Abstract
Objectives
Paternal preconception health is recognized as an important contributor to pregnancy outcomes. Nonetheless, pregnancy outcomes of partners of men with inflammatory arthritis (IA) have never been studied. Our objective was to describe the pregnancy outcomes of partners of men diagnosed with IA.
Methods
We performed a multicentre cross-sectional retrospective study conducted in the Netherlands. Men with IA who were over 40 years old that reported at least one positive pregnancy test were included. To analyse the impact of IA on pregnancy outcomes, pregnancies were classified into two groups: pregnancies conceived after the diagnosis of IA and before the diagnosis of IA.
Results
In total, 408 male participants diagnosed with IA reported 897 singleton pregnancies that resulted in 794 live births. Pregnancies conceived after the diagnosis of IA had higher rate of miscarriage (12.27 vs 7.53%, P = <0.05). This increased risk was still present after adjusting for confounders [OR 2.03 (95% CI 1.12, 3.69) P = 0.015].
Conclusions
This is the largest study to describe the pregnancy outcomes of partners of men diagnosed with IA and the first to demonstrate that paternal IA is associated with a higher risk of miscarriage. Notwithstanding, the overall rate of miscarriage reported in our study could be comparable to previously reported population estimates.
Keywords: inflammatory arthritis, rheumatoid arthritis, spondyloarthritis, pregnancy outcomes
Rheumatology key messages.
Poor preconception paternal health has been associated with a higher risk of pregnancy loss.
Paternal inflammatory arthritis was associated with an increased risk of miscarriage.
Research to understand how paternal preconception inflammatory arthritis impacts pregnancy outcomes is urgently needed.
Introduction
Paternal preconception health is recognized as an important contributor to pregnancy outcomes [1]. It has been shown that increased abnormalities in sperm DNA [2], low sperm quality [3], oxidative stress [4] and epigenetic changes in sperm [5] are potential mechanisms that could lead to worse pregnancy outcomes. Furthermore, it has been suggested that pregnancies conceived by men diagnosed with chronic diseases are at higher risk of ending in losses (miscarriage, ectopic pregnancy or stillbirth) [6]. However, the impact of paternal inflammatory arthritis (IA) on pregnancy outcomes has never been studied.
RA, JIA, PsA and AS are frequent causes of IA that have been associated with impaired male fertility [7, 8]. Because human reproduction failure ranges from the inability to conceive to the incapacity to maintain pregnancy after successful conception [9], it is of the utmost importance to evaluate the impact of paternal IA on pregnancy outcomes.
Therefore, our objective was to describe the pregnancy outcomes of partners of men diagnosed with IA.
Methods
Study design and patient selection
This study is part of the iFAME-Fertility study [8]. Briefly, the iFAME-Fertility study is a multicentre cross-sectional study that was primarily designed to evaluate the impact of IA on the male fertility rate (total number of children per man). Men diagnosed by their rheumatologists with IA (RA, JIA, PsA and AS), who at the time of inclusion were 40 years or older and who indicated that their ‘family size’ was completed participated in the study. Participants completed a self-reported questionnaire that included demographic, medical history, family planning and fertility related questions. Additionally, participants who reported at least one pregnancy (‘any positive pregnancy test, even if it did not result in a live born child’) completed a different questionnaire that included questions regarding pregnancy outcomes.
To evaluate the impact of paternal IA on pregnancy outcomes, we classified pregnancies into two groups: pregnancies conceived after the diagnosis of IA and before the diagnosis of IA.
Data collection
A self-reported questionnaire developed for this study was used. The design of this questionnaire was based on the ‘fertility experiences questionnaire’ [10]. A miscarriage was defined as a pregnancy loss that occurred before the 16th week of gestation and a stillbirth as a pregnancy loss at or after 16 weeks of gestation.
Statistical analysis
Comparisons between the two groups were tested. Categorical variables were presented as number (percentage), and continuous variables are reported as mean (s.d.) or median (IQR), as appropriate. Continuous variables were compared using a paired t test and Wilcoxon rank. Categorical variables were compared using χ2 tests and Fisher’s exact tests.
To control for confounders, the multivariate logistic regression model was used. All clinically considered important potential confounders [paternal age at conception, paternal and maternal smoking exposure, paternal medication preconception exposure, diagnosis of IA, conception by assisted reproductive technology (ART) and consecutive pregnancy number] were fitted into the model. The level of significance was set as a two-tailed P ≤ 0.05, and statistical analyses were completed using Stata V.15 (StataCorp-LP, College Station, Texas).
Ethics
This study was specifically reviewed and approved by the ethics review boards of all participating centres in compliance with the Declaration of Helsinki (Erasmus MC—Ethics Committee: MEC-2018–1418, Admiraal de Ruyter Hospital—Ethics Committee: ADRZ2019-010 iFAME-Fertility, Franciscus Hospital—Ethics Committee: T-110. 4, Leiden University Medical Center, Reinier de Graaf Hospital, Haga Hospital—Ethics Committee: N19.081. 5, Maasstad Hospital—Ethics Committee: L2020040). All patients gave their written informed consent.
Results
Between September 2019 and January 2021, a total of 1841 men were invited to participate. In total, 628 male participants diagnosed with IA were included in the iFAME-Fertility study. Of them, 408 men reported at least one positive pregnancy test and were included in this part of the study. In total, these men reported 897 singleton pregnancies that resulted in 794 live births.
A detailed description of the differences in pregnancy characteristics between the study groups is presented in Table 1. In line with our previous findings [8], pregnancies after IA diagnosis were characterized by a statistically significant longer time to pregnancy (TTP) [6.99 (s.d. 11.79) vs 4.83 (s.d. 8.71), P = 0.002] and by a statistically significant larger rate of pregnancies that were conceived by ART [20 (9.09%) vs 23 (3.40%), P = 0.0001]. As expected, paternal and maternal age at conception were statistically significant higher in pregnancies after IA diagnosis [34.27 (s.d. 6.08) vs 30.49 (s.d. 5.34) and 30.69 (s.d. 5.16) vs 28.45 (s.d. 4.83), respectively, both P = 0.0001]. The percentage of pregnancies with paternal exposure to anti-rheumatic drugs 3 months before conception was statistically significant higher in pregnancies after IA diagnosis [110 (50.23%) vs 40 (5.91%), P = 0.0001].
Table 1.
Pregnancy characteristics and outcomes
| All pregnancies | Pregnancy after IA diagnosis | Pregnancy before IA diagnosis | P-value | |
|---|---|---|---|---|
| Total number of pregnancies | 897 | 220 | 677 | — |
| 1st pregnancy, n (%) | 408 (45.48) | 103 (46.82) | 305 (45.05) | P = 0.87 |
| Year of pregnancy, mean (s.d.) | 1990 (12.76) | 1996 (12.96) | 1989 (12.12) | P ≤ 0.05 |
| TTP, months (s.d.) | 5.35 (9.59) | 6.99 (11.79) | 4.83 (8.71) | P ≤ 0.05 |
| Spontaneous pregnancies, n (%) | 854 (95.21) | 200 (90.91) | 654 (96.60) | P ≤ 0.05 |
| Conceived by ART, n (%) | 43 (4.79) | 20 (9.09) | 23 (3.40) | P ≤ 0.05 |
| Pregnancy duration, weeks (s.d.) | 38.31 (4.06) | 38.57 (3.22) | 38.23 (4.30) | P = 0.66 |
| Paternal demographic characteristics | ||||
| Paternal age at conception, mean (s.d.) | 31.31 (5.72) | 34.27 (6.08) | 30.49 (5.34) | P ≤ 0.05 |
| Paternal age at conception >40 years, n (%) | 78 (8.70) | 53 (24.09) | 25 (3.69) | P ≤ 0.05 |
| Paternal education higher than bachelor, n (%) | 363 (40.47) | 94 (42.73) | 269 (39.73) | P = 0.44 |
| Paternal preconception exposure a | ||||
| Paternal smoking exposure, n (%) | 344 (38.39) | 67 (30.59) | 277 (40.92) | P ≤ 0.05 |
| Paternal alcohol exposure, n (%) | 613 (68.42) | 136 (62.10) | 447 (70.46) | P = 0.07 |
| Paternal medication exposure, n (%) | 150 (16.74) | 110 (50.23) | 40 (5.91) | P ≤ 0.05 |
| Paternal diagnosis of IA | ||||
| Age at diagnosis IA, years (s.d.) | 42.99 (12.65) | 26.99 (7.85) | 47.59 (9.69) | P ≤ 0.05 |
| Diagnosis RA, n (%) | 451 (50.28) | 82 (37.27) | 369 (54.51) | P ≤ 0.05 |
| Diagnosis JIA, n (%) | 15 (1.67) | 15 (6.82) | 0 (0.00) | – |
| Diagnosis AS, n (%) | 181 (20.18) | 75 (34.09) | 106 (15.66) | P ≤ 0.05 |
| Diagnosis PsA, n (%) | 286 (31.88) | 68 (30.91) | 218 (32.20) | P = 0.72 |
| Paternal fertility evaluation outcomes a | ||||
| Fertility evaluation, n (%) | 138 (15.38) | 57 (25.91) | 81 (11.96) | P ≤ 0.05 |
| Low sperm quality, n (%) | 45 (5.02) | 20 (35.09) | 25 (30.85) | P = 0.60 |
| Maternal demographic characteristics | ||||
| Maternal age at conception, mean (s.d.) | 29.00 (5.00) | 30.69 (5.16) | 28.45 (4.83) | P ≤ 0.05 |
| Maternal age at conception >40 years, n (%) | 10 (1.11) | 5 (2.27) | 5 (0.74) | P = 0.06 |
| Maternal preconceptiona and pregnancy exposure | ||||
| Maternal preconception smoking exposure, n (%) | 171 (19.08) | 32 (14.61) | 139 (20.53) | P = 0.06 |
| Maternal smoking exposure during pregnancy, n (%) | 58 (6.47) | 13 (5.94) | 45 (6.65) | P = 0.93 |
| Maternal alcohol exposure, n (%) | 329 (36.72) | 73 (33.33) | 256 (37.81) | P = 0.31 |
| Maternal alcohol exposure during pregnancy, n (%) | 53 (5.92) | 16 (7.31) | 37 (5.47) | P = 0.45 |
| Maternal fertility evaluation outcomes b | ||||
| Fertility evaluation, n (%) | 116 (12.93) | 40 (18.18) | 76 (11.23) | P ≤ 0.05 |
| P = 0.07 | ||||
| Female infertility secondary to known cause,bn (%) | 37 (31.90) | 19 (47.50) | 18 (57.89) | P ≤ 0.05 |
| Female infertility secondary to unknown cause, n (%) | 11 (9.48) | 6 (15.00) | 5 (6.58) | P ≤ 0.05 |
| Pregnancy outcomes | ||||
| Live births, n (%) | 794 (88.52) | 190 (86.36) | 604 (89.22) | P = 0.05 |
| Miscarriage, n (%) | 78 (8.70) | 27 (12.27) | 51 (7.53) | P ≤ 0.05 |
| Induced abortion, n (%) Medical indication Personal reasons |
25 (2.78) 5 (20.00) 20 (80.00) |
3 (1.36) 0 (0.00) 3 (100.00) |
22 (3.25) 5 (22.73) 17 (77.27) |
P = 0.13 |
| Stillbirths, n (%) | 6 (0.67) | 0 (0.00) | 6 (0.89) | P = 0.16 |
| Pregnancy outcomes related to maternal and neonatal morbidity c | ||||
| Pre-term birth, n (%) | 149 (16.61) | 31 (14.09) | 118 (17.43) | P = 0.24 |
| Hypertensive disorders (hypertension, pre/eclampsia), n (%) | 41 (4.57) | 8 (3.64) | 33 (4.87) | P = 0.45 |
| Gestational diabetes mellitus, n (%) | 11 (1.28) | 2 (0.94) | 9 (1.38) | P = 0.62 |
| Intrauterine growth restriction, n (%) | 12 (1.34) | 1 (0.45) | 11 (1.65) | P = 0.19 |
| Maternal anaemia, n (%) | 15 (1.67) | 6 (2.73) | 9 (1.33) | P = 0.16 |
3 months before conception.
Endometriosis, fallopian tube obstruction, polycystic ovary syndrome, uterine abnormality, early menopause.
Maternal and neonatal morbidity reported for pregnancies ≥ 16 weeks of gestation (n = 806).
ART: assisted reproductive technology; IA: inflammatory arthritis; TTP: time to pregnancy.
Pregnancy outcomes
The rate of miscarriage was statistically significant higher in pregnancies after IA diagnosis [27 (12.27%)] compared with pregnancies before IA diagnosis [51 (7.53%), P = 0.030] (see Table 1). The rate of live births was lower in pregnancies before IA diagnosis, but this difference was not statistically significant when compared with pregnancies after IA diagnosis [190 (86.36%) vs 604 (89.22%), P = 0.053]. The rates of induced abortions, stillbirths and pre-term births were statistically similar between the two groups.
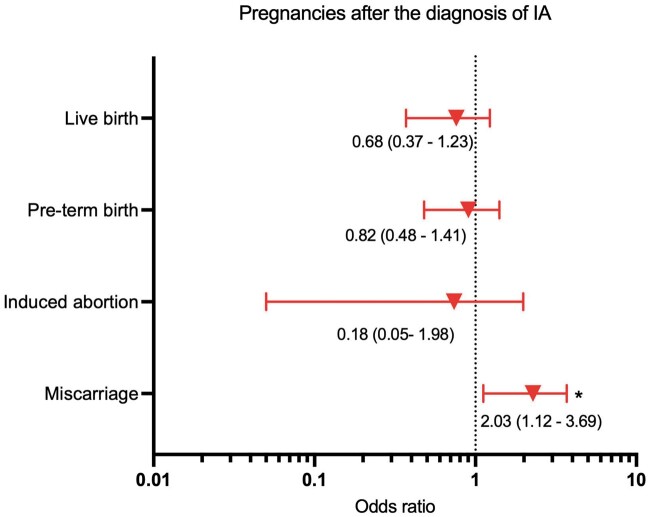
After adjusting for confounders, compared with pregnancies before IA diagnosis, the difference remained significant OR miscarriage in pregnancies after IA diagnosis [2.03 (95% CI 1.12, 3.69)] (see Fig. 1). Furthermore, a subgroup analysis based on the specific diagnosis of IA (RA, AS, PsA) revealed that the adjusted OR for a miscarriage was highest in pregnancies after the diagnosis of PsA and RA [4.35 (95% CI 1.65, 11.49) and 2.96 (95% CI 1.19, 7.36), respectively] (see Supplementary Fig. S1, available at Rheumatology online).
Fig. 1.
Adjusted OR (point estimate and 95% CI) for pregnancy outcomes with paternal IA exposure
Multivariate logistic regression models adjusted for confounders (paternal age at conception, paternal and maternal smoking exposure, paternal medication preconception exposure, diagnosis of IA, conception by ART, year of pregnancy and consecutive pregnancy number). Because of a high degree of correlation between paternal and maternal age at conception (r = 0.70), only paternal age at conception was included in the model. *Statistically significant, P < 0.05.
The rates of pregnancy outcomes related to maternal and neonatal comorbidity were calculated for all pregnancies with a gestational age ≥16 weeks (n = 806) and were not statistically significant different between our groups (see Table 1).
Discussion
Consistent with findings that have suggested that impaired preconception paternal health is associated with adverse pregnancy outcomes [6], our study is the first of its kind to demonstrate that paternal IA is significantly associated with a higher risk of miscarriage. Notably, this was independent of traditional risk factors for miscarriage such as advanced paternal and maternal age. The risk of miscarriage was highest in pregnancies after the diagnosis of PsA and RA.
The mechanism responsible for an increased risk of miscarriage in pregnancies after IA diagnosis is complex and probably multifactorial. First, abnormal sperm DNA is considered as one of the most important paternal factors associated with pregnancy loss [11]. In this regard, it has been shown that systemic inflammation and certain andrological comorbidities frequently present in men with IA (i.e. hypogonadism or varicocele) [7] have been associated with abnormal sperm DNA and low sperm quality [12, 13].
Second, because drug exposure has also been associated with abnormal sperm DNA and low sperm quality [14], paternal preconception exposure to anti-rheumatic drugs warrants discussion. Based on limited paternal exposure data, it has been concluded that paternal anti-rheumatic drug exposure is not associated with an increased risk for adverse pregnancy outcomes [15, 16]. Nonetheless, pregnancy loss and specifically miscarriage were not assessed as a pregnancy outcome.
Third, it has been shown that poor paternal health can negatively impact pregnancy outcomes [6]. Epigenetic changes in sperm can impact reproductive health [17] and were suggested as a potential cause of this association. Although epigenetic changes in several immune cells have been identified as important mechanisms associated with the pathogenesis and progression of RA [18], the occurrence of epigenetic changes on spermatozoa of men diagnosed with IA has not been studied before.
Our study is the first large study (897 pregnancies) to evaluate the impact of paternal IA exposure on pregnancy outcomes, including miscarriage. In addition, we used an extensive questionnaire to gain insight into most of the factors that might have influenced our results. Nonetheless, our study has important limitations. First, the study was not primarily designed to evaluate pregnancy outcomes. Second, men who experience negative pregnancy outcomes might be more willing to participate in these types of studies and this is a potential source of selection bias in our study. Furthermore, some pregnancies occurred >30 years ago which can lead to non-differential misclassification bias. For this same reason, and to minimize the risk of misclassification bias regarding paternal preconception anti-rheumatic drug exposure, we did not collect information on specific preconception anti-rheumatic drug exposure. Therefore, the potential impact of specific paternal pharmacological therapy on pregnancy outcomes was not assessed in our study. Third, although some of the most important maternal risk factors for miscarriage were taken into account in our analysis (maternal age, smoking and alcohol exposure), our study lacks information on other maternal factors such as anatomical abnormalities and relevant comorbidities. Lastly, our subgroup analysis revealed that the OR for miscarriage was different among the different diagnosis of IA. Although this finding is relevant for future research and for the clinical setting, the total number of pregnancies per diagnosis was low and these findings should be treated with caution.
Albeit these findings need to be corroborated by large prospective studies, rheumatologists should be aware that paternal IA may increase the risk of miscarriage. Although in various studies, different incidences of miscarriage have been described (dependent on the population studied and methodology used), the overall incidence of miscarriages found in our study is in line with data from a large Danish cohort that included >1 221 546 pregnancies and reported that 10.9% of clinically recognized pregnancies end in a miscarriage [19].
For specific advice and interventions on minimizing the negative impact of paternal IA on reproductive health (fertility and pregnancy outcomes), basic, translational and epidemiological studies are urgently needed. These studies should focus on understanding how inflammation (i.e. disease activity at the time of conception), pharmacological treatment, epigenetics and other factors associated with paternal IA influence pregnancy outcomes and male reproductive health. Until more is known about the potential effect of paternal IA on pregnancy outcomes, we recommend that all men diagnosed with arthritis and a wish to conceive should receive, at a minimum, general preconception counselling [20].
In conclusion, this study shows an association between paternal preconception IA and an increased risk of miscarriage. Notwithstanding, the overall rate of miscarriage reported in our study could be comparable to previously reported population estimates. Multiple biological mechanisms can be responsible for this association and more research is urgently needed to understand how paternal preconception IA influences pregnancy outcomes and ultimately to improve the quality of care for men diagnosed with IA and a desire for fatherhood.
Supplementary Material
Acknowledgements
We extend our gratitude to Ron Buijs, data manager of the department of Rheumatology, Erasmus MC for his invaluable help with regard to technical support, data collection and data management. Additionally, we would like to thank Annemarie Kraan-Donker, Ellis Niemantsverdriet and Marieke Holtrop for their important administrative and logistical contributions to the study.
All authors met the authorship criteria, they had a substantial contribution to the conception or design of the work (L.F.P-G., E.R., H.T.W.S., J.M.W.H., B.P.K., R.J.E.M.D.) or the acquisition (L.F.P-G., E.R., R.J.E.M.D.), analysis (L.F.P-G., H.T.W.S. and R.J.E.M.D.) or interpretation of data for the work (all authors) and were involved in revising a draft of this work, gave final approval of this version to be published, and are accountable for all aspects of the work in ensuring accuracy and integrity.
Funding: This work was supported by research grants from the Dutch Arthritis Foundation (ReumaNederland) (project number: 16–3-402), The Netherlands Organization for Health Research and Development (ZonMw) (project number 849200009) and Consejo Nacional de Ciencia y Tecnologia (CONACYT) (project number 601574). All are non-profit organizations.
Disclosure statement: L.F.P-G. Consultant of: Galapagos; M.R.K. Consultant of: Novartis, Grant/research support from: Novartis Speakers bureau: Galapagos; R.J.E.M.D. Speakers bureau: UCB, Roche, Abbvie, Genzyme, Novartis, Consultant of: Galapagos, Grant/research support from: UCB.
Patient and public involvement: Patients were involved in the design of the questionnaire and the invitation letter. We carefully assessed the burden on participating patients. We intend to share the results to participating patients and will appropriately disseminate the results.
Contributor Information
Luis F Perez-Garcia, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam.
Esther Röder, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam.
Hieronymus T W Smeele, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam.
Robbert Goekoop, Department of Rheumatology, Haga Hospital, The Hague.
Johanna M W Hazes, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam.
Marc R Kok, Department of Rheumatology and Clinical Immunology, Maasstad Hospital, Rotterdam.
Ilja Tchetverikov, Department of Rheumatology, Albert Schweitzer Hospital, Dordrecht.
Annette van der Helm-van Mil, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam; Department of Rheumatology, Leiden University Medical Center, Leiden.
Jos van der Kaap, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam; Department of Rheumatology, Admiraal de Ruyter Hospital, Goes.
Petra Kok, Department of Rheumatology, Reinier de Graaf Hospital, Delft.
Bouwe P Krijthe, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam; Department of Rheumatology, Sint Franciscus Vlietland Group, Rotterdam, Netherlands.
Radboud J E M Dolhain, Department of Rheumatology, Erasmus MC, University Medical Center, Rotterdam.
Data availability statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Soubry A. POHaD: why we should study future fathers. Environ Epigenet 2018;4:dvy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon L, Murphy K, Shamsi MB. et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod 2014;29:2402–12. [DOI] [PubMed] [Google Scholar]
- 3. Lee SH, Song H, Park YS. et al. Poor sperm quality affects clinical outcomes of intracytoplasmic sperm injection in fresh and subsequent frozen-thawed cycles: potential paternal effects on pregnancy outcomes. Fertil Steril 2009;91:798–804. [DOI] [PubMed] [Google Scholar]
- 4. Wright C, Milne S, Leeson H.. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online 2014;28:684–703. [DOI] [PubMed] [Google Scholar]
- 5. Abbasi J. The paternal epigenome makes its mark. JAMA 2017;317:2049–51. [DOI] [PubMed] [Google Scholar]
- 6. Kasman AM, Zhang CA, Li S. et al. Association between preconception paternal health and pregnancy loss in the USA: an analysis of US claims data. Hum Reprod 2021;36:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez-Garcia LF, Te Winkel B, Carrizales JP. et al. Sexual function and reproduction can be impaired in men with rheumatic diseases: a systematic review. Semin Arthritis Rheum 2020;50:557–73. [DOI] [PubMed] [Google Scholar]
- 8. Perez-Garcia LF, Röder E, Goekoop RJ. et al. Impaired fertility in men diagnosed with inflammatory arthritis: results of a large multicentre study (iFAME-Fertility). Ann Rheum Dis 2021;80:1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choudhury SR, Knapp LA.. Human reproductive failure I: immunological factors. Human Reproduction Update 2001;7:113–34. [DOI] [PubMed] [Google Scholar]
- 10. Thomas FS, Stanford JB, Sanders JN. et al. Development and initial validation of a fertility experiences questionnaire. Reprod Health 2015;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson L, Gallos ID, Conner SJ. et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908–17. [DOI] [PubMed] [Google Scholar]
- 12. Finelli R, Pallotti F, Cargnelutti F. et al. Sperm DNA damage and cytokines in varicocele: a case-control study. Andrologia 2021;53:e14023. [DOI] [PubMed] [Google Scholar]
- 13. Finelli R, Leisegang K, Finocchi F. et al. The impact of autoimmune systemic inflammation and associated medications on male reproductive health in patients with chronic rheumatological, dermatological, and gastroenterological diseases: a systematic review. Am J Reprod Immunol 2021;85:e13389. [DOI] [PubMed] [Google Scholar]
- 14. Sakkas D, Alvarez JG.. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027–36. [DOI] [PubMed] [Google Scholar]
- 15. Wallenius M, Lie E, Daltveit AK. et al. Brief report: no excess risks in offspring with paternal preconception exposure to disease-modifying antirheumatic drugs. Arthritis Rheumatol 2015;67:296–301. [DOI] [PubMed] [Google Scholar]
- 16. Perez-Garcia LF, Dolhain RJEM, Vorstenbosch S. et al. The effect of paternal exposure to immunosuppressive drugs on sexual function, reproductive hormones, fertility, pregnancy and offspring outcomes: a systematic review. Hum Reprod Update 2020;26:961–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcho C, Oluwayiose OA, Pilsner JR.. The preconception environment and sperm epigenetics. Andrology 2020;8:924–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nemtsova MV, Zaletaev DV, Bure IV. et al. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Front Genet 2019;10:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M.. Maternal age and fetal loss: population based register linkage study. BMJ (Clin Res Ed) 2000;320:1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frey KA, Navarro SM, Kotelchuck M, Lu MC.. The clinical content of preconception care: preconception care for men. Am J Obstet Gynecol 2008;199:S389–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.