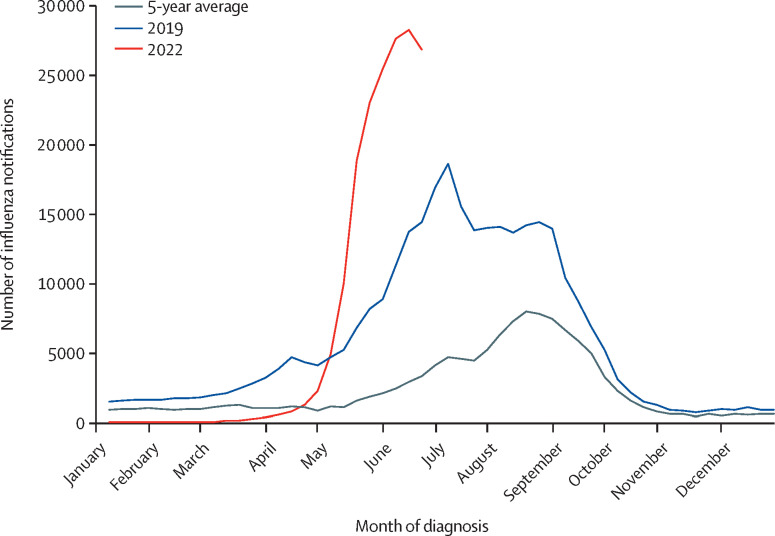
Non-pharmaceutical interventions for COVID-19 have resulted in very low levels of circulating influenza globally. However, now that these pandemic control measures have been abandoned in the UK, the return of influenza as a major public health issue appears inevitable. There has been a rapid rise in influenza A notifications in Australia, which started earlier than usual and as of writing are tracking at record high numbers (figure ). These data from Australia help predict what is to come in the northern hemisphere in the winter of 2022–23.
Figure.
Notifications of laboratory-confirmed influenza in Australia
The 5-year average is based on notifications from Jan 1, 2017, to June 19, 2022. The figure was adapted from the Australian Government Department of Health and Aged Care.1
As of June 19, 2022, 85% of influenza cases in Australia were due to influenza A (H3N2),1 which is known to cause more severe epidemics.2 The sharp increase in cases was probably driven by relaxation of measures put in place to mitigate the COVID-19 pandemic and the low proportion of the population vaccinated against influenza. In addition, there has been little natural influenza infection for the past 2 years. As a result, herd immunity against currently circulating viruses is probably substantially lower compared with previous years, a situation exacerbated by the entire cohort of children younger than 2 years who have never been exposed to influenza.
Uptake of the seasonal influenza vaccine has been declining in Australia and the UK, including in those at risk of severe disease, such as pregnant women and children.3, 4 Influenza vaccination rates also dropped in UK health-care workers from 77% in 2020–21 to 61% in 2021–22, when the vaccine was offered concomitantly with the COVID-19 booster.3 Public attention is focused on COVID-19 and potential autumn booster vaccinations, and safety concerns and mistrust of COVID-19 vaccines might result in enhanced hesitancy towards the influenza vaccine. Barriers to vaccination should be addressed by engaging with communities, including members of minority ethnic groups, and training vaccinators to emphasise the safety and efficacy of coadministering COVID-19 and influenza vaccines.
The measures taken to control the COVID-19 pandemic have created new challenges for managing seasonal influenza. The Australian data provide a warning for an earlier and more severe influenza season in the northern hemisphere. The UK Joint Committee on Vaccination and Immunisation decision to remove those aged 50–64 and 11–15 years from the groups eligible for the 2022–23 influenza vaccine should be reconsidered.5 Children are responsible for most influenza transmission, as in Australia, where 10–14 year olds currently have one of the highest infection rates.1 Therefore, vaccinating children aged 11–15 years is particularly important. However, for this to be effective, vaccine campaigns should start early and disparities in the vaccination of groups at high risk of infection (eg, health-care workers and children) must be addressed.
DP reports a doctoral research fellowship from the National Institute for Health and Care Research. IB reports shares in CSL. SGS reports support from WHO “to conduct a systematic review, appraisal and grading of evidence on repeat seasonal influenza vaccination” and the National Institutes of Health (R01AI141534); OptumLabs research credits through the University of California (no funding received, but access to data granted for 1 year); participation in advisory boards (no remuneration received) for influenza vaccines at Seqiris and Sanofi; serving as an unpaid member of the WHO Strategic Advisory Group of Experts on Immunization Working Group on Influenza from 2017 to 2021; being, since 2011, an observer or invited member of the National Influenza Surveillance Committee (unpaid); and funding to her employer, the WHO Collaborating Centre for Reference and Research on Influenza, for the development of influenza vaccines from Sanofi and International Federation of Pharmaceutical Manufacturers and Associations. TWC reports consulting fees from Biofire/BioMerieux, QIAGEN, Cepheid, Sanofi, and Roche/Shionogi; payment from QIAGEN, Biofire/BioMerieux, and Janssen for a diagnostics educational event and presentations at conferences; participating on a data and safety monitoring board for Roche/Shionogi for an influenza antiviral trial; and receiving equipment from Biofire/BioMerieux and QIAGEN for independent trials of respiratory virus diagnostics. JWT and MP report an investigator-led grant paid to their institution from Sanofi, outside the submitted work. MP reports grants paid to their institution, from Gilead Sciences and consulting fees from QIAGEN, outside the submitted work. All other authors declare no competing interests.
References
- 1.Australian Government Department of Health and Aged Care Australian influenza surveillance report—no 06—fortnight ending 19 June 2022. June 24, 2022. https://www1.health.gov.au/internet/main/publishing.nsf/Content/ozflu-surveil-no06-22.htm
- 2.Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999–2018. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Health Security Agency National flu immunisation programme 2022 to 2023 letter. 2022. https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan/national-flu-immunisation-programme-2022-to-2023-letter
- 4.Van Buynder PG, Newbound A, MacIntyre CR, Kennedy AT, Clarke C, Anderson J. Australian experience of the SH21 flu vaccination program during the COVID-19 vaccine program. Hum Vaccin Immunother. 2021;17:4611–4616. doi: 10.1080/21645515.2021.1967042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint Committee on Vaccination and Immunisation Reimbursable vaccines and eligible cohorts for the 2022/23 NHS Seasonal Influenza (flu) Vaccination Programme. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1081646/Tripartite_annual_flu_letter_2022_to_2023_V2.pdf