Abstract
Objective
To investigate the clinical performance, safety, and patient-reported outcomes of an active osseointegrated steady-state implant system that uses piezoelectric technology.
Study Design
A prospective, multicenter, open-label, single-arm, within-subject clinical investigation.
Setting
Three tertiary referral clinical centers located in Melbourne, Sydney, and Hong Kong.
Patients
Twenty-nine adult subjects, 24 with mixed hearing loss or conductive hearing loss and 5 with single-sided sensorineural deafness.
Intervention
Implantation with the Cochlear Osia 2 System.
Main Outcome Measures
Audiological threshold evaluation and speech recognition in quiet and in noise. Patient satisfaction and safety.
Results
At 6-month follow-up after surgery, a mean improvement in pure-tone average of 26.0 dB hearing level and a mean improvement of 8.8 dB signal-to-noise ratio in speech reception threshold in noise was achieved with the investigational device as compared with the unaided situation. Usability of the investigational device was rated 71.4/100 mm for sound processor retention and 81.4/100 mm for overall comfort using a visual analog scale.
Conclusion
These outcomes confirm the clinical safety, performance, and benefit of an innovative active transcutaneous bone conduction implant using a piezoelectric transducer design in subjects with conductive hearing loss, mixed hearing loss, or single-sided sensorineural deafness.
Key Words: Active transcutaneous bone conduction implant, Conductive and mixed hearing loss, Piezoelectric, Safety, Semi-implantable hearing device, Single-sided deafness, Speech recognition in quiet, Speech recognition in noise
INTRODUCTION
Bone conduction hearing implants (BCHIs) are an established means of aural habilitation/rehabilitation for individuals with conductive hearing loss (CHL), mixed hearing loss (MHL), or single-sided deafness (SSD). Both percutaneous and transcutaneous systems are commercially available, with the choice of system dependent on the type and severity of hearing loss and user preference. Percutaneous systems have been demonstrated to provide more efficient sound transmission compared with passive transcutaneous systems (1) in which some transmission losses occur because of skin attenuation.
More recently, active transcutaneous BCHIs have been introduced. These systems have been designed to combine the benefits of transcutaneous solutions with the more efficient transfer capabilities of percutaneous systems (2). In addition, a major benefit is reducing the risk of implant site infections compared with percutaneous systems (3). Similar to passive transcutaneous systems, active transcutaneous systems consist of a sound processor (SP) that is held in position on the skin using an external SP magnet and an implant magnet. However, with active transcutaneous systems, the transducer is implanted under the skin rather than residing within the external SP, thus eliminating the potential for soft tissue attenuation, which can significantly reduce sound transfer, particularly at higher frequencies important for speech understanding (4). This technological innovation allows active transcutaneous devices to provide greater amplification for patients who require it yet prefer a transcutaneous system for esthetic and/or care reasons (5). However, unlike passive systems, active systems typically require that the transducer is recessed into the skull bone (6), which may limit optimal placement of the implant and may require preoperative computed tomographic scanning during surgical planning (7). The implant used in this study uses a piezoelectric transducer, instead of an electromagnetic transducer (2). This design innovation results in a thinner transducer that does not need to be recessed and can be fixed to the bone surface via established osseointegrated implant technology (8).
This study aimed to assess the clinical performance, safety, and patient-reported outcomes (PROs) of a new active transcutaneous osseointegrated steady-state bone conduction (BC) system, in which the redesigned implant has a monolithic design in comparison to the flexible design implemented in the first generation of the system (2).
MATERIALS AND METHODS
This study was an open, prospective, multicenter clinical investigation, conducted at three cochlear implant centers, two in Australia and one in Hong Kong. The investigation was approved by respective local ethics committees as per local regulations and conducted in accordance with the Declaration of Helsinki (9) and ISO14155:2011 (10). The study was registered on ClinicalTrials.gov with identifier NCT04041700. Cochlear Bone Anchored Solutions AB (Mölnycke, Sweden) acted as study sponsor. Remote and on-site monitoring visits were performed by IQVIA RDS, Hong Kong, and Cochlear, Mölnlycke and Avania BV, Sydney, Australia. Statistical analyses were performed by Statistiska Konsultgruppen (Göteborg, Sweden), and data coding was performed by AVANIA BV (Bilthoven, the Netherlands).
Inclusion and Exclusion Criteria
Inclusion criteria included the following: adult subjects with CHL or MHL in the ear to be implanted, with BC threshold pure-tone average (PTA4; mean of thresholds at 0.5, 1, 2, and 4 kHz) of less than or equal to 55 decibel hearing level (dBHL) or with SSD and air-conduction threshold PTA4 (mean of thresholds at 0.5, 1, 2, and 3 kHz) of 20 dBHL or less in the contralateral ear. Subject exclusion criteria were as follows: uncontrolled diabetes, insufficient bone quality/quantity, use of ototoxic drugs that may affect hearing, previous/planned radiotherapy in the implant area, inability to follow investigational procedures, and a condition that could jeopardize osseointegration and/or wound healing (e.g., osteoporosis, psoriasis, long-term systemic use of corticosteroids) or may have an impact on the study outcome as judged by the investigator.
Investigational Device
The investigational device was the Cochlear Osia 2 System (Cochlear Ltd., Sydney, Australia). The system consists of an external SP (Osia 2 SP) magnetically retained on the skin over the site of an internal implant (OSI200 Implant) fixated to the temporal bone with an osseointegrating implant (BI300 screw fixture, 3 or 4 mm). The SP was individually fitted to each subject's hearing loss using Osia Fitting Software 2.0.
Surgical Technique
The single-component design of the OSI200 Implant allows a simpler surgical procedure than was used with the OSI100 Implant. The appropriate site for the transducer and coil, which transfers the signal and power between SP and implant, was planned using a nonsterile silicone template. The incision was made 1.5 cm clear of the implant margin and a scalp flap elevated lateral to periosteum adequate to expose the entire transducer site. A subperiosteal pocket was created for the coil component of the device and positioning confirmed with a sterile silicone template. For subjects with thick scalps, the coil could be positioned lateral to periosteum or even lateral to temporalis muscle. The BI300 was then placed in the usual manner at the center of the planned transducer site. The bone bed indicator clearance tool was then attached to the BI300 screw fixture, and soft tissue and bone were removed as necessary to allow free rotation and confirm clearance. The OSI200 implant was then positioned, secured to the BI300 screw fixture using the torque wrench, and the incision closed.
Study Schedule and Assessments
At the screening and baseline visit, baseline characteristics and medical history were recorded, and complete audiograms were obtained. The subcutaneous components of the investigational device were implanted unilaterally or bilaterally at a subsequent visit: for the one subject implanted bilaterally, one side was preoperatively selected as test ear for efficacy evaluations. Postoperative visits were carried out at 2 weeks (suture removal), 4 weeks (SP fitting), 6 weeks (SP check), 3 months (primary efficacy and safety evaluation), and 6 months (end of study) after surgery. Surgical study parameters evaluated included the following: soft tissue thickness, soft tissue thinning at the SP location (mandated if thickness >9 mm), BI300 screw fixture location, type of anesthesia, surgery time, any bone polishing/removal at the actuator site, and type/location of surgical incision.
Audiological assessments were performed unaided and using a Cochlear Baha 5 Power SP on a Baha Softband fitted using Baha Fitting Software 5.4 (Cochlear Bone Anchored Solutions AB, Mölnlycke, Sweden) to ascertain expected benefits at the baseline visit and with the investigational device at the time of activation (first fitting of SP on implant) and all subsequent visits. The tests were performed in a sound-insulated audiometric booth using calibrated equipment with the nontest ear blocked in case of normal or near-normal hearing or a large asymmetry between ears. During testing, SPs were set to fixed directionality mode. Threshold audiometry was performed using warble tones presented via a loudspeaker located 1 m in front of the subjects at 0-degree azimuth, with 1 m of free space surrounding the test subject. The speech test in quiet was performed using phonetically balanced monosyllabic words presented in free field through a speaker placed 1 m in front of the seated subject (0-degree azimuth) at 50-, 65-, and 80 dB speech pressure level (SPL). Scores were recorded as percentage of correctly repeated words at each presentation level, and a change in score of at least 10 percentage points can be considered as clinically relevant, based on clinical consensus. During speech in noise testing, both speech and noise were presented in free field at 0-degree azimuth (front). In Melbourne and Sydney, the AuSTIN test was administered and sentences were presented at a constant level of 65 dB SPL throughout the test, and babble noise was adapted stepwise according to the software used to establish the signal-to-noise ratio (SNR) providing a 50% level of correctly repeated morphemes. In Hong Kong, the CHINT methodology was used, where noise was maintained at a constant 65 dB SPL, and speech was adapted stepwise according to the software used to establish the SNR where the test subject repeated 50% of the material correctly. Changes of greater than 3 dB SNR are reported as clinically relevant because it has been demonstrated that changes of greater than 3 dB are required to be reliably discriminable (11).
PROs were collected at baseline and at 3 and 6 months after implantation using validated questionnaires: the Health Utilities Index (HUI3) (12,13), the Abbreviated Profile of Hearing Aid Benefit (APHAB) (14,15), and the Speech, Spatial and Qualities of Hearing Scale 12 (SSQ12) (16,17). HUI3 evaluates eight health-related quality of life (QoL) dimensions (vision, hearing, speech, walking/mobility, dexterity, self-care, emotion, cognition) and a comprehensive health state attribute. A change in global HUI3 score of 0.03 or higher is considered clinically relevant (18). APHAB is a hearing-related PRO instrument, which includes four subscales (ease of communication, reverberation, background noise, aversiveness) and a global score. A change in score of higher than 10 for global score is generally regarded as clinically relevant (19). SSQ12 is a short (12-item) version of the original 49-item SSQ questionnaire (20) that measures the self-reported auditory disability in everyday life across three subdomains (speech, spatial, and qualities of hearing). Changes of 1.0 unit or greater on SSQ subscales indicate a clinically relevant change (20).
Patient-reported daily usage, wearing comfort, and retention were collected at all study visits after activation. Daily use was reported as the average hours of daily SP use during the period preceding the visit. Comfort and retention were assessed by indication on a visual analog scale consisting of a straight line running from 0 to 100 mm (a mark placed at 0 mm indicated no comfort at all/insufficient retention, and a mark placed at 100 mm indicated the most comfortable situation imaginable/excellent retention). The primary safety analysis was performed 3 months after surgery, but safety parameters were recorded throughout the investigation.
The primary efficacy endpoints in the investigation were the improvement in 1) the mean free-field thresholds (PTA4) and 2) speech reception threshold (SRT) in noise (dB SNR) with the investigational device at 3 months as compared with preoperative unaided hearing. Complete analysis of the primary and all secondary efficacy endpoints was performed with the data at 3 and 6 months.
Statistical Analyses
Statistical analyses were performed according to a predefined statistical analysis plan. Efficacy analyses were performed on the intention-to-treat population (all implanted subjects) and per-protocol population (all subjects who completed the investigation without major protocol deviations). Safety analyses were performed on the safety population (all surgically treated subjects). The primary and secondary efficacy analysis was performed on the total population. Audiological results were analyzed against the subjects' unaided hearing and against the preoperative performance with Baha 5 Power on a Softband. Health-related QoL (HUI) with the investigational device was compared with the subject's preoperative situation (with or without previous hearing amplification). Hearing-related PROs (APHAB, SSQ) were compared with preoperative unaided hearing whether or not the subject used a hearing aid preoperatively.
All statistical analyses were paired and nonparametric. The Fisher's nonparametric permutation test for paired observations was used for most of the paired analyses of continuous variables (when this test failed to approximate the p value, Wilcoxon-signed rank test was used). For paired analysis of dichotomous and ordered categorical variables, the Sign test was used. All significance tests were two-tailed and performed at the 0.05 significance level. Effect sizes (ESs) for speech performance measures were calculated by dividing the standardized test statistic by the square root of the sample size. ESs for health outcome questionnaires are represented by the standardized response mean, which was calculated by dividing the change in score by the standard deviation (SD) of the change in score. Both measures can be interpreted using Cohen's standard of ESs.
Demographics, baseline characteristics, surgical variables, daily use, comfort, adverse events, and device deficiencies were only analyzed descriptively.
The required sample size was calculated based on 6-month safety data from 51 subjects with MHL, CHL, or SSD implanted with the predecessor Osia System at five clinics in a multicenter clinical investigation (2). Using these data, it was estimated that 30 subjects enrolled across three clinics would be sufficient to detect any safety issues attributed to the Investigational device and to detect significant changes in the primary performance evaluations: mean audiometric thresholds (PTA4) and SRT in noise (SNR, dB SNR) with a power of 0.99.
RESULTS
Thirty-four adult subjects signed an informed consent form, and 29 of these were implanted with the investigational device. Five subjects did not undergo surgery and were withdrawn from the investigation after the preoperative baseline visit. All 29 implanted subjects were included in the intention-to-treat and safety population, and 16 subjects were included in the per-protocol population. Because of the COVID-19 pandemic, some subjects were prevented from attending clinic visits, and complete fitting data were obtained for 27 subjects. In addition, 10 subjects were unable to complete audiological assessment at 3-month follow-up. However, these subjects did report experiencing improvements with the investigational device, which was supported when data were collected from these subjects at 6-month follow-up. Presented data therefore include outcomes at 6 months for the primary efficacy endpoint and for all safety and performance endpoints, because the majority of subjects attended the clinic at this time point. Two subjects were unable to complete audiological assessments at 6 months, where missing data were carried forward from the previous follow-up visit, as supported by a sensitivity analysis. Because the study was not powered for individual indications and only five SSD subjects were recruited, p values are presented for the whole sample. Patient demographics can be seen in Table 1.
TABLE 1.
Patient demographics, baseline characteristics, and recruitment site
| Variable | Total (n = 29) |
Mixed/Conductive Hearing Loss (n = 24) |
SSD (n = 5) |
|---|---|---|---|
| Age, mean (SD; range), yr | 46.7 (19.7; 21–82) | 44.9 (18.8; 21–76) | 55.2 (23.8; 23–82) |
| Sex | |||
| Male | 13 (44.8%) | 12 (50.0%) | 1 (20.0%) |
| Female | 16 (55.2%) | 12 (50.0%) | 4 (80.0%) |
| Race | |||
| Asian | 3 (10.3%) | 3 (12.5%) | 0 (0.0%) |
| Australian Aboriginal | 1 (3.4%) | 1 (4.2%) | 0 (0.0%) |
| White | 23 (79.3%) | 18 (75.0%) | 5 (100.0%) |
| Other | 2 (6.9%) | 2 (8.3%) | 0 (0.0%) |
| Site | |||
| Royal Victorian Eye and Ear Hospital | 20 (69.0%) | 17 (70.8%) | 3 (60.0%) |
| Sydney Cochlear Implant Centre | 6 (20.7%) | 4 (16.7%) | 2 (40.0%) |
| Prince of Wales Hospital, Shatin | 3 (10.3%) | 3 (12.5%) | 0 (0.0%) |
SD indicates standard deviation; SSD, single-sided deafness.
Surgery and Safety Evaluation
The mean (SD) time for surgery was 52.8 (13.3) minutes (range, 30–84 min), and all subjects were implanted under general anesthesia. Bone polishing was performed in 12 of 29 subjects, and all subjects received the 4-mm BI300 Implant. Soft tissue reduction was not required in any of the subjects, and the mean (SD) soft tissue thickness was 6.6 (1.29) mm (range, 5–9 mm). A C-shaped surgical incision was used when implanting 12 subjects, an S-shaped incision was used when implanting in 12 subjects, and 5 subjects received an alternative incision type. Mean (SD) incision length was 71.4 (23.1) mm (range, 30.0–120.0 mm), and the location of the surgical incision in relation to the implant was anterior in 26 (89.7%) subjects and posterior in the remaining 3 (10.3%) subjects. Finally, the coil was placed in a periosteal pocket for 24 (82.8%) subjects, on top of the periosteum for 3 (10.3%) of the subjects, and on top of the muscle for 2 (6.9%) of the subjects.
Regarding safety, 27 adverse events were reported in 15 subjects. Five events were device related, 12 were related to the surgical procedure, and a further 10 events were related to both device and procedure. A majority of these events were minor, such as itching and mild discomfort, with 24 being classified as mild in severity and the remaining 3 events classified as moderate. The events of moderate severity were pain in one subject and wound infections in two subjects. Four subjects received antibiotics to treat wound infections, including both subjects with infections of moderate severity, all were resolved by the 3-month visit. Discomfort and itchiness around the incision reported in one subject were not resolved by study end; however, all other adverse events were resolved.
Audiological Evaluation
Baseline audiograms for subjects with MHL/CHL and SSD are shown in Figure 1, and data used to plot audiograms and mean PTA4 hearing thresholds for both implanted and nonimplanted ears are available in Supplemental Digital Content (Supplementary Tables 1–3, http://links.lww.com/MAO/B462). Aided hearing thresholds for patients with CHL/MHL or patients with SSD can be seen in Figure 2, A and B, where changes are both clinically relevant and statistically significant (p <0.05) at all frequencies, except at 8 kHz. The improvement in mean PTA4 hearing thresholds at 6 months compared with the preoperative unaided hearing situation was statistically significant (p <0.05), and the ES was large (>0.8; Table 2). The mean PTA4 improvement in all subjects was also greater than 10 dB and therefore clinically relevant. Similarly, mean improvements in speech tests in quiet (Fig. 2C) were statistically significant (p <0.001) compared with unaided hearing in all test conditions (Table 2). Individually, these improvements were clinically important for 86, 89, and 89% of subjects at 50, 65, and 80 dB SPL, respectively. The mean improvement in the SRT in noise (Fig. 2D; Table 2) was also statistically significant (p <0.05) and clinically relevant. Clinically relevant improvements of greater than 3 dB SNR were recorded for 19 of the 27 subjects. Mean (SD) improvements in speech recognition scores in quiet of 10.7 (18.3; range, −28.0 to 48.0), 8.8 (15.2; range, −18.0 to 58.0), and 4.0 (8.26; range, −12.9 to 30.0) percentage points were also measured with the investigational device compared with the preoperative situation with Baha 5 Power on a Softband at 50, 65, and 80 dB SPL, respectively. These changes were all statistically significant (p <0.05), and clinically relevant at 50 dB SPL. A mean (SD) improvement of 0.85 (1.59) dB SNR (range, −3.50 to 2.50 dB SNR) was also measured with the investigational device at 6-month follow-up compared with the preoperative situation using Baha 5 Power on a Softband when listening in noise (p <0.05), which did not reach clinical relevance.
FIG. 1.
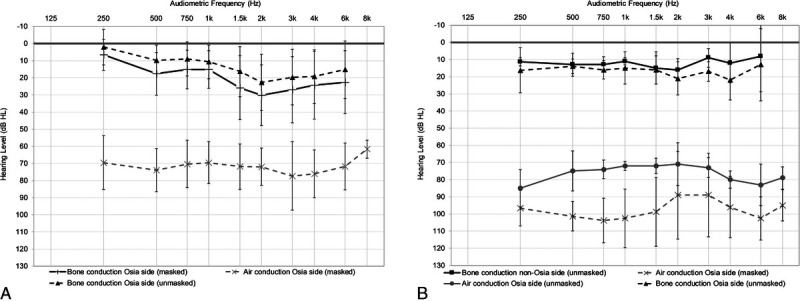
Mean baseline audiograms for subjects with mixed or conductive hearing loss (n = 23; A) or single-sided sensorineural deafness (n = 4; B). Error bars represent the standard deviation of the mean.
FIG. 2.
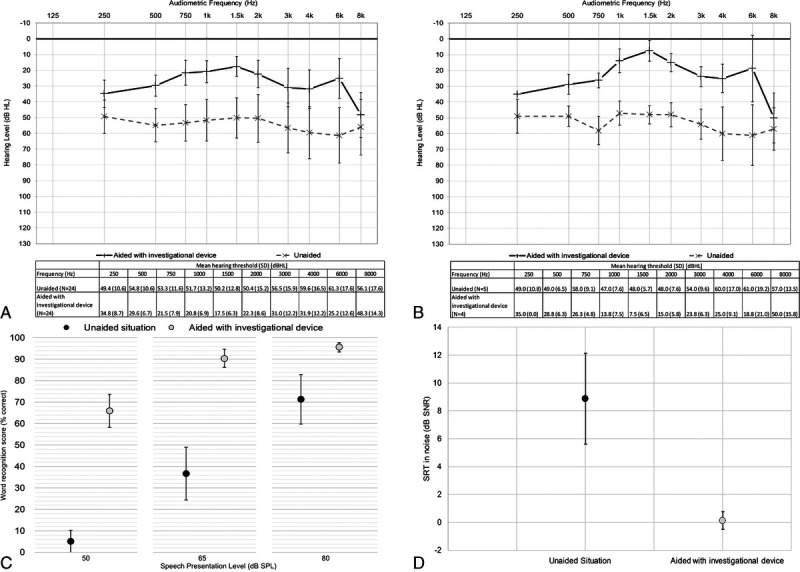
Free-field thresholds measured preoperatively and with the investigational device 6 months after surgery in patients with MHL or CHL (n = 23; A) and patients with SSD (n = 4; B), where error bars represent the standard deviation of the mean. Word recognition scores in quiet (n = 27; mean and 95% CIs; C) and SRTs in noise (n = 27; mean and 95% CIs; D), both measured preoperatively unaided and with the investigational device at 6 months. CHL indicates conductive hearing loss; CI, confidence interval; MHL, mixed hearing loss; SRTs, speech reception thresholds.
TABLE 2.
Mean change in audiometric results from to the preoperative unaided situation to the postoperative aided situation with the investigational device at 6 months
| Variable | Improvement from Unaided to 6 mo Aided, Mean (SD; Range) |
|---|---|
| Free-field hearing thresholds, PTA4, dBHL | 28.4 (9.6; 10 to 46.3), n= 27, p < 0.001, ES 0.87 |
| Speech reception threshold in noise, dB SNR | 8.84 (7.88; −1.4 to 21.8), n = 27, p < 0.001, ES 0.83 |
| Word recognition score in quiet, 50 dB SPL (% correct) | 62.3 (22.1; −4.0 to 84.0), n= 27, p < 0.001, ES 0.85 |
| Word recognition score in quiet, 65 dB SPL (% correct) | 54.0 (29.8; −6.0 to 98.0), n= 27, p < 0.001, ES 0.85 |
| Word recognition score in quiet, 80 dB SPL (% correct) | 24.3 (28.0; −7.0 to 92.0), n= 27, p < 0.001, ES 0.80 |
ES, effect size; HL, hearing level; PTA4, pure-tone average (thresholds at 0.5, 1, 2, and 4 kHz); SD, standard deviation; SNR, signal-to-noise ratio; SPL, speech pressure level.
Patient-Reported Outcomes
The changes in the PROs between the preoperative and 6-month postoperative conditions are presented in Tables 2 and 3. HUI revealed a clinically relevant and statistically significant improvement (p <0.05; ES 0.55) in a comprehensive health state for the total population. A statistically significant (p <0.05, ES 0.41) and clinically relevant improvement in HUI3 score of 0.13 was also recorded in the hearing domain for the total population. Compared with the unaided situation, statistically significant (p <0.001, ES >0.80) and clinically relevant mean improvements were obtained for all SSQ subdomains: total score, speech score, spatial score, and quality score. Mean APHAB global score and all subscale scores, except the aversiveness score, also showed a statistically significant (p <0.001, ES >0.80) and clinically relevant improvements in the total population. Mean (SD) daily device usage at the end of the study was 8.6 h/d (4.7) h/d (range, 0.0–17.0 h/d) for the total population, with an estimated mean (SD) battery lifetime of 24.3 (12.4) hours (range, 4.0–56.0 hours). One patient with SSD did not use their SP during the follow-up period because of a change in lifestyle attributed to the COVID-19 pandemic. This patient mostly remained at home during the investigation and did not engage in typical activities in which they would normally wear their SP. The mean (SD) reported retention score was 71.4/100 (26.7) mm (range, 7.0–100.0 mm), and the mean (SD) reported comfort level was 81.4/100 (20.0) mm (range, 29.0–100.0 mm) for the total sample.
TABLE 3.
Mean change in HUI3, APHAB, and SSQ from the preoperative situation to the postoperative situation after 6 months
| Variable | Change From Unaided to 6 mo Aided, Mean (SD; Range) |
|---|---|
| HUI, comprehensive health state | 0.091 (0.165; −0.173 to 0.397), ES 0.55, n = 27 |
| HUI, hearing attribute | 0.129 (0.318; −0.290 to 0.710), ES 0.41, n = 27 |
| APHAB, global | 25.9 (26.2; −29.9 to 70.8), ES 0.99, n = 27 |
| APHAB, ease of communication | 27.4 (31.4; −43.7 to 82.2), ES 0.87, n = 27 |
| APHAB, background noise | 28.7 (28.1; −41.3 to 74.7), ES 1.02, n = 27 |
| APHAB, reverberation | 31.9 (19.2; 4.7 to 74.8), ES 1.66, n = 27 |
| SSQ, total | 2.50 (1.66; −0.58 to 5.50), ES 1.51, n = 27 |
| SSQ, speech | 2.68 (1.89; −1.90 to 6.08), ES 1.42, n = 27 |
| SSQ, spatial | 2.30 (2.42; −0.93 to 7.00), ES 0.95, n = 27 |
| SSQ, qualities | 2.41 (1.81; −1.00 to 6.68), ES 1.33, n = 27 |
Statistically significant (p ˂ 0.05) changes are reported.
ES, effect size; HL, hearing level; PTA4, pure-tone average (thresholds at 0.5, 1, 2, and 4 kHz); SD, standard deviation; SNR, signal-to-noise ratio; SPL, speech pressure level.
DISCUSSION
A new design active transcutaneous BCHI using piezoelectric stimulation for rehabilitation of patients with CHL, MHL, or SSD was clinically evaluated in this international, multicenter clinical investigation. Audiological outcomes, PROs, and safety data collected during the 6-month follow-up period demonstrate that the system is safe and that it provides excellent aural habilitation/rehabilitation. The low profile of the implant, made possible by the slim design of the piezoelectric actuator, allows for minimal bone removal compared with other active transcutaneous systems, which require the electromagnetic actuator to be recessed. This ability to place the actuator on the bone surface negates the requirement for it to be recessed, making the surgery more straightforward (21). The single-component design of the implant also permits for some surgical versatility, and it was possible to successfully position the coil above the muscle in some patients, further simplifying the surgery and reducing operating time. Soft tissue thinning was not required in any subject as the digital link between coil and SP can transmit over distances of up to 10 mm. Mean surgery time from first incision to closure was 53 minutes, with the shortest operations taking 30 minutes. It is expected that surgery times will reduce as surgeons become more familiar with the procedure. Transcutaneous systems typically result in lower complication rates compared with percutaneous systems (6), and this was reflected in our safety data, where operations were uneventful, complications were mostly mild in severity and postoperative infections only occurred in four patients. All infections resolved after treatment with antibiotics, and there were no implant losses.
The device provided a statistically significant and clinically relevant improvement of 28.4 dB in the PTA compared with the unaided situation. Improvements were particularly prominent at higher frequencies, which is the hallmark of this piezoelectric active transcutaneous BC system (2,5), and it provided an average gain of 36.1 dB at 6000 Hz compared with unaided and 13.9 dB compared with the Baha 5 Power on a Softband. Although the study was not powered to test superiority of the investigational system, compared with using a Baha 5 Power SP on a Softband, statistically significant (p <0.05) mean improvements in aided thresholds of 3.4, 4.6, 7.3, and 13.9 dB with the investigational device were recorded at 1.5, 2, 4, and 6 kHz, respectively. Although statistically significant, only the final measure is clinically relevant. The outcomes highlight the importance of trialing a Baha 5 SP on a Softband to provide an impression of the anticipated benefit that can be achieved with the Osia system before surgery and thus support the potential Osia candidate in their decision and shape their postimplant expectations. However, superior performance should be expected with the Osia system compared with passive transcutaneous systems because of the lack of skin attenuation and the ability to place the implant closer to the cochlea (22). The Osia system also utilizes a piezoelectric transducer, which can provide greater output at higher frequencies compared with electromagnetic transducers (23). This study showed that the device provided statistically significant and clinically relevant improvements in speech recognition in noise and in quiet compared with the unaided situation. Statistically significant improvements (p <0.05) in speech recognition in quiet and in noise were also observed compared with the preoperative condition with a Baha 5 Power SP on a Softband. These changes were clinically important at 50 dB SPL, but not when listening in noise. The statistically significant and clinically relevant audiological results obtained with the investigational device for patients with MHL/CHL or SSD echo those obtained in other studies using a previous generation of the system (2,5,21). These studies also present statistically significant and clinically relevant improvements in aided hearing thresholds compared with the unaided situation and show statistically significant improvements in aided speech recognition compared with the unaided situation (2,5,21). One study (2) presenting HUI3 data captured a comparable mean improvement in hearing attribute of 0.149 at 12-month follow-up. This study also presented a mean improvement in global APHAB score of 26.3 compared with our score of 25.9 (2). SSQ-12 results also align with two studies presenting SSQ-12 data, in which patients reported that the investigational device reduced their hearing difficulties (2,5).
PROs showed clinically relevant subjective improvements in hearing benefit and health-related QoL compared with the preoperative situation. APHAB results demonstrated significant improvements in all measured domains other than aversiveness, in which decreasing scores have been correlated with increasing hearing loss (24). In addition, in the SSQ12 questionnaire, patients reported statistically significant improvements in all parameters: speech, spatial, and quality. These results were reflected in HUI3 data, where a statistically significant and clinically relevant improvement in comprehensive health state was captured, which was primarily due to improvements in the hearing domain, but mean improvements were also captured across all other subdomains other than walking.
Comfort and battery life are considered important by patients requiring a hearing device (25). Mean comfort of the system was rated 81/100 mm, indicating that recipients considered the device to be comfortable. The average daily usage time reported at 6-month follow-up was 8.6 h/d, indicating a device usage between battery changes of 1 day and up to 2.5 d. High levels of compliance indicate that subjects were satisfied with the device and that they found the system beneficial, as supported by other outcome measures.
Key limitations of the present study should be addressed. Insufficient recruitment of patients with SSD limited our ability to perform a robust subanalysis on this subgroup. Therefore, conclusions regarding benefits in the subgroup of patients with SSD should be interpreted with caution. However, this group of patients has shown favorable hearing and QoL outcomes with a previous generation of the system (2). In addition, follow-up is limited to 6 months, and further studies are required to map the long-term outcomes and safety profile of the investigational device. Finally, future studies should aim to compare active implantable transcutaneous systems with both passive implantable transcutaneous systems and percutaneous systems, as more robust data are needed to guide decision making regarding these categories of implant.
CONCLUSIONS
This clinical investigation has demonstrated that the Osia 2 System, using an innovative piezoelectric transducer, is a safe and effective treatment option for patients with CHL, MHL (up to BC thresholds of 55 dBHL), and SSD. The system provides significant clinical benefits in terms of improved hearing performance and QoL. The outcomes from this study correspond well with the benefits seen in earlier studies using the OSI100 implant.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Kammy Yeung and Dr. Wai Tsz Chang for their contributions to the work conducted at The Chinese University of Hong Kong; Rachelle Hassarati, Jonathan Kong, Alex Saxby, and Lori Sword for their contributions to the work conducted at Next Sense (formerly SCIC/RIDBC) Sydney; and Prof. Stephen O'Leary, Dr. Markus Dahm, Dr. Jeanmarc Gerard, Dr. Claire Iseli, and Evelyn Do for their contributions to the work conducted at The Royal Victorian Eye and Ear Hospital Melbourne and Adrienne Paterson at HEARnet Melbourne. The authors would also like to thank statistician Mattias Molin and team at Statistiska Konsultgruppen for their support with statistical analysis.
Footnotes
Sources of support and disclosure of funding: This study was sponsored by Cochlear, a manufacturer and supplier of hearing devices. A.T.L. and A.Ö. are current Cochlear employees.
Supplemental digital content is available in the text.
Contributor Information
Catherine S. Birman, Email: Catherine.Birman@nextsense.org.au.
Nicholas Baulderstone, Email: Nick.baulderstone@nextsense.org.au.
Aaran T. Lewis, Email: aalewis@cochlear.com.
Iris H.Y. Ng, Email: irisng@ent.cuhk.edu.hk.
Anna Östblom, Email: aostblom@cochlear.com.
Alex Rousset, Email: Alex.Rousset@eyeandear.org.au.
Sylvia Tari, Email: sylvia.tari@eyeandear.org.au.
Michael C.F. Tong, Email: mtong@ent.cuhk.edu.hk.
Robert Cowan, Email: r.cowan@unimelb.edu.au.
REFERENCES
- 1.Rigato C Reinfeldt S Håkansson B, et al. Effect of transducer attachment on vibration transmission and transcranial attenuation for direct drive bone conduction stimulation. Hear Res 2019;381:107763. [DOI] [PubMed] [Google Scholar]
- 2.Mylanus EAM Hua H Wigren S, et al. Multicenter clinical investigation of a new active osseointegrated steady-state implant system. Otol Neurotol 2020;41:1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberlies NR Castaño JE Freiser ME, et al. Outcomes of BAHA connect vs BAHA attract in pediatric patients. Int J Pediatr Otorhinolaryngol 2020;135:110125. [DOI] [PubMed] [Google Scholar]
- 4.Motlagh Zadeh L Silbert NH Sternasty K, et al. Extended high-frequency hearing enhances speech perception in noise. Proc Natl Acad Sci 2019;116:23753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goycoolea M Ribalta G Tocornal F, et al. Clinical performance of the Osia™ system, a new active osseointegrated implant system. Results from a prospective clinical investigation. Acta Otolaryngol 2020;140:212–9. [DOI] [PubMed] [Google Scholar]
- 6.Magele A, Schoerg P, Stanek B, Gradl B, Sprinzl GM. Active transcutaneous bone conduction hearing implants: Systematic review and meta-analysis. PLoS One 2019;14:e0221484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plontke SK, Götze G, Wenzel C, Rahne T, Mlynski R. Implantation of a new active bone conduction hearing device with optimized geometry. HNO 2020;68:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelissen RC den Besten CA Faber HT, et al. Loading of osseointegrated implants for bone conduction hearing at 3 weeks: 3-Year stability, survival, and tolerability. Eur Arch Otorhinolaryngol 2016;273:1731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association . World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 10.ISO . Clinical investigation of medical devices for human subjects—Good clinical practice. 2011. Available at: https://www.iso.org/standard/45557.html. Accessed August 19, 2020.
- 11.McShefferty D, Whitmer WM, Akeroyd MA. The just-noticeable difference in speech-to-noise ratio. Trends Hear 2015;19:2331216515572316. doi: 10.1177/2331216515572316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feeny D Furlong W Torrance GW, et al. Multiattribute and single-attribute utility functions for the Health Utilities Index Mark 3 System. Med Care 2002;40:113–28. [DOI] [PubMed] [Google Scholar]
- 13.Mok WK Wong WH Mok GT, et al. Validation and application of Health Utilities Index in Chinese subjects with down syndrome. Health Qual Life Outcomes 2014;12:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox RM, Alexander GC. The abbreviated profile of hearing aid benefit. Ear Hear 1995;16:176–86. [DOI] [PubMed] [Google Scholar]
- 15.Kam AC, Tong MC, van Hasselt A. Cross-cultural adaptation and validation of the Chinese abbreviated profile of hearing aid benefit. Int J Audiol 2011;50:334–9. [DOI] [PubMed] [Google Scholar]
- 16.Noble W, Jensen NS, Naylor G, Bhullar N, Akeroyd MA. A short form of the Speech, Spatial and Qualities of Hearing Scale suitable for clinical use: the SSQ12. Int J Audiol 2013;52:409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou H, Wen B, Perreau A, Kim E, Tyler R. Validation of the Chinese translation of the Spatial Hearing Questionnaire and its short form. Am J Audiol 2016;25:25–33. [DOI] [PubMed] [Google Scholar]
- 18.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): Concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox RM. Administration and application of the APHAB. Hear J. 1997;50:32–48. [Google Scholar]
- 20.Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol 2004;43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau K Scotta G Wright K, et al. First United Kingdom experience of the novel Osia active transcutaneous piezoelectric bone conduction implant. Eur Arch Otorhinolaryngol 2020;277:2995–3002. [DOI] [PubMed] [Google Scholar]
- 22.Beros S Dobrev I Farahmandi TS, et al. Transcutaneous and percutaneous bone conduction sound propagation in single-sided deaf patients and cadaveric heads. Int J Audiol 2021;7:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Park IY, Shimizu Y, O'Connor KN, Puria S, Cho JH. Comparisons of electromagnetic and piezoelectric floating-mass transducers in human cadaveric temporal bones. Hear Res 2011;272(1–2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Löhler J Akcicek B Wollenberg B, et al. The influence of frequency-dependent hearing loss to unaided APHAB scores. Eur Arch Otorhinolaryngol 2016;273:3587–93. [DOI] [PubMed] [Google Scholar]
- 25.Kochkin S. MarkeTrak V: “Why my hearing aids are in the drawer” The consumers' perspective. Hear J 2000;53:34, 36, 39–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


