detectable mTSS change
| Analysis | SELECT-EARLY, response rate (95% CI) |
SELECT-COMPARE, response rate (95% CI) |
||||
|---|---|---|---|---|---|---|
| MTX | Upadacitinib 15 mg QD | Upadacitinib 30 mg QD | Placebo | Upadacitinib 15 mg QD | Adalimumab 40 mg eow | |
| LE analysis | ||||||
| Month 6 | 90.2 (86.6, 93.7), n = 264 | 96.4 (94.3, 98.6)**, n = 280 | 97.8 (96.1, 99.5)***, n = 273 | 85.4 (82.5, 88.2), n = 601 | 96.0 (94.4, 97.6)***, n = 596 | 94.9 (92.5, 97.4), n = 297 |
| Year 1 | 86.2 (82.1, 90.3), n = 268 | 94.8 (92.2, 97.3)***, n = 287 | 96.8 (94.8, 98.9)***, n = 283 | 80.1 (76.9, 83.3), n = 599 | 93.9 (92.0, 95.8)***, n = 604 | 93.6 (90.9, 96.4), n = 299 |
| As-observed analysis | ||||||
| Month 6 | 90.3 (86.8, 93.8), n = 268 | 96.4 (94.3, 98.6)**, n = 281 | 97.8 (96.1, 99.5)***, n = 275 | 88.9 (86.4, 91.5), n = 588 | 96.2 (94.7, 97.8)***, n = 585 | 96.2 (94.0, 98.4), n = 291 |
| Year 1 | 89.9 (86.1, 93.6), n = 247 | 94.3 (91.6, 97.1), n = 265 | 97.3 (95.4, 99.3)***, n = 262 | 89.4 (86.9, 91.9), n = 575 | 94.6 (92.7, 96.4)**, n = 571 | 94.6 (91.9, 97.2), n = 276 |
Smallest detectable change defined as change from baseline in mTSS ≤1.5 and ≤1.8 (SELECT-EARLY) or ≤1.7 and ≤1.8 (SELECT-COMPARE) at 6 months and 1 year, respectively.
eow, every other week.
P < 0.001, **P < 0.01 upadacitinib vs MTX or placebo.
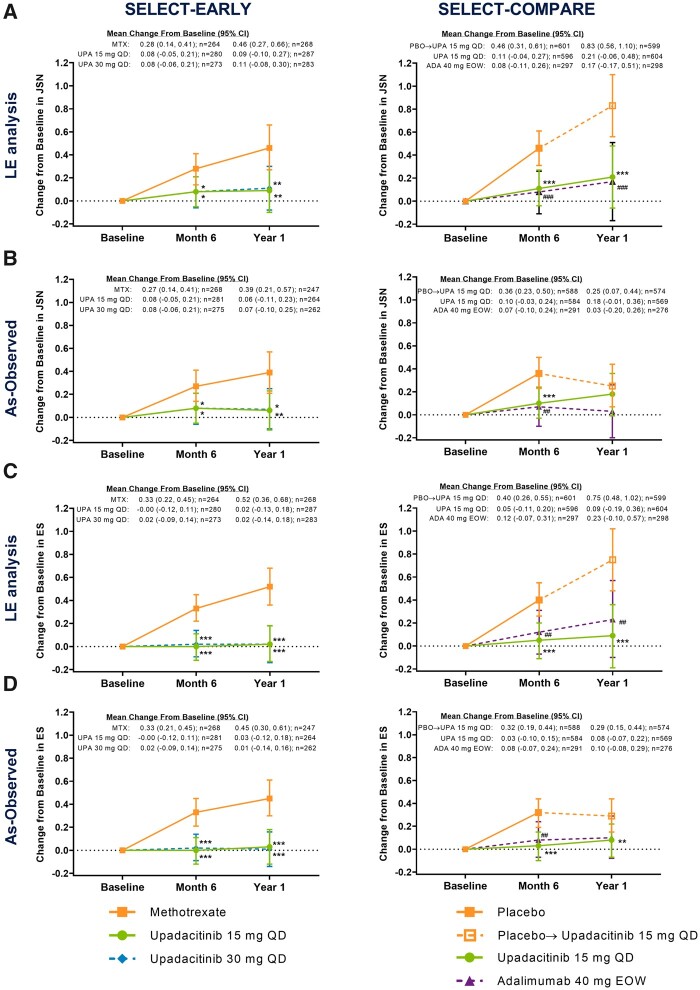
Similar results were observed in joint-space narrowing and erosion scores, both of which were significantly reduced from baseline with upadacitinib 15 mg and 30 mg monotherapy vs MTX monotherapy at 6 months and 1 year in the LE, LOCF and as-observed analyses (all P ≤ 0.05; Fig. 4, Supplementary Fig. S1, available at Rheumatology online).
Fig. 4.
Change from baseline in (A, B) joint-space narrowing and (C, D) erosion scores
The (A and C) LE and (B and D) as-observed analyses. In SELECT-COMPARE, at week 26 (6-month visit), patients receiving placebo were switched to upadacitinib. The month 6 analysis was conducted at week 24 in SELECT-EARLY and week 26 in SELECT-COMPARE; the year 1 analysis was conducted at week 48 in both studies. eow: every other week; ES: erosion score; JSN: joint-space narrowing score; PBO: placebo; UPA: upadacitinib. ***P < 0.001, **P < 0.01, *P < 0.05 upadacitinib vs MTX or placebo. ###P < 0.001 and ##P < 0.01 adalimumab vs placebo.
Upadacitinib treatment was also associated with inhibition of progression of structural joint damage over 1 year among MTX-IR patients from SELECT-COMPARE. The mean changes from baseline in mTSS were 0.16 for upadacitinib 15 mg QD (+ background MTX) and 0.94 for placebo (+ background MTX) at 6 months and 0.28 and 1.73, respectively, at 1 year based on LE analysis [treatment difference −0.79 (95% CI −1.07, −0.51) at 6 months and −1.44 (−1.95, −0.93) at 1 year; all P < 0.001; Fig. 1A]. Similar and statistically significant results were also shown in the LOCF analysis (Supplementary Fig. S1, available at Rheumatology online).
Significantly higher proportions of patients in the upadacitinib 15 mg group did not experience radiographic progression compared with the placebo group at 6 months and 1 year in both the LE and as-observed analyses (all P < 0.001; Fig. 2). The cumulative probability of change from baseline to year 1 in mTSS was in accordance with these data (Fig. 3) and significantly more patients in the upadacitinib 15 mg group also showed changes from baseline in mTSS less than or equal to SDC at 6 months and 1 year vs the placebo group (Table 1).
Furthermore, significantly reduced progression of joint-space narrowing and erosion scores with upadacitinib 15 mg QD (+ background MTX) vs placebo (+ background MTX) were observed at 6 months [treatment difference −0.34 (95% CI −0.50, −0.18) and −0.36 (−0.52, −0.20); both P < 0.001] and 1 year [−0.62 (−0.91, −0.34) and −0.66 (−0.95, −0.37), respectively; both P < 0.001; Fig. 4A and C]. Similar and statistically significant results were also shown in the LOCF analysis (Supplementary Fig. S1, available at Rheumatology online). Consistently significant results were seen in the as-observed analysis at month 6; progression of radiographic damage slowed in the placebo group after all patients switched to upadacitinib at month 6 (Figs 1B, 4B and D).
The results observed with adalimumab 40 mg every other week vs placebo (+ background MTX) were overall in line with those observed with upadacitinib 15 mg QD vs placebo (+ background MTX) at 6 months and 1 year (Figs 1–4, Supplementary Fig. S1, available at Rheumatology online).
Excellent agreement between the two primary readers was observed in both studies; the cross-sectional interreader reliability across mTSS and joint-space narrowing and erosion scores and across the time points varied from 0.91 to 0.97 among MTX-naïve patients in SELECT-EARLY and 0.92 to 0.97 in MTX-IR patients in SELECT-COMPARE.
In the subgroup analysis that excluded patients rescued to combination therapy, the results were similar among patients who remained on MTX or upadacitinib monotherapy compared with the overall population at 6 months and 1 year in MTX-naïve patients from SELECT-EARLY (Fig. 5). In SELECT-COMPARE, the progression rate at 1 year was low overall for patients who remained on continuous upadacitinib and adalimumab without therapy switch (Fig. 5). In general, when compared with results at 6 months, patients who were rescued or switched to upadacitinib in either study generally experienced limited further radiographic progression at 1 year (Supplementary Table S4, available at Rheumatology online).
Fig. 5.
Change in (A) mTSS, (B) joint-space narrowing, (C) erosion score and (D) percentage of non-progressors
Analyses among non-switchers in the SELECT-EARLY and SELECT COMPARE (as-observed analysis). SELECT-EARLY at 6 months: MTX, n = 232; upadacitinib 15 mg, n = 263; upadacitinib 30 mg, n = 266; and at 1 year: MTX, n = 215; upadacitinib 15 mg, n = 249; upadacitinib 30 mg, n = 255. SELECT-COMPARE at 6 months: upadacitinib 15 mg, n = 348; adalimumab 40 mg, n = 136; and at 1 year: upadacitinib 15 mg, n = 342; adalimumab 40 mg, n = 132. Non-switchers were patients who remained on randomized therapy. eow: every other week; ES: erosion score; JSN: joint-space narrowing score; PBO: placebo; UPA: upadacitinib.
Among the top 10% of radiographic progressors in each treatment arm, the mean change from baseline to year 1 in mTSS was higher in the MTX group [8.27 (95% CI 5.31, 11.23)] vs upadacitinib 15 mg QD [2.86 (1.61, 4.11)] and upadacitinib 30 mg QD [2.71 (0.95, 4.47); based on LE analysis] groups in SELECT-EARLY (Supplementary Fig. S2, available at Rheumatology online) and in the placebo group [12.98 (9.42, 16.53)] vs upadacitinib 15 mg QD [3.70 (2.48, 4.92)] and adalimumab 40 mg every other week [4.31 (2.03, 6.59); based on LE analysis] groups in SELECT-COMPARE (Supplementary Fig. S3, available at Rheumatology online). In MTX-naïve RA, the number needed to treat to prevent one additional patient from developing erosion progression over that expected with MTX was 6.7 for upadacitinib 15 mg and 6.6 for upadacitinib 30 mg (based on LE; Supplementary Table S5, available at Rheumatology online).
Discussion
This analysis of the SELECT-EARLY and SELECT-COMPARE studies demonstrated that treatment with upadacitinib monotherapy (15 or 30 mg QD) or combination therapy with background MTX (15 mg QD) was associated with significantly reduced progression of structural joint damage assessed by mTSS through 1 year in patients with active RA who were either MTX-naïve or had an inadequate response to MTX. The results for the LE and as-observed analyses were consistent, as were change scores for overall mTSS and its joint-space narrowing and erosion score components. Baseline and 6-month radiographs were reread as part of the scoring of the year 1 data, and consistency between reading sessions was demonstrated [9–11]. In line with the main radiographic findings of the two studies, most of the top 10% of radiographic progressors were from the control groups (MTX or placebo). In addition, a significantly higher proportion of patients receiving upadacitinib showed no radiographic progression compared with MTX or placebo, consistent with the mTSS change scores. Progression was also low in the subgroups of patients who received continuous upadacitinib therapy or received upadacitinib rescue therapy after switch/rescue. Our findings also demonstrated that patients originally randomized to placebo and then switched to upadacitinib 15 mg QD at or prior to 6 months had more structural progression compared with patients receiving upadacitinib 15 mg QD from baseline, indicating that early intervention with upadacitinib is more beneficial over the long term. However, progression of radiographic damage slowed once patients receiving placebo switched to active treatment with upadacitinib. This is in agreement with previous studies that have demonstrated that earlier disease control is associated with reduced radiographic progression in patients with RA [15–17].
Inhibition of radiographic progression has also been observed in other JAK inhibitor studies; however, results have not always been consistent between use as monotherapy or in combination with MTX [18–25]. The results in the SELECT-EARLY study showed consistent structural benefit vs MTX with both upadacitinib doses (15 mg and 30 mg QD) in an MTX-naïve population. In addition, in the SELECT-COMPARE study, upadacitinib 15 mg QD (+ background MTX) reduced radiographic progression vs placebo in a similar manner as adalimumab 40 mg every other week (+ background MTX) vs placebo in an MTX-IR population. The efficacy of adalimumab in inhibiting structural progression is well established [15, 26–28], and results from a long-term integrated analysis demonstrated that cumulative radiographic progression was significantly less in patients originally treated with adalimumab plus MTX compared with patients treated with MTX monotherapy alone for the first few years, highlighting the importance of early optimization of disease control [15].
The limitations of this analysis included that all patients receiving placebo in SELECT-COMPARE were rescued or switched to upadacitinib by week 26, so there was no true placebo comparator group up to 1 year. The placebo duration was limited to 6 months owing to ethical considerations. LE analysis was used to impute missing data and data after rescue/treatment switch, which is based on the assumption that the rate of change in radiographic progression is linear, and being a single imputation approach, does not account for uncertainty in the imputed values, which may lead to underestimation of the standard errors. Of note, LE is considered a valid methodology to estimate progression over time and is commonly used [29], but given the limitations of LE, it is relevant that both the conservative LOCF analysis and the as-observed data provided consistent results. Another limitation was that SELECT-COMPARE was a head-to-head study that assessed the efficacy of upadacitinib vs adalimumab for prespecified clinical endpoints but was not planned or powered to compare upadacitinib vs adalimumab for radiographic endpoints. However, although no formal comparisons were made, radiographic data observed with upadacitinib were overall in line with adalimumab data. Also, typical for radiographic progression studies in RA, only a subset of patients accounted for most of the progression in both arms, highlighting the need for biomarkers that could better identify patients likely to progress. Although the results from both studies could be confounded by their respective rescue treatments, similar outcomes were evident in the subgroup analyses of patients receiving continuous therapy (i.e. non-switchers).
The strengths of this analysis are that it involved a large population of both MTX-naïve and MTX-IR patients with active RA and characteristics known to be associated with progression. All radiographs were scored by two central readers who were blinded to radiographic sequence and all clinical information. In addition, both LE and as-observed analyses were conducted and demonstrated consistent results, with the exception of the year 1 as-observed data from SELECT-COMPARE that did not reach significance vs the placebo to upadacitinib switch group. However, this was not unexpected, given the slowing of progression following the switch from placebo to upadacitinib by week 26.
Conclusions
Upadacitinib significantly inhibited the progression of structural joint damage through 1 year in patients with active RA who were at increased risk for joint damage, both as monotherapy in MTX-naïve patients and in combination with background MTX in MTX-IR patients. Consistent results were observed with both upadacitinib doses (15 mg and 30 mg QD), in the LE and as-observed analysis, when assessing mTSS, joint-space narrowing, joint erosion scores or percentage of non-progressors. These findings provide further support that upadacitinib is an effective treatment for the management of RA.
Supplementary Material
Acknowledgement
AbbVie and the authors thank the patients, study sites and investigators who participated in this clinical study. The authors would also like to thank Mark C. Genovese for his contributions to the study. All authors had access to relevant data and participated in the drafting, review and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided by Maria Hovenden and Janet Matsuura, of ICON (North Wales, PA, USA) and was funded by AbbVie.
Funding: AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing and approval of the publication.
Disclosure statement: C.G.P. has received consulting fees from AbbVie, Acerta, Aclaris, Amgen, AstraZeneca, Bristol-Myers Squibb, Centrexion, Daiichi Sankyo, Deciphera, Five Prime Therapeutics, Genentech, Hoffmann-La Roche, Istesso, Janssen, Lilly USA, MedImmune, Merck, Novartis, Plexxikon, Pfizer, Sanofi, Salix-Santarus and Samsung; has received speakers bureau fees from Amgen; and is a founder and CEO of Spire Sciences, which provides imaging services to multiple pharmaceutical companies. V.S. has received consulting fees from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celltrion, Celgene, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sanofi and UCB. S.H. has received research grants and consultancy fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Pfizer, UCB and Novartis. E.M. has received research grants and speakers bureau fees from AbbVie, Bristol-Myers Squibb, Roche, Eli Lilly, Novartis, Janssen and Pfizer. P.D. has received fees for participation in speakers bureaus for Bristol-Myers Squibb, Celltrion, Galapagos and Eli Lilly. X.B. has received fees for participation in speakers bureaus from AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, MSD, Novartis, Pfizer, Roche and UCB. I.-H.S., A.F., T.S., Y.L. and S.C. are full-time employees of AbbVie and own AbbVie stock or stock options. J.V.E. is a former employee of AbbVie and may own AbbVie stock or stock options.
Contributor Information
Charles G Peterfy, Spire Sciences, Boca Raton, FL.
Vibeke Strand, Division of Immunology and Rheumatology, Stanford University, Palo Alto, CA.
Alan Friedman, AbbVie, North Chicago, IL, USA.
Stephen Hall, Cabrini Health and Emeritus Research, Monash University, Malvern, VIC, Australia.
Eduardo Mysler, Department of Rheumatology, Organización Médica de Investigación, Buenos Aires, Argentina.
Patrick Durez, Rheumatology, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Institut de Recherche Expérimentale et Clinique, Brussels, Belgium.
Xenofon Baraliakos, Rheumatology, Herne and Ruhr-University Bochum, Herne, Germany.
Jeffrey V Enejosa, AbbVie, North Chicago, IL, USA.
Tim Shaw, AbbVie, North Chicago, IL, USA.
Yihan Li, AbbVie, North Chicago, IL, USA.
Su Chen, AbbVie, North Chicago, IL, USA.
In-Ho Song, AbbVie, North Chicago, IL, USA.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Wolfe F, Sharp JT.. Radiographic outcome of recent-onset rheumatoid arthritis: a 19-year study of radiographic progression. Arthritis Rheum 1998;41:1571–82. [DOI] [PubMed] [Google Scholar]
- 2. Sokka T. Long-term outcomes of rheumatoid arthritis. Curr Opin Rheumatol 2009;21:284–90. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 4. Combe B, Lula S, Boone C, Durez P.. Effects of biologic disease-modifying anti-rheumatic drugs on the radiographic progression of rheumatoid arthritis: a systematic literature review. Clin Exp Rheumatol 2018;36:658–67. [PubMed] [Google Scholar]
- 5. Murray E, Ellis A, Butylkova Y. et al. Systematic review and network meta-analysis: effect of biologics on radiographic progression in rheumatoid arthritis. J Comp Eff Res 2018;7:959–74. [DOI] [PubMed] [Google Scholar]
- 6. Parmentier JM, Voss J, Graff C. et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol 2018;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RINVOQ (upadacitinib). Full prescribing information. North Chicago, IL: AbbVie, 2019. [Google Scholar]
- 8.RINVOQ (upadacitinib). Summary of product characteristics. Weisbaden, Germany: AbbVie Deutschland, 2019. [Google Scholar]
- 9. Fleischmann R, Pangan AL, Song IH. et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. [DOI] [PubMed] [Google Scholar]
- 10. Vollenhoven R, Takeuchi T, Pangan AL. et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately to severely active rheumatoid arthritis (SELECT-EARLY): a randomized, double-blind, active-comparator, multi-center, multi-country trial. Arthritis Rheumatol 2020;72:1607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleischmann RM, Genovese MC, Enejosa JV. et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis 2019;78:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Heijde DM, van Leeuwen MA, van Riel PL. et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum 1992;35:26–34. [DOI] [PubMed] [Google Scholar]
- 13. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 1999;26:743–5. [PubMed] [Google Scholar]
- 14. Bruynesteyn K, Boers M, Kostense P, van der Linden S, van der Heijde D.. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis 2005;64:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landewe R, van Tubergen A.. Clinical tools to assess and monitor spondyloarthritis. Curr Rheumatol Rep 2015;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Nies JA, Krabben A, Schoones JW. et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014;73:861–70. [DOI] [PubMed] [Google Scholar]
- 17. Kyburz D, Gabay C, Michel BA, Finckh A; for the physicians of the SCQM-RA. The long-term impact of early treatment of rheumatoid arthritis on radiographic progression: a population-based cohort study. Rheumatology (Oxford) 2011;50:1106–10. [DOI] [PubMed] [Google Scholar]
- 18. van der Heijde D, Dougados M, Chen YC. et al. Effects of baricitinib on radiographic progression of structural joint damage at 1 year in patients with rheumatoid arthritis and an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open 2018;4:e000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Heijde D, Schiff M, Tanaka Y. et al. Low rates of radiographic progression of structural joint damage over 2 years of baricitinib treatment in patients with rheumatoid arthritis. RMD Open 2019;5:e000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Westhovens R, Rigby W, van der Heijde D. et al. SAT0158 Efficacy and safety of filgotinib in methotrexate-naïve patients with rheumatoid arthritis: FINCH 3 52-week results. Ann Rheum Dis 2020;79:1019–20. [Google Scholar]
- 21. Combe B, Kivitz A, Tanaka Y. et al. Efficacy and safety of filgotinib for patients with rheumatoid arthritis with inadequate response to methotrexate: FINCH1 primary outcome results. Arthritis Rheumatol 2019;71(Suppl 10):abstract 506. [Google Scholar]
- 22. Fleischmann R, Schiff M, van der Heijde D. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Heijde D, Tanaka Y, Fleischmann R. et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 24. Lee EB, Fleischmann R, Hall S. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 25. Westhovens R, Taylor PC, Alten R. et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis 2017;76:998–1008. [DOI] [PubMed] [Google Scholar]
- 26. Keystone EC, Kavanaugh AF, Sharp JT. et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11. [DOI] [PubMed] [Google Scholar]
- 27. Breedveld FC, Weisman MH, Kavanaugh AF. et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. [DOI] [PubMed] [Google Scholar]
- 28. Kavanaugh A, Fleischmann RM, Emery P. et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Markusse IM, Landewe R, Wolterbeek R. et al. Linear extrapolation of missing radiographic change scores in clinical trials does not spuriously overestimate group radiographic changes in rheumatoid arthritis. Rheumatology (Oxford) 2016;55:1295–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.