Abstract
BACKGROUND:
Few prognostic markers are available for patients with non-small-cell lung cancer (NSCLC) undergoing neurosurgical resection of symptomatic brain metastases.
OBJECTIVE:
We investigated whether tumor mutation status (EGFR, KRAS, ALK, ROS1, and BRAF) and treatment history were associated with survival after neurosurgery.
METHODS:
We reviewed the electronic health records of 104 patients with NSCLC with genomic profiling who underwent neurosurgical resection for symptomatic brain metastases at an academic institution between January 2000 and January 2018. We used multivariate Cox proportional hazards regression models to evaluate the association between overall survival (OS) after neurosurgery and clinicopathologic factors, including mutation status.
RESULTS:
Mean age of patients in this study was 61 (±12) years, and 44% were men. The median OS after neurosurgery was 24 months (95% confidence interval, 18–34 months). Our multivariate analysis showed that the presence of an EGFR mutation in the tumor was significantly associated with improved OS (hazard ratio [HR], 0.214; P = 0.029), independent of tyrosine kinase inhibitor use. Presence of KRAS, ALK, ROS1, and BRAF alterations was not associated with survival (all P > 0.05). Conversely, older age (HR, 1.039; P = 0.029), a history of multiple brain irradiation procedures (HR, 9.197; P < 0.001), and presence of extracranial metastasis (HR, 2.556; P = 0.016) resulted in increased risk of mortality.
CONCLUSIONS:
Patients requiring surgical resection of an epidermal growth factor receptor–mutated NSCLC brain metastasis had an associated improved survival compared with patients without this mutation, independent of tyrosine kinase inhibitor use. Decreased survival was associated with older age, multiple previous brain radiation therapies, and extracranial metastasis.
Keywords: Adenocarcinoma, Brain metastases, Brain surgery, EGFR mutation, Lung cancer
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths worldwide, accounting for >1.6 million deaths annually.1 The 5-year survival inclusive of all tumor stages remains low at 18%,2 and the development of brain metastases decreases overall survival (OS) and quality of life. Among patients with non-small-cell lung cancer (NSCLC), brain metastases are present at initial diagnosis in 7% of patients and can develop in the course of the disease in up to 30% of patients, with even higher rates observed in patients with metastatic NSCLC and driver mutations (i.e., EGFR and ALK).3,4 From both the patient’s and neurosurgeon’s perspective, it is important to understand the factors affecting prognosis when considering neurosurgical intervention for symptomatic brain metastases.
Systemic therapy for metastatic NSCLC directly depends on the presence or absence of targetable genetic driver mutations. In the United States, tyrosine kinase inhibitors (TKIs), including osimertinib, erlotinib, gefitinib, and afatinib, are Food and Drug Administration (FDA)–approved first-line therapies for epidermal growth factor receptor (EGFR)-mutated NSCLC.5–11 Similarly, crizotinib, ceritinib, and alectinib are approved by the Food and Drug Administration for first-line treatment of anaplastic lymphoma kinase (ALK)-rearranged NSCLC.12–14 The advent of these targeted therapies has resulted in significant improvements in progression-free survival and tumor response rates, with many also showing intracranial activity.12,15–17 There have been studies evaluating the prognostic role of mutation status in patients with NSCLC with brain metastasis.18–23 There have also been studies examining the usefulness of radiation for brain metastasis in patients with known targetable driver mutations (i.e., EGFR), given the availability of systemic therapies that have the potential to provide intracranial disease control.24,25 However, to our knowledge, there have been no studies specifically examining the prognostic role of mutation status in patients treated with neurosurgical resection.
Given that treatment and prognosis both rely on the mutational profile for metastatic lung cancer, our primary objective was to investigate how mutational status affects prognosis in patients undergoing neurosurgical resection for symptomatic brain metastases. In our retrospective cohort study, we evaluated the association of clinicopathologic factors, with a particular focus on NSCLC mutational status, and OS in patients with symptomatic brain metastasis requiring neurosurgery.
METHODS
Patient and Tumor Characteristics
Our cohort included 104 patients with NSCLC who underwent surgical resection for symptomatic brain metastasis at an academic institution from January 2000 to January 2018. Patient information was accessed through our institutional bioinformatics platform, which integrates clinical data from the electronic medical records. The study was approved by the institutional review board of our institution. Individual patient consent was not required in this study because it was retrospective.
We queried clinical and pathologic factors including patient demographics (age, sex, and race), lung cancer diagnosis (histology, staging at diagnosis, and any evidence of active systemic disease), treatment history (previous lung resection, lines of therapy, and radiation), nature of brain metastasis (number, location and size of brain metastases, and time from identification of brain metastasis to neurosurgery), and mutation status (EGFR, KRAS, ALK/ROS1 rearrangement, and BRAF). Brain metastasis was considered synchronous if it was diagnosed at the same time as the initial lung cancer diagnosis, or metachronous if it was diagnosed >2 months after the initial lung cancer diagnosis.26,27 We calculated recursive partitioning analysis (RPA) class and graded prognostic assessment (GPA) scores to stratify patient risks.28–30 RPA class I was defined to include patients <65 years old with Karnofsky Performance Status (KPS) ≥70, controlled lung primary (lesion is resected or stable with chemoradiation), and no extracranial metastases. Class III included patients with KPS <70. Class II included remaining patients not meeting the criteria for class III and I. GPA scores for each patient were calculated based on 4 features as follows: 1) age, with age <50 years (1 point), 50–60 years (0.5 points), >60 years (0 points); 2) KPS score, with KPS <70 (0 points), KPS 70–80 (0.5 points), KPS >90 (1 point); 3) number of brain metastases, with 1 brain metastasis (1 point), 2–3 brain metastases (0.5 points), >3 brain metastases (0 points); and 4) presence or absence of extracranial metastases (0 or 1 point, respectively). Tumor staging was classified according to the seventh edition of the American Joint Committee on Cancer staging criteria.31
Whole-brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) were designated as postoperative therapy if they were administered after neurosurgery and before local or distant progression of brain metastasis. To be considered postoperative, SRS must also have specifically targeted the resection cavity. All WBRT and SRS that occurred before neurosurgery were designated as previous radiation therapies. WBRT was generally administered as 30 Gy in 10–15 fractions. SRS was administered using the CyberKnife system (Accuracy Inc., Sunnyvale, California, USA). The prescribed dose and fractionation schedule for CyberKnife SRS were based on number, size, and location of the metastatic lesions, patient’s radiation history, and generally ranged from 18 to 24 Gy over 1–3 fractions.
Mutational analyses of NSCLC tumor samples were performed as part of routine care, and the results were accessed through the STRIDE database. Multiplex polymerase chain reaction and exome sequencing were used to identify EGFR, KRAS, and BRAF mutations, whereas fluorescence in situ hybridization was used to identify ALK and ROS1 gene rearrangements. Subtypes of mutations were described when possible except when the genotyping study was performed at another institution.
The primary outcome was OS, defined as the duration from the date of neurosurgery to the date of death. Patients were censored if they were lost to follow-up at the date they were last known to be alive.
Statistical Analysis
Descriptive statistics were calculated for baseline characteristics with discrete variables compared using the χ2 test and continuous variables compared using 1-way analysis of variance. Patients were grouped according to the mutation status of the primary lung cancer into 4 mutually exclusive groups: EGFR, KRAS, or ALK, or Wild Type (defined as EGFR, KRAS, or ALK testing negative even if other mutations may have been found on broader next-generation sequencing testing). Cox proportional hazards regression models were constructed to estimate the crude and adjusted hazard ratios (HRs), and the 95% confidence interval (CI). Survival curves were generated using the Kaplan-Meier approach and were analyzed using a log-rank test. Univariate analysis was performed to determine the variables of inclusion for multivariate analysis (P < 0.1 for inclusion). All results were evaluated at a 2-sided significance level of 5%. All analyses were performed using the software R v3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline Characteristics
Baseline characteristics for our study cohort are shown in Table 1. The study cohort included 56 women and 48 men, with a mean age of 61 ± 12 years at the time of neurosurgery. Thirty-four patients (33%) had EGFR mutations, 14 patients (13%) had KRAS mutations, 5 patients (5%) had ALK rearrangement, 2 patients (2%) had ROS1 rearrangement, and 4 patients (4%) had BRAF mutations. Because of the low number of patients with ALK/ROS1 rearrangements and BRAF mutations, we combined these patients because these mutations confer response to targeted therapy and are thus actionable. Forty-five patients (43%) were tested negative for these mutations (referred to as WT hereafter). The most common EGFR mutations in this cohort were L858R (n = 16, 47%), exon 19 deletion (n = 13, 38%), and exon 20 insertion (n = 1, 3%). Two patients (6%) developed T790M mutation in addition to the exon 19 deletion. The mutation locations were not known in 4 patients because the genotyping was performed at another institution. The most common KRAS mutations were in codon 12, 13, and 61, including G12F (n = 1, 7%), G12D (n = 1, 7%), G12V (n = 2, 14%), G12C (n = 6, 38%), G13D (n = 1, 7%), Q61R (n = 1, 7%), and undocumented (n = 2, 14%). BRAF mutations included V600E (n = 2, 50%), G466A (n = 1, 25%), and G464V (n = 1, 25%). We compared clinical characteristics among the 4 tumor mutation profile groups and observed significant differences in race (P = 0.042), smoking status (P < 0.001), median number of brain metastases (P = 0.036), presence of extracranial metastases (P = 0.023), GPA score (P < 0.001), TKI use (P < 0.001), and immunotherapy use (P = 0.015).
Table 1.
Patient Characteristics at Baseline and Across Groups
| Cohort | WT | EGFR Mutant | KRAS Mutant | Other Actionable Mutations | P | |
|---|---|---|---|---|---|---|
| Number of patients | 104 | 45 | 34 | 14 | 11 | |
| Age mean (standard deviation) | 61 (12) | 61 (11) | 60 (12) | 64 (15) | 61 (13) | 0.590 |
| Female | 56 (53.8) | 24 (53.3) | 17 (50.0) | 6 (42.9) | 9 (81.8) | 0.226 |
| Race | ||||||
| White | 61 (58.7) | 32 (71.1) | 16 (47.1) | 9 (64.3) | 4 (36.4) | 0.003 |
| Asian | 33 (31.7) | 9 (20.0) | 18 (52.9) | 2 (14.3) | 4 (36.4) | |
| Hispanic | 10 (9.6) | 4 (8.9) | 0 (0.0) | 3 (21.4) | 3 (27.3) | |
| Current/past smoker | 70 (67.3) | 31 (83.8) | 11 (39.3) | 11 (100.0) | 0 (0.0) | <0.001 |
| Histology | ||||||
| Adenocarcinoma | 96 (92.3) | 39 (86.7) | 33 (97.1) | 13 (92.9) | 11 (100.0) | 0.274 |
| Others | 8 (7.7) | 6 (13.3) | 1 (2.9) | 1 (7.1) | 0 (0.0) | |
| Primary stage | ||||||
| I–III | 26 (25.0) | 13 (28.9) | 6 (17.6) | 5 (35.7) | 2 (18.2) | 0.526 |
| IV | 71 (68.3) | 28 (62.2) | 25 (73.5) | 9 (64.3) | 9 (81.8) | |
| Onset of brain metastasis | ||||||
| Synchronous | 62 (59.6) | 25 (55.6) | 20 (58.8) | 8 (57.1) | 9 (81.8) | 0.748 |
| Metachronous | 42 (40.4) | 20 (44.4) | 14 (41.2) | 6 (42.9) | 2 (18.2) | |
| Time to neurosurgery >1 month | 28 (26.9) | 11 (24.4) | 13 (38.2) | 1 (7.1) | 3 (27.3) | 0.153 |
| Median number of brain metastases (interquartile range) | 2 (3) | 2 (2) | 3 (4) | 3 (3) | 3 (4) | 0.036 |
| Maximum diameter of resected brain metastasis (cm) | ||||||
| >3 | 38 (36.5) | 21 (46.7) | 10 (29.4) | 3 (21.4) | 4 (36.4) | 0.270 |
| Location of resected brain metastasis | ||||||
| Supratentorial | 90 (86.5) | 41 (91.1) | 27 (79.4) | 12 (85.7) | 10 (90.9) | 0.507 |
| Infratentorial | 14 (13.5) | 4 (8.9) | 7 (20.6) | 2 (14.3) | 1 (9.1) | |
| Extracranial metastasis | ||||||
| Yes | 41 (39.4) | 13 (28.9) | 20 (58.8) | 3 (21.4) | 5 (45.5) | 0.023 |
| Previous resection of lung primary | ||||||
| Yes | 17 (10.6) | 8 (17.8) | 6 (17.6) | 2 (14.3) | 1 (9.1) | 0.902 |
| Tyrosine kinase inhibitor therapy | ||||||
| Yes | 46 (44.2) | 3 (6.7) | 33 (97.1) | 1 (7.1) | 9 (81.8) | <0.001 |
| Immunotherapy | ||||||
| Yes | 12 (11.5) | 10 (22.2) | 0 (0.0) | 2 (14.3) | 0 (0.0) | 0.015 |
| Line of therapy at time of neurosurgery | ||||||
| Treatment naive | 56 (53.8) | 26 (57.8) | 13 (38.2) | 10 (71.4) | 7 (63.6) | 0.159 |
| First line | 31 (29.8) | 13 (28.9) | 13 (38.2) | 4 (28.6) | 1 (9.1) | |
| Second line | 10 (9.6) | 3 (6.7) | 6 (17.6) | 0 (0.0) | 1 (9.1) | |
| Third or fourth line | 6 (5.8) | 2 (4.4) | 2 (5.9) | 0 (0.0) | 2 (18.2) | |
| Recursive partition analysis class | ||||||
| I | 8 (7.7) | 4 (8.9) | 1 (2.9) | 2 (14.3) | 1 (9.1) | 0.293 |
| II | 83 (79.8) | 36 (80.0) | 31 (91.2) | 9 (64.3) | 7 (63.6) | |
| III | 13 (12.5) | 5 (11.1) | 2 (5.9) | 3 (21.4) | 3 (27.3) | |
| Graded prognostic assessment score | ||||||
| 0–1.0 | 18 (17.3) | 4 (8.9) | 6 (17.6) | 3 (21.4) | 5 (45.5) | <0.001 |
| 1.5–2.0 | 42 (40.4) | 18 (40.0) | 20 (58.8) | 3 (21.4) | 1 (9.1) | |
| 2.5–3.5 | 44 (42.3) | 23 (51.1) | 8 (23.5) | 8 (57.1) | 5 (45.5) | |
| History central nervous station XRT before neurosurgery | ||||||
| None | 81 (77.9) | 37 (82.2) | 23 (67.6) | 13 (92.9) | 8 (72.7) | 0.732 |
| SRS | 14 (13.5) | 5 (11.1) | 7 (20.6) | 1 (7.1) | 1 (9.1) | |
| WBRT | 5 (4.8) | 2 (4.4) | 2 (5.9) | 0 (0.0) | 1 (9.1) | |
| SRS and WBRT | 4 (3.8) | 1 (2.2) | 2 (5.9) | 0 (0.0) | 1 (9.1) | |
| Postoperative XRT | ||||||
| None | 14 (13.5) | 5 (11.1) | 7 (20.6) | 1 (7.1) | 1 (9.1) | 0.824 |
| SRS | 74 (71.2) | 33 (73.3) | 22 (64.7) | 10 (71.4) | 9 (81.8) | |
| WBRT | 16 (15.4) | 7 (15.6) | 5 (14.7) | 3 (21.4) | 1 (9.1) | |
| Patient status | ||||||
| Alive | 39 (37.5) | 16 (35.6) | 13 (38.2) | 6 (42.9) | 4 (36.4) | 0.970 |
Values are number (%) except where indicated otherwise.
Other histology includes squamous cell carcinoma (n = 5) and large cell carcinoma (n = 3).
Other actionable mutations include ALK rearrangement (n = 5), ROS1 rearrangement (n = 2), BRAF mutation (n = 4).Significant values are bolded.
Significant values are bolded.
XRT, radiotherapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy.
Lines of Therapy
We documented the most recent line of systemic chemotherapy that the patient was receiving or had received near the time of neurosurgery as well as the use of any TKI or immunotherapy (Table 1). Most patients (n = 56, 53.8%) were treatment naive and had not received any systemic therapy, indicating that the brain metastasis was likely synchronous. TKI was used in almost all cases in which actionable mutations were found. Of patients with EGFR mutation, 97.1% received erlotinib, gefitinib, and/or osimertinib; 81.8% of patients with other actionable mutations received crizotinib (ALK/ROS1), alectinib (ALK/ROS1), and/or dabrafenib (BRAF). Immunotherapy such as pembrolizumab and nivolumab was given in a small subset of patients with WT status (n = 10, 22.2%) and KRAS mutation status (n = 2, 14.3%).
Symptoms
All patients were symptomatic at the time of neurosurgery and their chief symptoms are listed in Table 2. The most common symptoms included headache, focal weakness, imbalance, cognitive changes, vision changes, nausea/vomiting, and speech difficulties.
Table 2.
Presenting Symptoms at Time of Neurosurgery
| Symptom | n (%) |
|---|---|
| Headache | 40 (38.5) |
| Focal weakness | 32 (30.8) |
| Imbalance | 22 (21.2) |
| Cognitive changes | 17 (16.4) |
| Vision changes | 17 (16.4) |
| Nausea/vomiting | 12 (11.5) |
| Speech changes | 17 (16.4) |
Survival Analysis
At the time of last follow-up, 39 of 104 patients (38%) were alive. The median duration of follow-up for patients who were alive was 29 months. The median survival after neurosurgery was 24 months with a 5-year OS of 22% (Figure 1). On univariate Cox proportional hazard analysis (Table 3), EGFR mutation trended toward better OS (HR, 0.602; 95% CI, 0.340–1.065; P = 0.081). The Kaplan-Meier survival curve is plotted after stratifying for mutation status (Figure 2). The median OS for WT, EGFR, KRAS, and other actionable mutations was 16, 50, 26, and 27 months, respectively. Postoperative SRS was also a predictor of improved OS (HR, 0.431; 95% CI, 0.221–0.840; P = 0.013) on univariate analysis. Patients with a history of primary tumor lung resection indicated significantly improved survival (HR, 0.463; 95% CI, 0.220–0.977; P = 0.043). In addition, patients with GPA score in the 1.5–2 (HR, 0.377; 95% CI, 0.200–0.709; P = 0.002) and 2.5–3.5 (HR, 0.325; 95% CI, 0.174–0.607; P < 0.001) category, which indicates better functional status and lower disease burden, showed improved OS. Predictors of worse OS included older age (HR, 1.020; 95% CI, 0.998–1.042; P = 0.070), presence of extracranial metastasis (HR, 1.656; 95% CI, 1.013–2.707; P = 0.044), a history of WBRT before neurosurgery (HR, 5.964; 95% CI, 2.234–15.925; P < 0.001), and history of WBRT and SRS before neurosurgery (HR, 6.452; 95% CI, 2.245–18.539; P < 0.001). Being on the third or fourth line of systemic therapy also trended toward worse survival (HR, 2.336; 95% CI, 0.902–6.048; P = 0.081). Immunotherapy use (HR, 0.750; 95% CI, 0.300–1.871; P = 0.537) and TKI use (HR, 0.723; 95% CI, 0.442–1.186; P = 0.199) were not associated with survival after neurosurgery.
Figure 1.
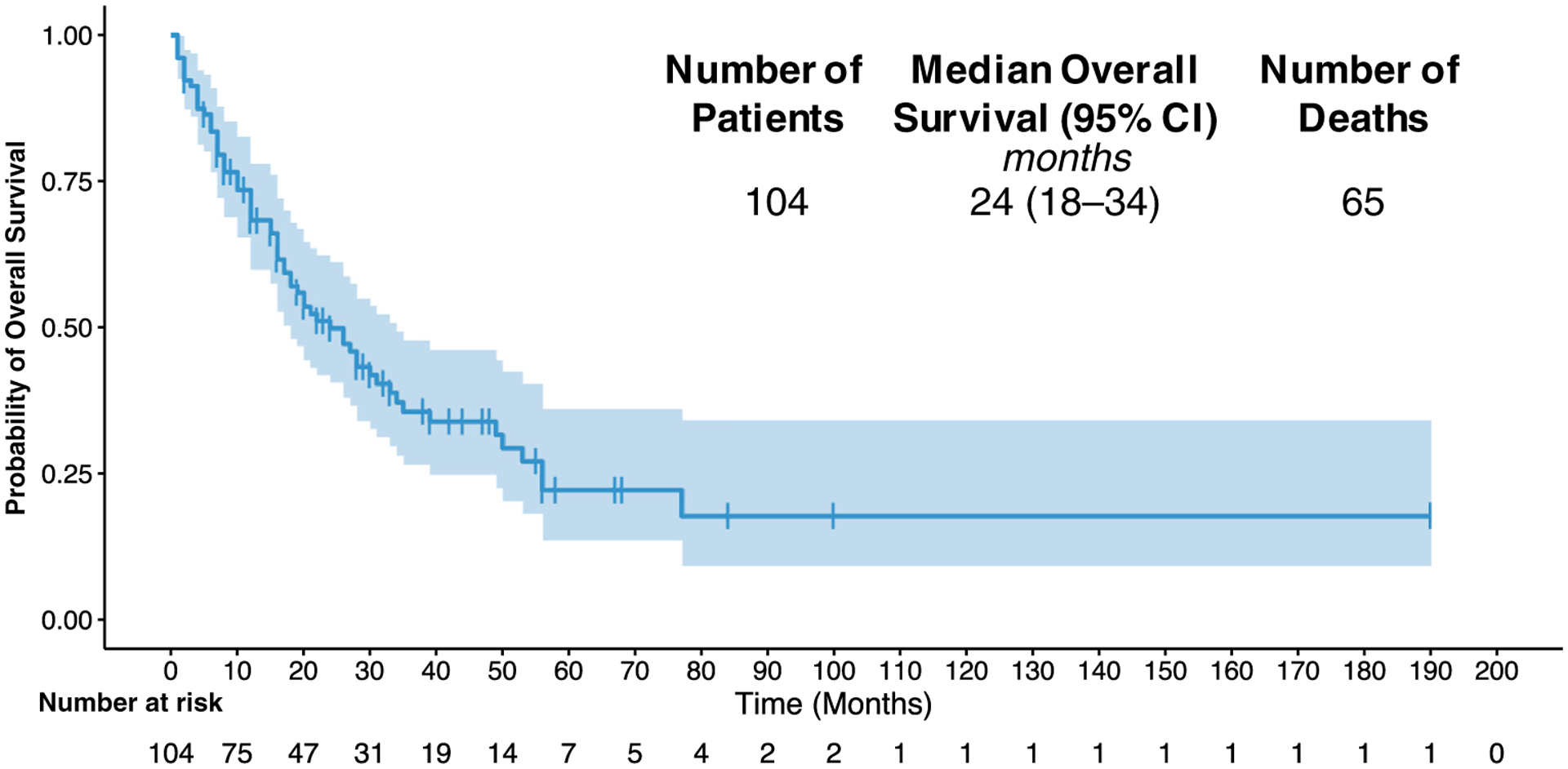
Kaplan-Meier curve for overall survival (OS) for the study cohort. CI, confidence interval.
Table 3.
Univariate Hazard Analysis for Mortality
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Crude Hazard Ratio | Lower | Upper | P | |
| Age | 1.020 | 0.998 | 1.042 | 0.070 |
| Male gender | 0.906 | 0.555 | 1.479 | 0.693 |
| Race | ||||
| White | Reference | |||
| Asian | 1.078 | 0.639 | 1.817 | 0.779 |
| Other | 1.240 | 0.485 | 3.167 | 0.654 |
| Current/past smoker | 1.186 | 0.711 | 1.978 | 0.513 |
| Adenocarcinoma | 0.940 | 0.377 | 2.348 | 0.895 |
| Stage IV at diagnosis | 1.208 | 0.672 | 2.169 | 0.528 |
| Synchronous brain metastasis | 0.838 | 0.511 | 1.377 | 0.486 |
| >1 month to neurosurgery | 1.300 | 0.754 | 2.243 | 0.345 |
| Number of brain metastases | 1.048 | 0.969 | 1.133 | 0.245 |
| Maximum diameter of resected brain metastasis >3 cm | 1.135 | 0.686 | 1.879 | 0.622 |
| Resection of supratentorial lesion | 1.555 | 0.671 | 3.608 | 0.303 |
| Evidence of extracranial metastasis | 1.656 | 1.013 | 2.707 | 0.044 |
| Previous resection of lung primary | 0.463 | 0.220 | 0.977 | 0.043 |
| Tyrosine kinase inhibitor use | 0.723 | 0.442 | 1.186 | 0.199 |
| Immunotherapy use | 0.750 | 0.300 | 1.871 | 0.537 |
| Line of therapy at time of neurosurgery | ||||
| Treatment naive | Reference | |||
| First line | 1.233 | 0.702 | 2.165 | 0.466 |
| Second line | 1.370 | 0.602 | 3.117 | 0.453 |
| Third or fourth line | 2.336 | 0.902 | 6.048 | 0.081 |
| Recursive partition analysis class | ||||
| I | Reference | |||
| II | 1.349 | 0.537 | 3.389 | 0.523 |
| III | 1.075 | 0.340 | 3.399 | 0.903 |
| Graded prognostic assessment score | ||||
| 0–1 | Reference | |||
| 1.5–2 | 0.377 | 0.200 | 0.709 | 0.002 |
| 2.5–3.5 | 0.325 | 0.174 | 0.607 | <0.001 |
| History of central nervous system before neurosurgery | ||||
| None | Reference | |||
| SRS | 1.511 | 0.737 | 3.101 | 0.260 |
| WBRT | 5.964 | 2.234 | 15.925 | <0.001 |
| SRS and WBRT | 6.452 | 2.245 | 18.539 | <0.001 |
| Postoperative radiotherapy | ||||
| None | Reference | |||
| SRS | 0.431 | 0.221 | 0.840 | 0.013 |
| WBRT | 0.592 | 0.251 | 1.399 | 0.232 |
| Mutation status | ||||
| WT | Reference | |||
| EGFR | 0.602 | 0.340 | 1.065 | 0.081 |
| KRAS | 0.815 | 0.372 | 1.784 | 0.608 |
| Other actionable mutations | 0.846 | 0.367 | 1.947 | 0.693 |
Other histology includes squamous cell carcinoma (n = 5) and large cell carcinoma (n = 3).
Other actionable mutations include ALK rearrangement (n = 5), ROS1 rearrangement (n = 2), BRAF mutation (n = 4).Significant values are bolded.
Significant values are bolded.
SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy.
Figure 2.
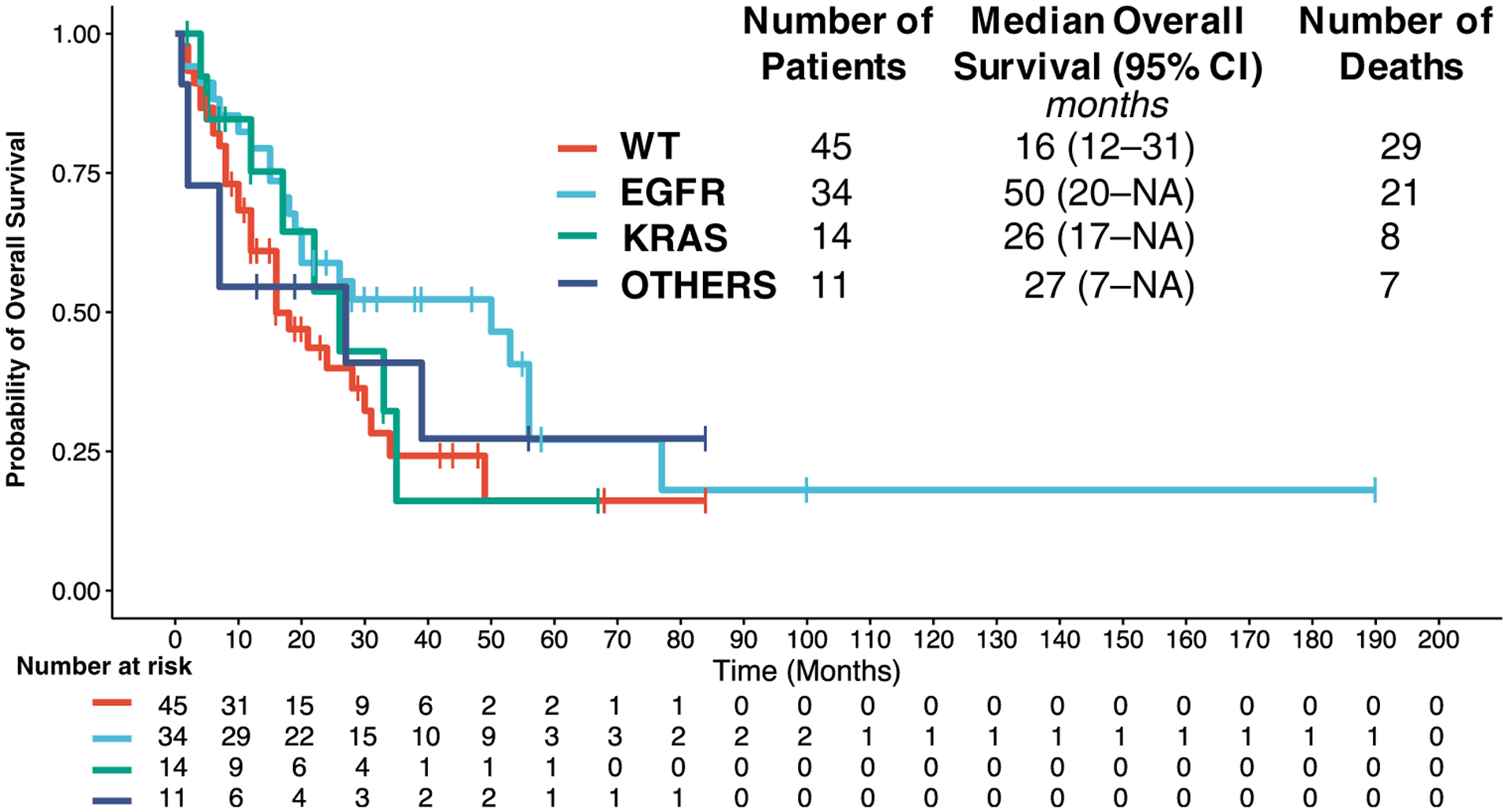
Kaplan-Meier curve for overall survival (OS) stratified by mutation status
Multivariate Analysis
We conducted a multivariate Cox regression analysis to adjust for potential confounding factors by including the variables that showed P < 0.10 based on our univariate analysis. These variables included age, mutation status, previous lung tumor resection, postoperative radiation, history of brain radiation, GPA, evidence of extracranial metastasis, and line of therapy (Table 4). We also included TKI use in the model to show that the effect of mutation status on survival can be explained by TKI use in these patients. The multivariate analysis showed that EGFR mutation is significantly associated with improved OS (HR, 0.214; 95% CI, 0.054–0.850; P = 0.029), independent of TKI use. Conversely, KRAS (HR, 0.774; 95% CI, 0.331–1.810; P = 0.559) and other actionable mutations including BRAF, ALK, and ROS1 (HR, 0.534; 95% CI, 0.160–1.776; P = 0.306) alterations were not significantly associated with OS. Furthermore, older age (HR, 1.0387; 95% CI, 1.008–1.070; P = 0.029) and a history of exposure to both WBRT and SRS, suggestive of multiple radiation sessions (HR, 9.197; 95% CI, 1.717–49.261; P = 0.001), were predictors of poor OS on multivariate analysis The presence of extracranial metastasis also resulted in greater risk of mortality (HR, 2.556; 95% CI, 1.193–5.475; P = 0.016).
Table 4.
Multivariate Hazard Analysis for Mortality
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Adjusted Hazard Ratio | Lower | Upper | P | |
| Age | 1.039 | 1.008 | 1.070 | 0.029 |
| Evidence of extracranial metastasis | 2.556 | 1.193 | 5.475 | 0.016 |
| Previous resection of lung primary | 0.504 | 0.214 | 1.187 | 0.117 |
| Tyrosine kinase inhibitor use | 1.549 | 0.413 | 5.808 | 0.516 |
| Line of therapy at time of neurosurgery | ||||
| Treatment naive | Reference | |||
| First line | 1.315 | 0.648 | 2.669 | 0.448 |
| Second line | 0.763 | 0.235 | 2.475 | 0.653 |
| Third or fourth line | 1.576 | 0.344 | 7.213 | 0.558 |
| Graded prognostic assessment score | ||||
| 0–1 | Reference | |||
| 1.5–2 | 0.513 | 0.211 | 1.244 | 0.140 |
| 2.5–3.5 | 0.766 | 0.293 | 2.001 | 0.586 |
| History of central nervous system XRT before neurosurgery | ||||
| None | Reference | |||
| SRS | 1.359 | 0.472 | 3.917 | 0.602 |
| WBRT | 3.419 | 0.929 | 12.581 | 0.064 |
| SRS and WBRT | 9.197 | 1.717 | 49.261 | 0.001 |
| Postoperative XRT | ||||
| None | Reference | |||
| SRS | 0.765 | 0.264 | 2.221 | 0.622 |
| WBRT | 1.509 | 0.420 | 5.416 | 0.528 |
| Mutation status | ||||
| WT | Reference | |||
| EGFR | 0.214 | 0.054 | 0.850 | 0.029 |
| KRAS | 0.774 | 0.331 | 1.810 | 0.555 |
| Other actionable mutations | 0.534 | 0.160 | 1.776 | 0.306 |
Other histology includes squamous cell carcinoma (n = 5) and large cell carcinoma (n = 3).
Other actionable mutations include ALK rearrangement (n = 5), ROS1 rearrangement (n = 2), BRAF mutation (n = 4).
Tyrosine kinase inhibitor was included in the multivariate model on clinical grounds.
Significant values are bolded.
XRT, radiotherapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy.
DISCUSSION
The prognosis of untreated brain metastases from lung cancer is poor, with a median OS of only 1–2 months.32 Neurosurgical resection of a single brain metastasis followed by WBRT has been shown to significantly improve survival over WBRT alone.33 More recently, SRS has emerged as an important treatment option for the control of brain metastasis in patients with few metastases.34 Neurosurgeons and patients require updated prognostic markers to guide decision making on surgical resection for symptomatic brain metastasis.
Previous studies on patients with NSCLC requiring neurosurgical resection of brain metastases yielded a few prognostic factors, which included age, tumor size, primary tumor stage, RPA, evidence of systemic disease, and synchronous brain metastasis.26,35–40 Before our work, the role of mutation status (EGFR, KRAS, and ALK) had been examined in patient cohorts receiving radiotherapy but not in patients undergoing neurosurgery for brain metastases.19,41 At our institution, the median OS after neurosurgery in patients with NSCLC was 24 months, with a 5-year survival of 22%. These findings are in the upper range of OS after neurosurgery reported in the literature.39 Our data suggest that the presence of an EGFR mutation is associated with improved OS after neurosurgical resection of brain metastasis, independent of other demographic and clinical factors. A recent multi-institutional study42 showed that EGFR mutation was associated with improved OS after diagnosis of brain metastasis. Our study indicates that EGFR mutation continues to be a prognostic factor at the time of neurosurgery for symptomatic brain metastasis. The presence of EGFR mutation, as with other actionable mutational targets, allows a greater array of drug choices to the patients, in particular TKIs such as erlotinib and osimertinib, which have been found to show substantial intracranial activity.43,44 The efficacy of TKI may explain in part the improved survival seen in this group of patients. However, in our multivariate model, EGFR mutation status remained significantly predictive of survival even after adjusting for TKI use. This finding suggests that tumors with EGFR mutation likely have less aggressive biology compared with the WT counterpart. This situation was not true for patients with ALK/ROS1 rearrangements and/or BRAF mutations, whose survival was similar to patients with WT status despite being treated with TKI.
Other predictors of survival identified in this study were previous radiation therapy and presence of extracranial disease. Patients with a history of WBRT and SRS procedures before the current neurosurgical resection had significantly worse prognosis. This finding suggests that the development of a new symptomatic brain lesion requiring surgical resection in the setting of previous radiation therapy likely represented more advanced neurologic disease, thus resulting in reduced OS. In a similar light, presence of extracranial disease indicates more aggressive disease in these patients.
Limitations of our study included a small sample size and the retrospective nature of the cohort. As with all retrospective studies, residual confounders may be present in the final model because no randomization process took place. We also acknowledge the relatively small sample size for patients with ALK/ROS1 rearrangements and BRAF mutations, which may have limited our ability to detect a significant difference in survival trends among these patients.
CONCLUSIONS
In patients undergoing neurosurgical resection of NSCLC brain metastasis, the presence of an EGFR activating mutation was associated with an increase in OS, independent of TKI use. Conversely, older age, a history of multiple radiation procedures, and extracranial metastasis were associated with a decrease in OS. These findings may help clinicians tailor counseling for patients with symptomatic NSCLC brain metastases.
Conflict of interest statement:
This study was supported by the U.S. National Institutes of Health (grant number K08 NS901527).
Abbreviations and Acronyms
- ALK
Anaplastic lymphoma kinase
- CI
Confidence interval
- EGFR
Epidermal growth factor receptor
- GPA
Graded prognostic assessment
- HR
Hazard ratio
- KPS
Karnofsky Performance Status
- NSCLC
Non-small-cell lung cancer
- OS
Overall survival
- RPA
Recursive partitioning analysis
- SRS
Stereotactic radiosurgery
- TKI
Tyrosine kinase inhibitor
- WBRT
Whole-brain radiation therapy
REFERENCES
- 1.Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Schuette W Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer. 2004;45: S253–S257. [DOI] [PubMed] [Google Scholar]
- 4.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378: 113–125. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y-L, Zhou C, Liam C-K, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–1889. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 10.Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y-L, Zhou C, Hu C-P, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 12.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377: 829–838. [DOI] [PubMed] [Google Scholar]
- 13.Solomon BJ, Mok T, Kim D-W, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 14.Soria J-C, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 15.Schuler M, Wu Y-L, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non–small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11:380–390. [DOI] [PubMed] [Google Scholar]
- 16.Costa DB, Shaw AT, Ou S-HI, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non–small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33: 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vansteenkiste J, Reungwetwattana T, Nakagawa K, et al. CNS response to osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFR-TKI sensitising mutation (EGFRm)-positive advanced non-small cell lung cancer (NSCLC): data from the FLAURA study. Ann Oncol. 2017;28(suppl 10). 10.1093/annonc/mdx782. [DOI] [Google Scholar]
- 18.Luo D, Ye X, Hu Z, et al. EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumor Biol. 2014;35:2437–2444. [DOI] [PubMed] [Google Scholar]
- 19.Lee D-W, Shin D-Y, Kim JW, et al. Additional prognostic role of EGFR activating mutations in lung adenocarcinoma patients with brain metastasis: integrating with lung specific GPA score. Lung Cancer. 2014;86:363–368. [DOI] [PubMed] [Google Scholar]
- 20.Wang TJC, Saad S, Qureshi YH, et al. Does lung cancer mutation status and targeted therapy predict for outcomes and local control in the setting of brain metastases treated with radiation? Neuro Oncol. 2015;17:1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai L, Zhu J, Zhang X, et al. A comparative analysis of EGFR mutation status in association with the efficacy of TKI in combination with WBRT/SRS/surgery plus chemotherapy in brain metastasis from non-small cell lung cancer. J Neurooncol. 2014;120:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasini P, Serdjebi C, Khobta N, et al. EGFR and KRAS mutations predict the incidence and outcome of brain metastases in non-small cell lung cancer. Int J Mol Sci. 2016;17:E2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperduto PW, Yang TJ, Beal K, et al. The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys. 2016;96:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor–mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol. 2016;95:673–679. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor–naïve epidermal growth factor receptor–mutant non–small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. [DOI] [PubMed] [Google Scholar]
- 26.Getman V, Devyatko E, Dunkler D, et al. Prognosis of patients with non-small cell lung cancer with isolated brain metastases undergoing combined surgical treatment. Eur J Cardiothorac Surg. 2004;25:1107–1113. [DOI] [PubMed] [Google Scholar]
- 27.Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. [DOI] [PubMed] [Google Scholar]
- 29.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol. 2008;70: 510–514. [DOI] [PubMed] [Google Scholar]
- 30.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol. 2010; 77:655–661. [DOI] [PubMed] [Google Scholar]
- 31.Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2007;2:686–693. [DOI] [PubMed] [Google Scholar]
- 32.Penel N, Brichet A, Prevost B, et al. Pronostic factors of synchronous brain metastases from lung cancer. Lung Cancer. 2001;33:143–154. [DOI] [PubMed] [Google Scholar]
- 33.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson B, Hanssens P, Wolff R, Söderman M, Lindquist C, Beute G. Thirty years’ experience with Gamma Knife surgery for metastases to the brain. J Neurosurg. 2009;111:449–457. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Zhang X, Jiang X, et al. Outcome of surgical resection for brain metastases and radical treatment of the primary tumor in Chinese non-small-cell lung cancer patients. Onco Targets Ther. 2015;8: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billing PS, Miller DL, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg. 2001;122:548–553. [DOI] [PubMed] [Google Scholar]
- 37.I H, Lee J Il, Nam DH, et al. Surgical treatment of non-small cell lung cancer with isolated synchronous brain metastases. J Korean Med Sci. 2006;21:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrews RJ, Gluck DS, Konchingeri RH. Surgical resection of brain metastases from lung cancer. Acta Neurochir (Wien). 1996;138:382–389. [DOI] [PubMed] [Google Scholar]
- 39.Modi A, Vohra HA, Weeden DF. Does surgery for primary non-small cell lung cancer and cerebral metastasis have any impact on survival? Interact Cardiovasc Thorac Surg. 2009;8:467–473. [DOI] [PubMed] [Google Scholar]
- 40.McPherson CM, Suki D, Feiz-Erfan I, et al. Adjuvant whole-brain radiation therapy after surgical resection of single brain metastases. Neuro Oncol. 2010;12:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases. JAMA Oncol. 2017;3:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405. [DOI] [PubMed] [Google Scholar]
- 44.Ballard P, Yates JWT, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. [DOI] [PubMed] [Google Scholar]


