Summary
The high efficacy of modern ART and the increase in non-infertile patients who are using ART for family building in the U.S. call into question the relevance of the standard, one-size-fits-all infertility evaluation. Herein we explore whether all patients presenting for ART need uterine cavity and tubal assessment and what tests are most appropriate, efficient, and cost-effective in current times.
Keywords: Saline infusion sonohysterography (SIS), hysterosalpingo-contrast sonography (HyCoSy), hysteroscopy, hysterosalpingogram (HSG), hydrosalpinx
Capsule
ART is highly effective and often indicated in non-infertile couples. New, less-invasive diagnostic modalities are available for evaluation of the uterine cavity and fallopian tubes. Given this, it is time to individualize diagnostic evaluation of patients planning ART.
Introduction
Many modalities for assessment of the uterine cavity and fallopian tubes pre-date high efficacy ART and should be re-evaluated in the modern patient. This is particularly true among the growing population of non-infertile women undergoing embryo transfer, e.g., to enable PGT, for isolated male factor, for ‘reciprocal IVF’ among same-sex female partners, or for women serving as gestational carriers.
Ultrasound has emerged as a potentially more efficient and less invasive tool for assessing both the uterine cavity and fallopian tubes. Saline ultrasound has emerged as similarly accurate and less invasive than diagnostic hysteroscopy. Proprietary contrast agents are increasingly used, especially in Europe, and may make for easier sonographic demonstration of tubal patency. However, operator and assessor skill significantly impact the utility of ultrasound for tubal assessment.
Herein we discuss currently available tools for uterine cavity and fallopian tube assessment in the era of high efficacy ART, with particular attention to appropriate evaluation of patients for whom anatomic pathology is not particularly suspected.
Pathology of the uterine cavity and ART
Uterine cavity abnormalities occur in 16.9% of women presenting with infertility, and nearly 40% of women presenting with abnormal uterine bleeding, most commonly in the form polyps (13%), submucous fibroids (2.9%), and adhesions (0.2%) (1).
Endometrial polyps are common (prevalence estimates ranging widely from 6–32% in women undergoing hysteroscopy prior to ART) and may have a negative impact on fertility (2). A small RCT showed higher pregnancy rates after polypectomy vs. diagnostic hysteroscopy with polyp biopsy in 215 infertile patients undergoing up to four cycles of IUI (63.4% vs. 28.2%, relative risk 2.1 95% CI 1.5–2.9) (3). While the benefit of polypectomy prior to embryo transfer remains unproven, endometrial polyps have been suggested to negatively impact endometrial receptivity (4) and polypectomy is generally thought to be safe and to have the potential to improve fertility outcomes (2) (5). One study reported an increased rate of biochemical pregnancies when endometrial polyps were left untreated in patients undergoing IVF (18.3% in the polyp group compared with 9.6% in the non-polyp group (p = .01); odds ratio = 2.12; 95% confidence interval, 1.09–4.12) (6). However, it is important to note that the prevalence of endometrial polyps in patients with normal menses (fertile or non-infertile) is unknown, as is the impact of polyps on live birth rates following embryo transfer and whether polypectomy improves live birth rates from embryo transfer (7) (2).
Other uterine cavity abnormalities have been shown to affect pregnancy rates in patients conceiving without ART. A study by Hooker et al demonstrated a significant increase in median time to conception leading to live birth in patients with intrauterine synechiae vs. women without (15 months vs. 5.0 months (HR 0.54 (5% CI: 0.30–0.97)) (8). Additionally, while there is lack of consensus regarding the management of intramural fibroids in the setting of infertility (9) (10), there is general consensus around the benefit of hysteroscopic resection of cavity distorting fibroids from a fertility perspective. While level I evidence is lacking, this practice likely improves outcomes (11) (12). Less common uterine abnormalities, such as Mullerian anomalies, also have a significant impact on obstetrical outcomes. Specifically, they can lead to recurrent miscarriage, preterm labor, breech presentation, intrauterine growth restriction, and abnormal placentation (13).
Uterine cavity assessment
Consensus is lacking as to the most appropriate first line diagnostic methodology to assess the uterine cavity prior to embryo transfer. This is particularly true for non-infertile patients undergoing ART. The gold standard of uterine cavity evaluation has historically been hysteroscopy; however, other tests, may be similarly accurate, less invasive, and less expensive.
Transvaginal ultrasound
Transvaginal ultrasound (TVUS) provides quick and inexpensive assessment of uterine anatomy. For the diagnosis of polyps specifically, TVUS has a wide range of reported diagnostic accuracy. TVUS is reported to have a sensitivity from 19–100%, specificity of 53–100%, positive predictive value (PPV) of 70–100%, and negative predictive value (NPV) of 87–97% (14). Adding color-flow doppler increased both sensitivity and specificity of TVUS for diagnosis of endometrial polyps (15) (16). For diagnosing uterine malformations, reported sensitivity of plain vaginal ultrasound was 44%. A very wide range of sensitivity (0–97%) has been reported for diagnosis of intrauterine adhesions using plain TVUS (17), suggesting that operator/assessor expertise and ultrasound resolution impact the ability of TVUS to detect adhesions without instillation of contrast.
The diagnostic accuracy of TVUS for uterine cavity defects also varies with menstrual cycle timing. One study reported low (28.2%) sensitivity during days 1–4 of the cycle, with improved detection during days 16–19. No lesion <1cm was detected by TVUS during the follicular phase (days 1–12) (18). TVUS provides excellent assessment of the myometrium and adnexal structures, including antral follicle count, making it indispensable to the pre-ART evaluation. Nevertheless, its sensitivity for uterine cavity assessment is suboptimal (1).
Sonohysterography or Saline Infusion Sonography
Sonohysterography (SHG), also known as Saline Infusion Sonography (SIS), involves instillation of saline into the uterine cavity during TVUS. First introduced in the late 1990s, this test outperforms TVUS and hystersalpingography (HSG) in its ability to diagnose uterine cavity defects (19) (20) (21), with high (>90%) PPV and NPV (1) (17) (22). The distension of the cavity with saline allows better visualization of the endometrial canal (21). SIS improved detection of intrauterine pathology from 67% to 87% relative to TVUS (23). For diagnosis of endometrial polyps, a recent meta-analysis concluded improved diagnostic accuracy of SIS over TVUS without saline infusion (24). A Cochrane meta-analysis did not find a significant difference in accuracy between 2D- and 3D-SIS in detecting endometrial polyps and myomas. Both methods approach the accuracy of diagnostic hysteroscopy in subfertile women (25).
That said 3D-SIS technology provides diagnostic advantages, even over hysteroscopy. 3D-SIS allows visualization of the whole uterus in three orthogonal planes, including the coronal view (Figure 1). The method is accurate and expedient in determining the appropriate surgical approach to myomas with submucosal components (Figure 2). Further, in experienced hands, it may be used for mapping and classification of severity of adhesions (26) (Figure 1B). 3D-SIS provides an accurate, reliable, and non-invasive tool for the classification of common congenital uterine malformations. Visualization of the external uterine contour constitutes a significant advantage of SIS over both diagnostic hysteroscopy and HSG, as it allows differentiation between bicornuate and septate pathology (Figure 1C). SIS also provides optimal ability to discern between normal, arcuate, and septate configurations -- diagnoses that are frequently debated by clinicians (27). The newest application of 3D-SIS is the calculation of uterine cavity volume using dedicated software (28). Further research is needed to determine the potential benefit of this parameter.
Figure 1: Three-dimensional saline infusion sonohysterography (3D-SIS) coronal view of uteri.
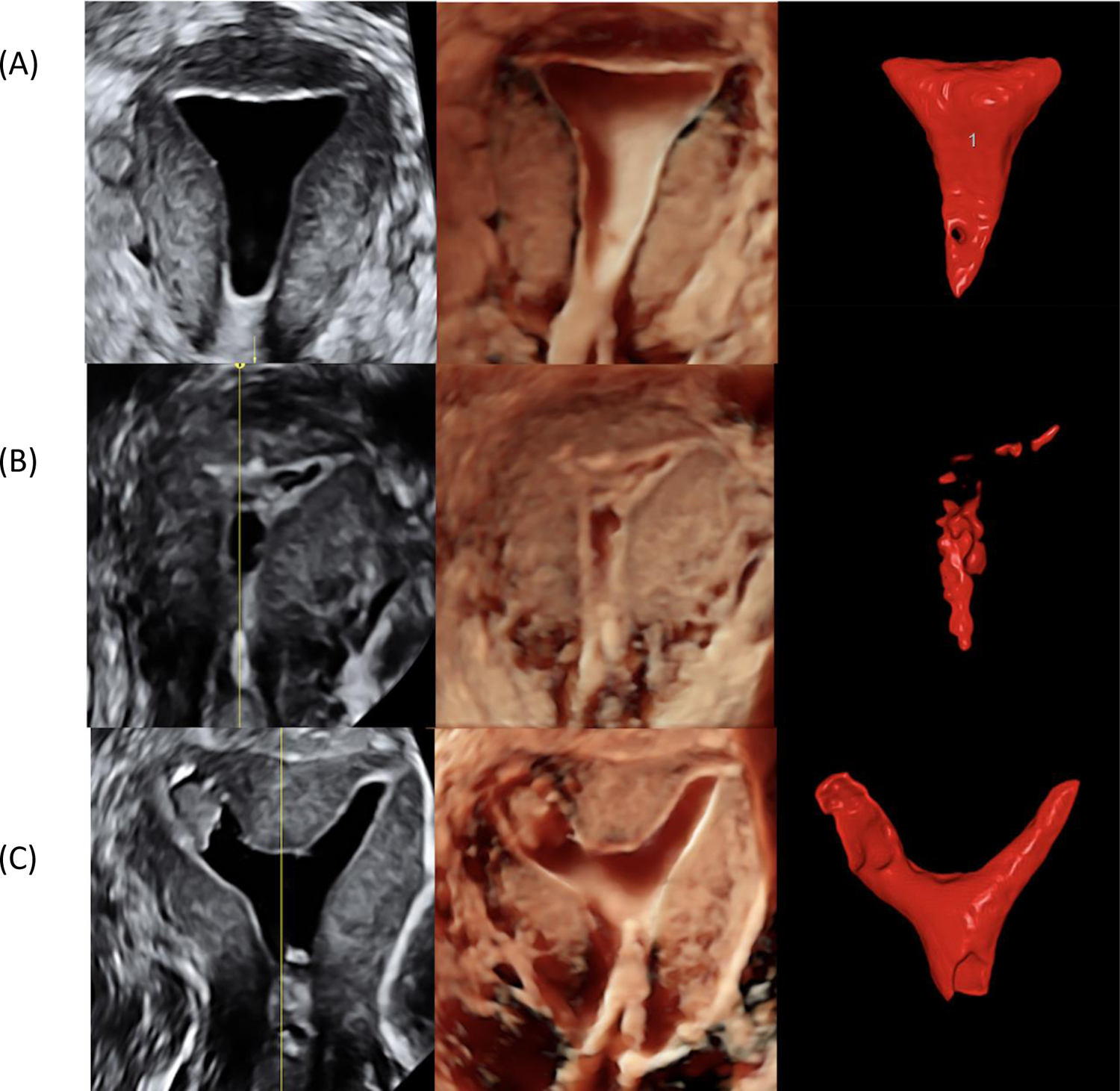
- Render modes: LEFT COLUMN: greyscale and Volume Contrast Imaging, CENTER COLUMN: HDlive, a.k.a. virtual hysteroscopy; RIGHT COLUMN: Automatic Volume Calculation software (SonoAVC)
- Cases: TOP ROW (A): Normal uterus with normal uterine cavity shape and volume; MIDDLE ROW (B): Intrauterine synechiae and small uterine cavity volume due to presence of adhesions; BOTTOM ROW (C): Septate uterus with endometrial polyp in left part of uterine cavity.
Figure 2: Three-dimensional saline infusion sonohysterography (3D-SIS) in assessment of submucosal myoma.
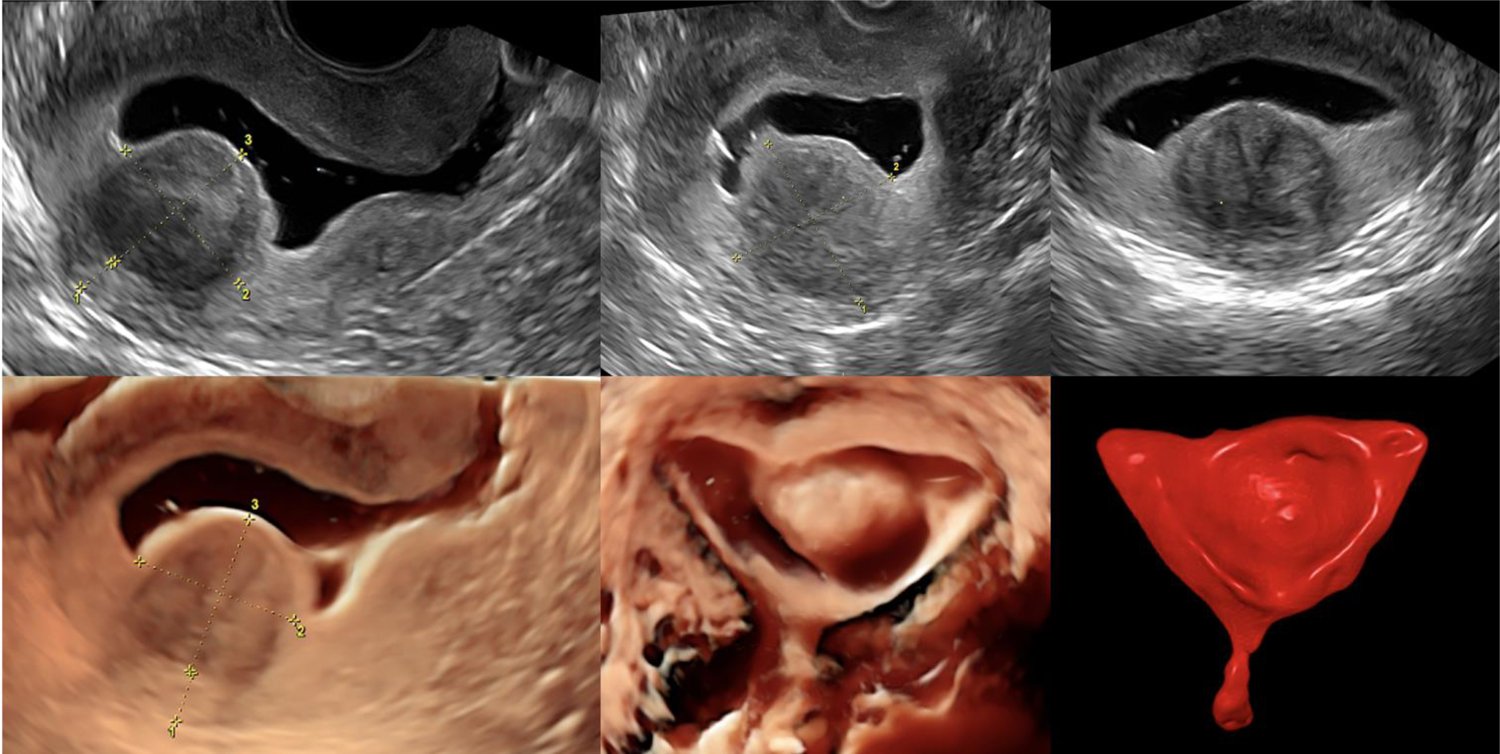
This method can determine suitability of submucosal myomas to hysteroscopic resection because it allows assessment of myoma penetration into myometrium, distance between myoma and uterine surface, and myoma size.
Hysteroscopy
Hysteroscopy has long been considered the gold standard and definitive method for diagnosis and treatment of intrauterine pathology (1) (29). This procedure provides direct visualization and the immediate treatment of abnormalities to restore normal anatomy, as well as the ability to obtain tissue samples for diagnosis. This test can be performed in the operating room with general anesthesia or sedation, or in the office with or without local anesthetic or sedation (30). Yet, there is important evidence indicating low inter-rater reliability of diagnostic hysteroscopy, which calls into question the method’s status as the gold standard (31, 32). Furthermore, diagnostic hysteroscopy is limited in its ability to discern among normal, arcuate, and the septate pathology (27, 33). While hysteroscopy has the benefit of incorporating diagnosis with immediate treatment (30), it may be more costly and invasive and may cause more pain and discomfort than SIS or HSG (1).
One study randomized subjects without a prior ‘uterine factor’ diagnosis, and without suspicion for intracavitary pathology based on TVUS and HSG, to hysteroscopy vs. no hysteroscopy prior to IVF/ICSI. The authors found that 43% of patients in the hysteroscopy group had abnormal uterine pathology, which was resected in real time. The clinical pregnancy rate was significantly higher in the hysteroscopy group (odds ratio, OR 2.77; 95% CI, 1.53–5.00) (34). However, this RCT was not prospectively registered and evaluated a small sample for each individual class of pathology. Another study compared patient preference between SIS and hysteroscopy. Patients reported a lower pain score with SIS; however, hysteroscopy was still preferred (35). Immediate treatment allows patients to avoid multiple steps, which can avoid delay in fertility treatment. A recent RCT compared SIS to office hysteroscopy in 100 patients planning embryo transfer and found that office hysteroscopy had similar (short) procedure times and patient satisfaction but fewer delays in initiation of fertility treatment and fewer secondary procedures were required (36).
On the other hand, discomfort, cost, and diagnostic limitations must be weighed heavily in considering generalization of diagnostic hysteroscopy in the evaluation of infertility and in patients planning embryo transfer. Physicians in Europe have launched the “Campaign Against Painful Hysteroscopy” and in 2020 sent an open letter to the Department of Health and Social Care in the United Kingdom (37). The largest and best quality RCTs, showed no improvement in IVF live birth rate following diagnostic hysteroscopy with treatment of detected abnormalities in patients with a normal TVUS of the uterine cavity either prior to first IVF (33) or in patients with a history of 2–4 prior embryo transfers without pregnancy (38). Based on these data, routine hysteroscopy in women with normal transvaginal ultrasound should not be offered prior to embryo transfer. Rather, SIS represents a highly accurate, cost-effective, patient friendly diagnostic modality in this setting.
Importance of Fallopian Tube Assessment in Patients Considering ART
While ruling out defects of the uterine cavity remains the keystone of diagnostic evaluation prior to embryo transfer in the era of highly effective ART, determining patency and normal caliber of fallopian tubes also plays an important role. Confirming patency remains vital in determining whether ART is indicated in the first place. 80% of couples desiring pregnancy will conceive within 1 year, and 50% of couples who do not conceive in the first year and are diagnosed with infertility will be pregnant in the second year without ART (39). Moreover, for women less than 40 years old with at least one patent fallopian tube, the cumulative pregnancy rate is over 50% after 6 cycles of intrauterine insemination, and up to 75% if further cycles of intrauterine insemination are undertaken (39).
Assessment of fallopian tube caliber warrants consideration even for patients who have clear indication(s) for IVF and embryo transfer. For such patients, fallopian tube patency is irrelevant. However, the presence of hydrosalpinx(ges) is associated with significantly lower IVF success rates. Since laparoscopic salpingectomy returns IVF success rates to those of patients without hydrosalpinges, diagnosis and treatment are crucial (40–42).
What follows is a discussion of how fallopian tubes should be assessed in the era of high-efficacy ART.
From X-ray hysterosalpingography, and laparoscopy with dye, to modern ultrasound
There are various tubal patency tests, which may be applied during fertility workup, with variable sensitivity and specificity, inter-rater reproducibility, invasiveness, and costs. The most traditional tests are X-ray hysterosalpingography (HSG) and laparoscopy with transcervical dye instillation. In the U.S., HSG is recommended as a first line test in the setting of infertility (1, 40). Laparoscopy with dye allows for direct visualization of fallopian tubes, pelvic cavity, and specific comorbidities including adhesions and endometriosis and is considered a reference for accuracy (39) (43) (44). Due to its invasive nature, laparoscopy is reserved for those women needing surgical treatment of suspected pelvic pathology or as a confirmatory tool in women with occluded tubes, especially when tubal surgery is planned (44). In the U.S., HSG remains the most utilized tool to assess for tubal patency, caliber, and para tubal loculations, despite suboptimal sensitivity (65%) and specificity (83%) (39). Pelvic pain, allergy, and exposure to radiation are drawbacks of HSG (45) (46). Infection is also a concern, but its incidence is exceedingly low (<0.5%). Overall complication rates of HSG were 1.8% where water-based contrast was used and 5.4% with oil-based contrast (47). Lastly, HSG requires X-ray, which is not available in all clinics and may necessitate referral to a hospital or radiology center.
The popularity of ultrasound-based tubal patency tests – known as hysterosalpingo-contrast sonography (HyCoSy) – is increasing, particularly in Europe (44), with notably slow uptake in the U.S. European authors have noted thatwith incorporation of HyCoSy, modern transvaginal ultrasound holds potential to become a ‘one-stop shop’ for comprehensive fertility assessment allowing targeted investigation of the uterus, myometrium, endometrium, uterine cavity, ovaries, follicle count, and fallopian tubes, including tubal patency assessment (44, 48)(38). The addition of tubal assessment to SIS is associated with minimal or no additional cost (particularly if air or saline contrast agent(s) are used). However, many fertility specialists, particularly in the U.S., are not familiar with ultrasonographic assessment of the fallopian tubes and how 3D imaging and Doppler have potential to increase the accuracy, reliability, as well as tolerability, and safety of tubal patency testing.
Evolving contrast agents for ultrasound tubal patency testing
Normal saline is a cheap, non-commercial contrast agent with many advantages. Because it is negative (anechoic), saline is an excellent agent for visualization of the uterine cavity; however, it cannot optimally delineate the fallopian tubes (44) (48). Saline infusion is sufficient to confirm that at least one tube is patent, by visualization of saline in the pouch of Douglas after infusion. Better contrasts to depict the fallopian tubes are those that generate hyperechoic (positive) images of medium flow through the tubes to the abdominal cavity. Since hyperechoic contrasts reduce the ability to identify hyperechoic intracavitary and endometrial lesions, it is reasonable to instill first saline for cavity assessment, then hyperechoic contrast for fallopian tube assessment.
Until recently, the only ultrasonographic contrast method for tubal patency approved by FDA and available in the U.S. was air/saline hysterosalpingo-contrast sonography (air/saline-HyCoSy). In this method, saline as anechoic component, and air bubbles as hyperechoic component, are infused simultaneously or alternately using the same equipment as for saline infusion sonohysterography (49). The main limitation of this method is that moving air bubbles and saline do not generate a clear and steady visualization of the tubes; therefore, an accurate and reliable diagnosis may require a high degree of expertise on the part of the interpreter. The most important diagnostic limitation of air/saline HyCoSy is low PPV/high false positive rate for tubal occlusion (50). Furthermore, the test is often inconclusive (44) (48). A strength of air/saline HyCoSy is that the method has a high NPV, i.e. that diagnostic accuracy of (a) patent tube(s) is high. Due its high NPV (low rate of false diagnoses of patent tube(s)) and low cost, HyCoSy deserves strong consideration for initial assessment of tubal patency.
Several commercial hyperechoic contrast agents have been proposed to increase accuracy of tubal patency testing. However, microbubble contrast agents: Echovist® (galactose microparticles; Bayer Schering Pharma AG, Berlin, Germany) and SonoVue® (sulfur hexafluoride; Bracco International BV, Amsterdam, The Netherlands), respectively, did not significantly increase diagnostic accuracy (43). Further, Echovist® has been removed from the market due to allergic reactions, while SonoVue is still available for off-label use. Foam as contrast has recently been introduced for sonographic assessment of fallopian tube patency (51). The ExemFoam® (hydroxyethylcellulose, glycerol and purified water; GynaecologIQ, Delft, The Netherlands) is the first such commercial agent. The contrast was launched ten years ago in the European market. In 2019, it became the first FDA-approved contrast agent for assessing tubal patency (52). HyCoSy method with use of foam is called HyFoSy (hysterosalpingo-foam sonography). Finally, as a considerably cheaper alternative, HyLiFoSy (hysterosalpingo-lignocaine foam sonography), using foam contrast created with widely accessible Lidocaine gel, air, saline, and power Doppler, has been described (53). As with microbubble contrast agents, the use of commercial foam contrast did not improve upon the diagnostic accuracy of HyCoSy in the hands of experienced practitioners, because air/saline-HyCoSy has close to perfect NPV (44). Therefore, HyCoSy stands as an excellent option, particularly in low-resource settings once appropriate training is completed.
2D and 3D HyFoSy, and Doppler HyFoSy
HyFoSy with 2-dimensional-transvaginal sonography is a basic option (Figure 3A) with foam offering potentially improved fallopian tube visualization (51). HyFoSy with 3-dimensional or Doppler imaging was described by expert users and suggested as a good option to standardize image acquisition without the need for skilled probe movements to include the fallopian tubes and ostia (44). For intermediate skill-level operators, 2D/3D HyFoSy with automatic scanning may be a better option, since it is less operator-dependent and less time-consuming. The first study evaluating inter-rater reproducibility of HyCoSy/HyFoSy recently showed that agreement in the diagnosis of tubal patency was only moderate with 2-D sonogaraphy using foam (κ = 0.55) and air/saline (κ = 0.67) but was very good with power Doppler (κ = 0.95) (54).
Figure 3: Ultrasound tubal patency testing with foam (HyFoSy):
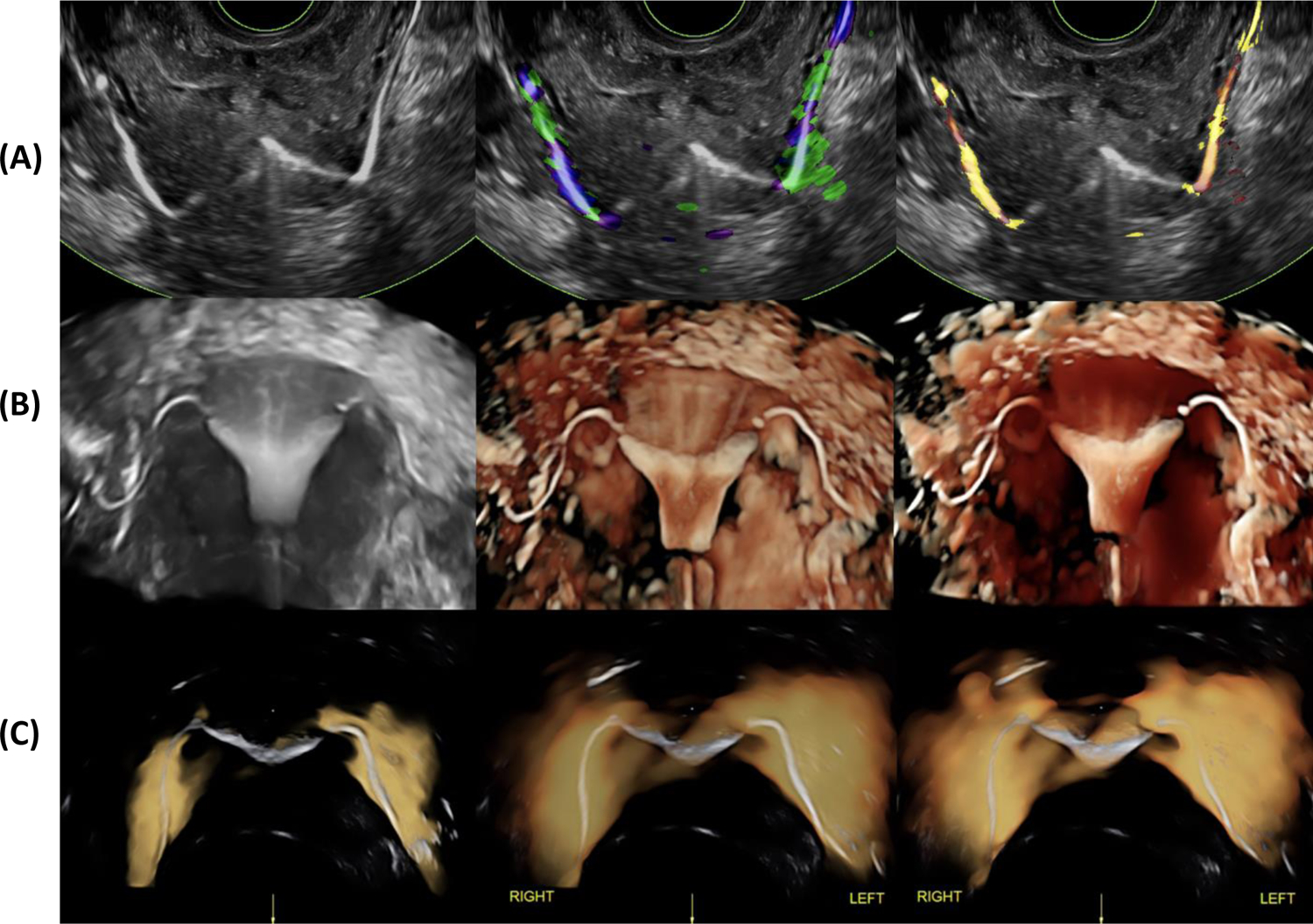
- (A) 2D-HyFoSy in grey scale (LEFT) and tissue Doppler imaging (CENTER and RIGHT);
- (B) 3D-HyFoSy in grey scale (LEFT), HDlive render mode (CENTER and RIGHT);
- (C) 3D-HyFoSy and power Doppler. Power Doppler generates a highly useful sign called flaming tubes sign and makes diagnosis very easy to interpret for low and medium experienced users (53).
The same group assessed the diagnostic accuracy of these methods in comparison to laparoscopy (44) (55). 2D air/saline HyCoSy and 2D/3D HyFoSy showed no significant differences in diagnostic accuracy of tubal patency relative to one another, and both methods had significantly lower accuracy than laparoscopy with dye instillation. However, the addition of Doppler with high-definition flow imaging improved the accuracy of HyFoSy to the level of laparoscopy. The addition of Doppler increased PPV, i.e. it reduced the odds of falsely diagnosing tubal occlusion in the setting of patency. With 2D and 3D HyFoSy alone, a thin sonogram of the tubes close to 1 mm is obtained in grayscale, and its flow is sometimes inconclusive. With Doppler HyFoSy, the flow of foam generates a thick (>0.5 cm) colored sonogram around the tubes. Thus, inconclusive impressions of flow in the gray scale may be confirmed by adding of Doppler (Figure 3). In summary, without Doppler, both HyFoSy and HyCoSy are less accurate than laparoscopy with dye instillation; however, Doppler improves accuracy for both HyFoSy and HyCoSy to that of laparoscopy. While data directly comparing the diagnostic accuracy of Doppler HyCo/HyFoSy to HSG are unavailable, indirect comparisons indicate that both methods are similarly accurate to HSG (44) (43). A recent RCT in which infertile subjects were managed based on the results of HyFoSy vs. HSG, showed non-inferiority of HyFoSy to HSG in terms of live birth within 12 months (46 vs 47%) (56).
HyCoSy and HyFoSy are generally well-tolerated (57) (58), and pre procedure analgesia may further reduce the rate moderate to severe procedure-related pain (28). Initially there were no significant complications after more than 350 000 procedures with ExemFoam (59) (60) (61). Recently, one case of diffuse skin immune reaction, diagnosed as cutaneous small-vessel vasculitis, was the first reported case of hypersensitivity reaction to ExemFoam and occurred in the setting of contrast intravasation (61). However, complications may be under-reported. The first case of intravasation during air/saline HyCoSy was described after two decades of its use (62).
Potential Therapeutic Benefit of HyCoSy / HyFoSy
It has been described that ‘flushing’ of the fallopian tubes in the setting of HSG is associated with an increase in naturally conceived pregnancies (63, 64). A higher rate of pregnancy has been reported using oil-based vs. water-based contrast for HSG (41% vs. 34%) (65) (66). Original studies with commercial contrasts agents for tubal patency testing and flushing for sonographic fallopian tube assessment have been performed with disclosed support from the manufacturers (45) (67) and have shown 5% increase in ongoing pregnancy rate in short term (80% vs. 75%) (68) and in long-term follow-up (45). A recent RCT indicated that basic HyFoSy (without 3D or power Doppler) was less painful but led to similar pregnancy outcomes relative to HSG (69). However, independent studies without risk of commercial bias and with different methodologies of tubal patency testing and flushing are needed. Flushing with air/saline may also increase the rate of natural pregnancies.
Assessment of Fallopian Tube Caliber
For patients with one patent tube and unilateral hydrosalpinx, surgical management increases odds of conceiving without IVF (70). For those patients with indication for ART, the presence of hydrosalpinx(ges) has a clear negative prognostic influence and should be mitigated surgically prior to embryo transfer (71). RCTs comparing IVF outcomes with or without surgical management of hydrosalpinges, found surgery to be effective, returning success rates to those of patients without hydrosalpinges (40–42).
Classic studies evaluating surgical management of hydrosalpinx prior to embryo transfer relied predominantly upon ultrasound diagnosis of hydrosalpinx. While plain ultrasound suffers from low sensitivity (72, 73), specificity of TVUS for hydrosalpinx, is high. It is not known whether surgical management increases the chances of an embryo to successfully implant, in the case of hydrosalpinx(ges) that are visible with contrast instillation (as in HSG, SIS, HyCoSy, or HyFoSy), but not visible on plain TVUS. While the AIUM state that hydrosalpinx is not an absolute contraindication to HyCoSy (74), most original studies consider the presence of hydrosalpinx(ges) as a relative contraindication to HyCoSy (75, 76). For this reason, many practitioners perform plain TVUS prior to instillation of contrast and stop the procedure without performing HyCoSy, if a hydrosalpinx is suspected based on TVUS findings. Contrast instillation may increase infection risk in the setting of hydrosalpinx, but risk of procedure related pelvic inflammatory disease remains exceedingly low. Many practitioners administer prophylactic antibiotics prior to HSG if hydrosalpinx is suspected based on history or administer post procedure antibiotics if an HSG is diagnosed incidentally, which may further reduce infection risk (47). The prevalence of unsuspected, clinically relevant hydrosalpinx(ges) that would be diagnosed on HSG or HyCoSy but not on plain TVUS is not known. However, it is likely quite low, particularly among the population of non-infertile patients planning ART. Therefore, while it is reasonable to undertake HSG to rule out hydrosalpinx in patients planning embryo transfer, when tubal factor is suspected (with consideration for antibiotic prophylaxis), or even in the setting of unexplained infertility, it is our recommendation not to perform HSG routinely among non-infertile patients, in whom no tubal pathology is suspected, and in whom TVUS/SIS do not raise suspicion for dilated fallopian tube(s).
Conclusion
In the setting of high efficacy ART, particularly when embryo transfer is planned to a non-infertile patient, data provide insufficient guidance for patients and practitioners regarding optimal assessment and treatment of the uterine cavity and fallopian tubes. Success rates from ART are high and seem to be approaching an asymptote. Therefore, there is heightened relevance of even the smallest improvements in the odds of achieving live birth following embryo transfer.
Higher quality studies are needed to confirm whether and to what extent modern ART outcomes are improved by identification and treatment of uterine cavity defects – especially polyps, which are highly prevalent (7) (2). This is even more true among the growing population of non-infertile patients undergoing ART. In the absence of data to guide us, we must consider the high prevalence of uterine cavity defects and that their removal is safe and may be cost effective (2) (77). Bearing in mind the high cost of ART and of donor sperm, as well as the medical risks undertaken in the ART process, we recommend evaluating the uterine cavity with SIS prior to embryo transfer in all patients.
Historically, it was recommended to perform diagnostic hysteroscopy routinely on all patients prior to ART (78, 79). Hysteroscopy has been considered by some as first line in patients with recurrent implantation failure (80) (81); however, recent RCT data contradict this practice (38). In clinics where office hysteroscopy is available, it has been argued to use this modality more broadly, and even as first line, since enables efficient treatment without delay (36); however, again, recent RCT data did not show a benefit in terms of live birth for patients undergoing hysteroscopy prior to first IVF (33). SIS has demonstrated diagnostic accuracy approaching that of hysteroscopy (23) (17, 82) (83) (82) and also enables visualization of the external uterine contour, myometrium and adnexae. Therefore, we recommend SIS, rather than diagnostic hysteroscopy, as the first line cavity assessment prior to embryo transfer in most settings and for most patients. The use of diagnostic hysteroscopy should be limited.
Fallopian tubes should be assessed for patency to determine the most appropriate first line treatment for infertility. While HSG with or without laparoscopy have been historically undertaken, recent data indicate that sonographic tubal assessment with contrast infusion has excellent accuracy and tolerability, particularly when performed at experienced centers. 3D rendering and high-frequency Doppler increase accuracy. While uptake of this diagnostic modality has been slow in the U.S., it holds potential as a ‘one-stop shop’ for comprehensive assessment of pelvic anatomy in the infertile patient and may increase natural fecundity similar to HSG. It is our opinion that 3D ultrasound training should be standard within Reproductive Endocrinology and Infertility (REI) training programs. Consideration should also be given for HyCoSy training. This modality holds potential to reduce reliance upon HSG, which though generally safe, entails an extra step with associated discomfort and cost.
The decision of whether and what tubal assessment is needed to rule out hydrosalpinx, in patients planning embryo transfer with no tubal pathology suspected, is a complex one, particularly in the current era of high-efficacy ART, where even small improvements in odds of success are at a premium. Studies demonstrating benefit with surgical treatment of hydrosalpinges were predominantly based on plain ultrasound diagnosis, and the prevalence of unsuspected hydrosalpinges that can be seen only with contrast instillation is likely low, particularly in a non-infertile population. Therefore, while it remains important to rule out hydrosalpinges prior to embryo transfer in patients with suspected tubal pathology, and even in patients with unexplained infertility, we recommend against the use of HSG prior to embryo transfer in non-infertile patients in whom tubal pathology is not suspected based on history and TVUS/SIS.
In the era of highly-efficacy ART, the future of diagnostic evaluation for infertility has turned in large part towards genetic, genomic, metabolomic, and proteomic evaluation of embryos, endometrium, and even sperm. Still, most sub-fertile couples prefer natural conception, and many maintain a good prognosis for achieving live birth without costly, invasive ART. Saline infusion sonohysterography is a highly sensitive tool for identification of uterine cavity defects (and provides the ability to simultaneously assess the myometrium and ovaries, including antral follicle count). The addition of air infusion with 3D image acquisition and high frequency doppler holds great potential to make transvaginal ultrasound a simple, cost-effective, one-stop shop for comprehensive anatomic assessment in the infertile female. Office hysteroscopy, where available, has advantages in its ability to treat uterine cavity defects in real time but did not show a benefit in terms of live birth.
Given the high cost and high success of IVF with PGT in the U.S., even small increases in the chances of achieving live birth are at a premium. Even so, unnecessary, unproven, and uncomfortable diagnostic tests and procedures should be avoided, and cost-effective, evidence-based modalities should be prioritized, as highlighted by the ongoing debate of ‘add-ons’ to IVF (84, 85). As reviewed here, the available evidence indicates that appropriate contemporary practice prior to embryo transfer includes ruling out significant uterine cavity defects and hydrosalpinges using sonographic techniques followed by individualized surgical treatment of any significant pathology that is uncovered. HSG and diagnostic hysteroscopy are likely over-used, particularly in a non-infertile population. Practitioners should increase their skills in performing and interpreting 3D-SIS prior to ART, and REI fellowship programs should incorporate training in the performance and interpretation of 3D-SIS. Hysteroscopic management of specific lesions should be individualized, and additional studies are needed to provide proof of the dogma that removal of the most common, minor intracavitary findings is truly beneficial before ART.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflicts of interest, commercial or otherwise.
References
- 1.Fertility evaluation of infertile women: a committee opinion. Fertility and sterility. 2021;116. [DOI] [PubMed] [Google Scholar]
- 2.Vitale SG, Haimovich S, Lagana AS, Alonso L, Di Spiezio Sardo A, Carugno J, et al. Endometrial polyps. An evidence-based diagnosis and management guide. Eur J Obstet Gynecol Reprod Biol. 2021;260:70–7. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Medina T, Bajo-Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod. 2005;20:1632–5. [DOI] [PubMed] [Google Scholar]
- 4.Kodaman PH. Hysteroscopic polypectomy for women undergoing IVF treatment: when is it necessary? Curr Opin Obstet Gynecol. 2016;28:184–90. [DOI] [PubMed] [Google Scholar]
- 5.Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand. 2010;89:992–1002. [DOI] [PubMed] [Google Scholar]
- 6.Elias RT, Pereira N, Karipcin FS, Rosenwaks Z, Spandorfer SD. Impact of newly diagnosed endometrial polyps during controlled ovarian hyperstimulation on in vitro fertilization outcomes. J Minim Invasive Gynecol. 2015;22:590–4. [DOI] [PubMed] [Google Scholar]
- 7.Bosteels J, van Wessel S, Weyers S, Broekmans FJ, D’Hooghe TM, Bongers MY, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12:CD009461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262–78. [DOI] [PubMed] [Google Scholar]
- 9.Yan L, Yu Q, Zhang YN, Guo Z, Li Z, Niu J, et al. Effect of type 3 intramural fibroids on in vitro fertilization-intracytoplasmic sperm injection outcomes: a retrospective cohort study. Fertil Steril. 2018;109:817–22 e2. [DOI] [PubMed] [Google Scholar]
- 10.Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin North Am. 2012;39:521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertility and sterility. 2017;108. [DOI] [PubMed] [Google Scholar]
- 12.Bulletti C, De Ziegler D, Polli V, Flamigni C. The role of leiomyomas in infertility. J Am Assoc Gynecol Laparosc. 1999;6:441–5. [DOI] [PubMed] [Google Scholar]
- 13.Taylor HS, Pal L, Seli E, & Fritz MA. Speroff’s clinical gynecologic endocrinology and infertility. 9th ed: Lippincott Williams & Wilkins; 2019. [Google Scholar]
- 14.Salim S, Won H, Nesbitt-Hawes E, Campbell N, Abbott J. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569–81. [DOI] [PubMed] [Google Scholar]
- 15.Jakab A, Ovari L, Juhasz B, Birinyi L, Bacsko G, Toth Z. Detection of feeding artery improves the ultrasound diagnosis of endometrial polyps in asymptomatic patients. Eur J Obstet Gynecol Reprod Biol. 2005;119:103–7. [DOI] [PubMed] [Google Scholar]
- 16.Timmerman D, Verguts J, Konstantinovic ML, Moerman P, Van Schoubroeck D, Deprest J, et al. The pedicle artery sign based on sonography with color Doppler imaging can replace second-stage tests in women with abnormal vaginal bleeding. Ultrasound Obstet Gynecol. 2003;22:166–71. [DOI] [PubMed] [Google Scholar]
- 17.Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine cavity diseases. Fertil Steril. 2000;73:406–11. [DOI] [PubMed] [Google Scholar]
- 18.Hajishaiha M, Ghasemi-Rad M, Karimpour N, Mladkova N, Boromand F. Transvaginal sonographic evaluation at different menstrual cycle phases in diagnosis of uterine lesions. Int J Womens Health. 2011;3:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicinelli E, Romano F, Anastasio PS, Blasi N, Parisi C, Galantino P. Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol. 1995;85:42–7. [DOI] [PubMed] [Google Scholar]
- 20.Sohaey R, Woodward P. Sonohysterography: technique, endometrial findings, and clinical applications. Semin Ultrasound CT MR. 1999;20:250–8. [DOI] [PubMed] [Google Scholar]
- 21.Yang T, Pandya A, Marcal L, Bude RO, Platt JF, Bedi DG, et al. Sonohysterography: Principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salle B, Gaucherand P, de Saint Hilaire P, Rudigoz RC. Transvaginal sonohysterographic evaluation of intrauterine adhesions. J Clin Ultrasound. 1999;27:131–4. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzler P, Concin H, Bosch H, Berlinger A, Wohlgenannt K, Collins WP, et al. An evaluation of sonohysterography and diagnostic hysteroscopy for the assessment of intrauterine pathology. Ultrasound Obstet Gynecol. 1998;11:337–42. [DOI] [PubMed] [Google Scholar]
- 24.Sanin-Ramirez D, Carriles I, Graupera B, Ajossa S, Neri M, Rodriguez I, et al. Two-dimensional transvaginal sonography vs saline contrast sonohysterography for diagnosing endometrial polyps: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2020;56:506–15. [DOI] [PubMed] [Google Scholar]
- 25.Nieuwenhuis LL, Hermans FJ, Bij de Vaate AJM, Leeflang MM, Brolmann HA, Hehenkamp WJ, et al. Three-dimensional saline infusion sonography compared to two-dimensional saline infusion sonography for the diagnosis of focal intracavitary lesions. Cochrane Database Syst Rev. 2017;5:CD011126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin TN S E, Jurkovic D. Ultrasound and intrauterine adhesions: a novel structured approach to diagnosis and management. Ultrasound Obstet Gynecol. 2015;46:131–9. [DOI] [PubMed] [Google Scholar]
- 27.Smit J, Kasius J, Eijkemans M, Veersema S, Fatemi H, EJ Sv, et al. The international agreement study on the diagnosis of the septate uterus at office hysteroscopy in infertile patients. Fertility and sterility. 2013;99. [DOI] [PubMed] [Google Scholar]
- 28.L I, M WP, N CO, L A. Pain Intensity During Ultrasound Assessment of Uterine Cavity and Tubal Patency With and Without Painkillers: Prospective Observational Study. Journal of minimally invasive gynecology. 2017;24. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton JA, Larson AJ, Lower AM, Hasnain S, Grudzinskas JG. Routine use of saline hysterosonography in 500 consecutive, unselected, infertile women. Hum Reprod. 1998;13:2463–73. [DOI] [PubMed] [Google Scholar]
- 30.Campo R, Santangelo F, Gordts S, Di Cesare C, Van Kerrebroeck H, De Angelis MC, et al. Outpatient hysteroscopy. Facts Views Vis Obgyn. 2018;10:115–22. [PMC free article] [PubMed] [Google Scholar]
- 31.Kasius JC, Department of Reproductive Medicine and Gynecology UMCU, 3584 CX Utrecht, The Netherlands, Broekmans FJM, Department of Reproductive Medicine and Gynecology UMCU, 3584 CX Utrecht, The Netherlands, Veersema S, Department of Reproductive Medicine and Gynecology SAh, Nieuwegein, The Netherlands, et al. Observer agreement in the evaluation of the uterine cavity by hysteroscopy prior to in vitro fertilization. Human Reproduction. 2022;26:801–7. [DOI] [PubMed] [Google Scholar]
- 32.D M, H IM, S P, J A, Ø G. Reproducibility of Endometrial Pathologic Findings Obtained on Hysteroscopy, Transvaginal Sonography, and Gel Infusion Sonography in Women With Postmenopausal Bleeding. Journal of minimally invasive gynecology. 2015;22. [DOI] [PubMed] [Google Scholar]
- 33.S JG, K JC, E MJC, K CAM, vG R, N AW, et al. Hysteroscopy before in-vitro fertilisation (inSIGHT): a multicentre, randomised controlled trial. Lancet (London, England). 2016;387. [DOI] [PubMed] [Google Scholar]
- 34.Elsetohy KA, Askalany AH, Hassan M, Dawood Z. Routine office hysteroscopy prior to ICSI vs. ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Arch Gynecol Obstet. 2015;291:193–9. [DOI] [PubMed] [Google Scholar]
- 35.van Dongen H, Timmermans A, Jacobi CE, Elskamp T, de Kroon CD, Jansen FW. Diagnostic hysteroscopy and saline infusion sonography in the diagnosis of intrauterine abnormalities: an assessment of patient preference. Gynecol Surg. 2011;8:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moustafa S, Rosen E, Goodman L. Patient and provider satisfaction with saline ultrasound versus office hysteroscopy for uterine cavity evaluation prior to in vitro fertilization: a randomized controlled trial. J Assist Reprod Genet. 2021;38:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hysteroscopy TCAP. Campaign Against Painful Hysteroscopy: Open letter to the Department of Health and Social Care (20 October 2020) UK2020 [Available from: https://www.pslhub.org/learn/patient-safety-in-health-and-care/womens-health/campaign-against-painful-hysteroscopy-open-letter-to-the-department-of-health-and-social-care-20-october-2020-r3435/.
- 38.E-T T, C R, K Y, T C, G L, G SS, et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): a multicentre, randomised controlled trial. Lancet (London, England). 2016;387. [DOI] [PubMed] [Google Scholar]
- 39.Recommendations | Fertility problems: assessment and treatment | Guidance | NICE: NICE; 2013. [Available from: https://www.nice.org.uk/guidance/cg156/chapter/recommendations.
- 40.Role of tubal surgery in the era of assisted reproductive technology: a committee opinion. Fertility and sterility. 2021;115. [DOI] [PubMed] [Google Scholar]
- 41.NP J, W M, MC S. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. The Cochrane database of systematic reviews. 2004. [DOI] [PubMed] [Google Scholar]
- 42.P C, E S, T T. Management of Hydrosalpinx in the Era of Assisted Reproductive Technology: A Systematic Review and Meta-analysis. Journal of minimally invasive gynecology. 2021;28. [DOI] [PubMed] [Google Scholar]
- 43.Maheux-Lacroix S, Boutin A, Moore L, Bergeron ME, Bujold E, Laberge P, et al. Hysterosalpingosonography for diagnosing tubal occlusion in subfertile women: a systematic review with meta-analysis. Hum Reprod. 2014;29:953–63. [DOI] [PubMed] [Google Scholar]
- 44.Ludwin I, Ludwin A, Wiechec M, Nocun A, Banas T, Basta P, et al. Accuracy of hysterosalpingo-foam sonography in comparison to hysterosalpingo-contrast sonography with air/saline and to laparoscopy with dye. Hum Reprod. 2017;32:758–69. [DOI] [PubMed] [Google Scholar]
- 45.Welie NV, Ludwin A, Martins WP, Mijatovic V, Dreyer K. Tubal Flushing Treatment for Unexplained Infertility. Semin Reprod Med. 2020;38(1):74–86. [DOI] [PubMed] [Google Scholar]
- 46.Saunders RD, Shwayder JM, Nakajima ST. Current methods of tubal patency assessment. Fertil Steril. 2011;95:2171–9. [DOI] [PubMed] [Google Scholar]
- 47.Roest I vW N, Mijatovic V, Dreyer K, Bongers M, Koks C, Mol BW. Complications after hysterosalpingography with oil- or water-based contrast: results of a nationwide survey. Hum Reprod Open. 2020;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ludwin I, Ludwin A, Nastri CO, Coelho Neto MA, Kottner J, Martins WP. Inter-Rater Reliability of Air/Saline HyCoSy, HyFoSy and HyFoSy Combined With Power Doppler for Screening Tubal Patency. Ultraschall Med. 2019;40:47–54. [DOI] [PubMed] [Google Scholar]
- 49.Jeanty P, Besnard S, Arnold A, Turner C, Crum P. Air-contrast sonohysterography as a first step assessment of tubal patency. J Ultrasound Med. 2000;19:519–27. [DOI] [PubMed] [Google Scholar]
- 50.L A, P K, L I, B T, K A. Two- and three-dimensional ultrasonography and sonohysterography versus hysteroscopy with laparoscopy in the differential diagnosis of septate, bicornuate, and arcuate uteri. Journal of minimally invasive gynecology. 2013;20. [DOI] [PubMed] [Google Scholar]
- 51.E MH, vV M, W M, E N. First experiences with hysterosalpingo-foam sonography (HyFoSy) for office tubal patency testing. Human reproduction (Oxford, England). 2012;27. [DOI] [PubMed] [Google Scholar]
- 52.@US_FDA. Drug Trials Snapshots: EXEM FOAM | FDA: @US_FDA; 2022. [Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-exem-foam.
- 53.Ludwin A, Nastri CO, Ludwin I, Martins WP. Hysterosalpingo-lidocaine-foam sonography combined with power Doppler imaging (HyLiFoSy-PD) in tubal patency assessment: ‘flaming tube’ sign. Ultrasound Obstet Gynecol. 2017;50:808–10. [DOI] [PubMed] [Google Scholar]
- 54.Ludwin I, Martins WP, Nastri CO, Ludwin A. Pain Intensity During Ultrasound Assessment of Uterine Cavity and Tubal Patency With and Without Painkillers: Prospective Observational Study. J Minim Invasive Gynecol. 2017;24:599–608. [DOI] [PubMed] [Google Scholar]
- 55.Van Schoubroeck D, Van den Bosch T, Meuleman C, Tomassetti C, D’Hooghe T, Timmerman D. The use of a new gel foam for the evaluation of tubal patency. Gynecol Obstet Invest. 2013;75:152–6. [DOI] [PubMed] [Google Scholar]
- 56.vW N, vR J, D K, vH MHA, B JP, V HR, et al. Can hysterosalpingo-foam sonography replace hysterosalpingography as first-choice tubal patency test? A randomized non-inferiority trial. Human reproduction (Oxford, England). 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boned-Lopez J, Alcazar JL, Errasti T, Ruiz-Zambrana A, Rodriguez I, Pascual MA, et al. Severe pain during hysterosalpingo-contrast sonography (HyCoSy): a systematic review and meta-analysis. Arch Gynecol Obstet. 2021;304:1389–98. [DOI] [PubMed] [Google Scholar]
- 58.Van Schoubroeck D, Van den Bosch T, Ameye L, Boes AS, D’Hooghe T, Timmerman D. Pain during Fallopian-tube patency testing by hysterosalpingo-foam sonography. Ultrasound Obstet Gynecol. 2015;45:346–50. [DOI] [PubMed] [Google Scholar]
- 59.Exalto N, Stassen M, Emanuel MH. Safety aspects and side-effects of ExEm-gel and foam for uterine cavity distension and tubal patency testing. Reprod Biomed Online. 2014;29:534–40. [DOI] [PubMed] [Google Scholar]
- 60.Exalto N, Emanuel MH. Clinical Aspects of HyFoSy as Tubal Patency Test in Subfertility Workup. Biomed Res Int. 2019;2019:4827376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ludwin A, Ludwin I, Szczeklik W, Martins WP. Cutaneous small-vessel vasculitis following hysterosalpingo-foam sonography (HyFoSy). Ultrasound Obstet Gynecol. 2019;54:831–4. [DOI] [PubMed] [Google Scholar]
- 62.Ludwin A, Ludwin I, Martins WP. Venous intravasation during evaluation of tubal patency by ultrasound contrast imaging. Ultrasound Obstet Gynecol. 2018;51:143–5. [DOI] [PubMed] [Google Scholar]
- 63.D K, vR J, M V, G M, V HR, vR IAJ, et al. Oil-Based or Water-Based Contrast for Hysterosalpingography in Infertile Women. The New England journal of medicine. 2017;376. [DOI] [PubMed] [Google Scholar]
- 64.W R, W A, J N, C K, F C, M BWJ, et al. Tubal flushing for subfertility. The Cochrane database of systematic reviews. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Welie N, Pham CT, van Rijswijk J, Dreyer K, Verhoeve HR, Hoek A, et al. The long-term costs and effects of tubal flushing with oil-based versus water-based contrast during hysterosalpingography. Reprod Biomed Online. 2021;42:150–7. [DOI] [PubMed] [Google Scholar]
- 66.van Rijswijk J, van Welie N, Dreyer K, Pham CT, Verhoeve HR, Hoek A, et al. Tubal flushing with oil-based or water-based contrast at hysterosalpingography for infertility: long-term reproductive outcomes of a randomized trial. Fertil Steril. 2020;114(1):155–62. [DOI] [PubMed] [Google Scholar]
- 67.Dreyer K, van Rijswijk J, Mijatovic V, Goddijn M, Verhoeve HR, van Rooij IAJ, et al. Oil-Based or Water-Based Contrast for Hysterosalpingography in Infertile Women. N Engl J Med. 2017;376:2043–52. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, van Welie N, van Rijswijk J, Johnson NP, Norman RJ, Dreyer K, et al. Effectiveness on fertility outcome of tubal flushing with different contrast media: systematic review and network meta-analysis. Ultrasound Obstet Gynecol. 2019;54:172–81. [DOI] [PubMed] [Google Scholar]
- 69.van Welie N, van Rijswijk J, Dreyer K, van Hooff MHA, Bruin JP, Verhoeve HR, et al. Can hysterosalpingo-foam sonography replace hysterosalpingography as first-choice tubal patency test? A randomized non-inferiority trial. Hum Reprod. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.S AW, L BA, M GL, R KS, C RJ, C AS, et al. Salpingectomy or proximal tubal occlusion of unilateral hydrosalpinx increases the potential for spontaneous pregnancy. Human reproduction (Oxford, England). 2003;18. [DOI] [PubMed] [Google Scholar]
- 71.DA S, F S, C J, P D, M L, P PB. Management of hydrosalpinx before IVF: a literature review. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2015;35. [DOI] [PubMed] [Google Scholar]
- 72.A M, T CN, B PM, A AE, GM K. Accuracy of endovaginal sonography for the detection of fallopian tube blockage. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1994;13. [DOI] [PubMed] [Google Scholar]
- 73.S AK, C R, DM P, P A, Z C, A M, et al. Role of ultrasonographic parameters for predicting tubal involvement in infertile patients affected by endometriosis: A retrospective cohort study. Journal of gynecology obstetrics and human reproduction. 2021;50(. [DOI] [PubMed] [Google Scholar]
- 74.AIUM Practice Parameter for the Performance of Sonohysterography and Hysterosalpingo-Contrast Sonography. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2021;40. [DOI] [PubMed] [Google Scholar]
- 75.L L, T J, B C, S A. Influence of HyCoSy on spontaneous pregnancy: a randomized controlled trial. Human reproduction (Oxford, England). 2009;24. [DOI] [PubMed] [Google Scholar]
- 76.S A, B T, B C, G S, A M, T J. The assessment of endometrial pathology and tubal patency: a comparison between the use of ultrasonography and X-ray hysterosalpingography for the investigation of infertility patients. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1999;14. [DOI] [PubMed] [Google Scholar]
- 77.Mouhayar Y, Yin O, Mumford SL, Segars JH. Hysteroscopic polypectomy prior to infertility treatment: A cost analysis and systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;213:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.LS GB, M R, D L, C C, S F. The role of diagnostic hysteroscopy and endometrial biopsy in assisted reproductive technologies. Fertility and sterility. 1998;70. [DOI] [PubMed] [Google Scholar]
- 79.Hinckley M, Milki A. 1000 office-based hysteroscopies prior to in vitro fertilization: feasibility and findings. JSLS : Journal of the Society of Laparoendoscopic Surgeons. 2004;8. [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Turki HA. Hysteroscopy as an investigation tool in recurrent implantation failure in vitro fertilization. Saudi Med J. 2018;39:243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod Biomed Online. 2004;8:590–4. [DOI] [PubMed] [Google Scholar]
- 82.Qazizadeh SH NA, Rashidi BH, Aqsa MM, Sohrabvand F, Tehraninejad S, et al. Comparison of sonohysterography and hysterosalpingography with hysteroscopy in the diagnosis of intrauterine lesions. Iran J Radiol. 2006;4(:37–41. [Google Scholar]
- 83.Kelekci S, Kaya E, Alan M, Alan Y, Bilge U, Mollamahmutoglu L. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril. 2005;84:682–6. [DOI] [PubMed] [Google Scholar]
- 84.vS-G A, M L, O R, P P, C AS, G N. Revisiting selected ethical aspects of current clinical in vitro fertilization (IVF) practice. Journal of assisted reproduction and genetics. 2022;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Glatthorn H, Decherney A. The efficacy of add-ons: selected IVF “add-on” procedures and future directions. Journal of assisted reproduction and genetics. 2022;39. [DOI] [PMC free article] [PubMed] [Google Scholar]


