Abstract
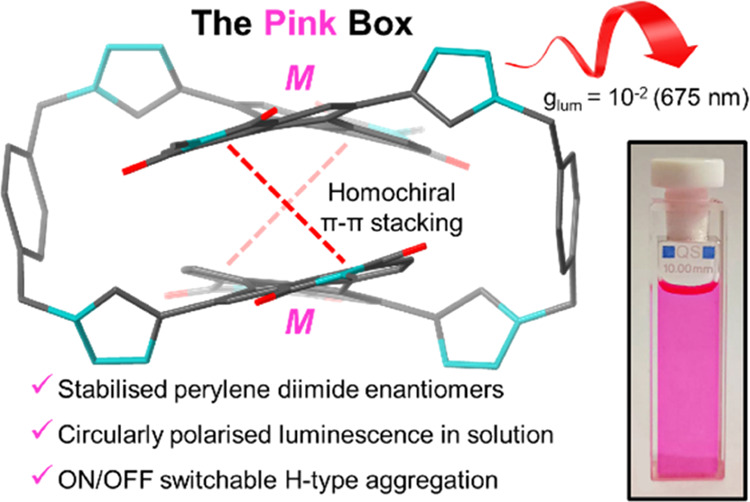
This work showcases chiral complementarity in aromatic stacking interactions as an effective tool to optimize the chiroptical and electrochemical properties of perylene diimides (PDIs). PDIs are a notable class of robust dye molecules and their rich photo- and electrochemistry and potential chirality make them ideal organic building blocks for chiral optoelectronic materials. By exploiting the new bay connectivity of twisted PDIs, a dynamic bis-PDI macrocycle (the “Pink Box”) is realized in which homochiral PDI–PDI π–π stacking interactions are switched on exclusively. Using a range of experimental and computational techniques, we uncover three important implications of the macrocycle’s chiral complementarity for PDI optoelectronics. First, the homochiral intramolecular π–π interactions anchor the twisted PDI units, yielding enantiomers with half-lives extended over 400-fold, from minutes to days (in solution) or years (in the solid state). Second, homochiral H-type aggregation affords the macrocycle red-shifted circularly polarized luminescence and one of the highest dissymmetry factors of any small organic molecule in solution (glum = 10–2 at 675 nm). Finally, excellent through-space PDI–PDI π-orbital overlap stabilizes PDI reduced states, akin to covalent functionalization with electron-withdrawing groups.
Introduction
Since Pedersen’s pioneering work on crown ethers,1 macrocycles have been the workhorses of supramolecular chemistry. Their unique shape persistence and tunability have enabled the full tool kit of noncovalent interactions to be explored.2 Beyond molecular recognition,3 macrocycles are now being exploited in a diverse range of fields, including sensing,4 catalysis,5 and organic electronics,6−8 using architectures that contain multiple π-conjugated components.9 Here, the preorganization of aromatic groups to promote inter-10 or intramolecular11 π–π stacking interactions is key to tuning macrocycle properties. Connecting two viologen units by rigid spacers, the “Blue Box” is an archetypal aromatic-based macrocycle12 that has found numerous applications in functional organic materials13 and machines.14 Other common π-conjugated components for macrocycles include porphyrins,15,16 tetrathiafulvalenes,17,18 and rylene diimides.19 In the latter class, perylene diimides (PDIs) are important targets because they are robust,20 economic organic dyes21 with a readily functionalizable scaffold.22 Coupled with their renowned electron-accepting and photophysical properties, PDIs are ubiquitous organic building blocks for next-generation semiconductor and optoelectronic materials.23−26
Complementary π–π stacking interactions are critical to advancing the performance and diversifying the applications of PDI-based electronic materials, motivating their integration into preorganized macrocyclic architectures.4,19,27−32 Importantly, functionalization of the PDI bay positions (1, 6, 7, 12) imparts a propeller-type contortion of the perylene core, generating axial chirality (denoted M or P).33 However, compared to π–π distance and relative orientation,34,35 axial chirality provides an underexploited handle for optimizing PDI–PDI π–π stacking interactions.36,37 In all but four bis-PDI macrocycles27,38−40 the dyes are connected via the imide positions, a strategy that necessitates the installation of up to four bulky groups in the bay region for solubility.4,19,28,29,31,32 However, we realized that these bay substituents can hamper homochiral PDI–PDI π–π stacking. This inspired us to exploit connectivity via two PDI bay positions (1, 7), realizing a new bis-PDI macrocycle (1), nicknamed the Pink Box due to its color (Figure 1). This macrocycle exhibits exclusive homochiral aromatic stacking between its PDI units, enhancing both chiroptical and electrochemical properties.
Figure 1.
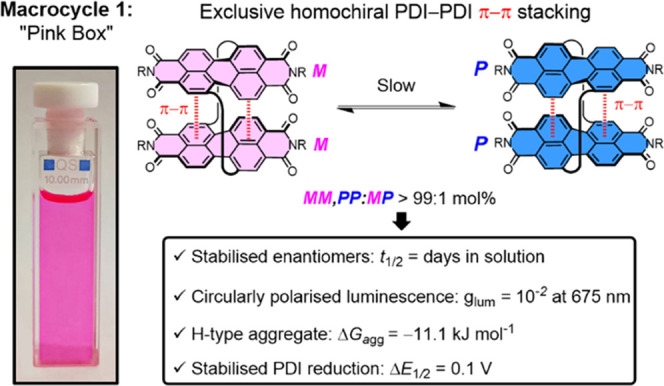
Exclusive homochiral π–π stacking in bis-PDI macrocycle 1, the Pink Box.
The linker choice is also key to our macrocycle design. The short aromatic spacers of 1 afford shape persistence and close PDI–PDI contacts (3.7 Å, Figure 2c), significantly closer than the two previous bay connected bis-PDI macrocycles (6 and 16 Å),27,40 which provides excellent electronic coupling. This is important because while the methylene groups afford some conformational flexibility, they prevent PDI–PDI through-bond conjugation.40 The crystal structure of macrocycle 1, the first of a PDI–triazole derivative, reveals that bay heterocycles cause substantial twisting of the aromatic framework (dihedral angle = 21°, Figure 2c), giving rise to enantiomers MM and PP, and the diastereomer MP, which is a meso-isomer due to a mirror plane between the two PDIs.40 Previous bis-PDI macrocycles have shown that MM and PP interconvert rapidly at room temperature (t1/2 = seconds–minutes)31,40−42viaMP,40 hampering applications in chiral optoelectronics. Therefore, our aim was to exploit strong homochiral π–π stacking in macrocycle 1 to stabilize PDI enantiomers for functional chiroptical materials. Although bay and imide connectivity have been used simultaneously to prevent stereoisomer interconversion in a bis-PDI macrocycle,43 we report an alternative strategy that uses only bay connectivity, making the imide positions available for future potential modifications.
Figure 2.
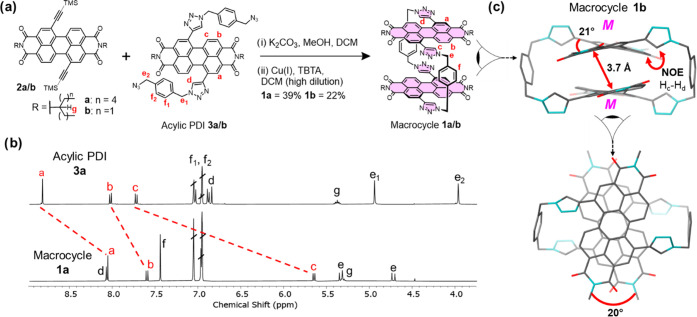
(a) Synthesis of bis-PDI macrocycles 1a/b. (b) Comparison of the 1H NMR spectra of macrocycle 1a and acyclic PDI 3a (toluene-d8, 373 K, 400 MHz). (c) X-ray crystal structure of macrocycle 1b (MM), viewed from the side and the top (alkyl side chains, hydrogens, and cocrystallized solvent molecules are omitted for clarity, see the Supporting Information, Section 3).
Herein, we show that complementary intramolecular π–π stacking interactions in macrocycle 1 (Figure 1) generate PDI enantiomers exclusively in solution (MM,PP:MP > 99:1 mol %). While complete selectivity for homochiral recognition between axially chiral PDIs has been reported,31,44 this is unprecedented in solely bay connected bis-PDI macrocycles.27,40 Exclusive homochirality affords three important advances for PDI chiral optoelectronics. First, for chirality, it raises the free energy barrier of PDI stereoisomer interconversion, extending MM/PP half-lives more than 400-fold, from minutes to days, enabling their resolution. Second, for photophysics, it generates an intramolecular H-type chiral aggregate. This is key to realizing the highest circularly polarized luminescence dissymmetry factor of any discrete PDI in solution (glum = 10–2 at 675 nm vs glum = 10–3 at 655 nm),45−49 which is also one of the highest of any small organic molecule in solution (Table S3).50−53 Third, for PDI electrochemistry, excellent through-space π–π electronic communication stabilizes PDI reduced states, matching the influence of conventional electron-withdrawing groups.34,54
Results and Discussion
Synthesis and Characterization
The bis-PDI macrocycle 1a was prepared using a multistage synthetic procedure, capitalizing on robust copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) “click” chemistry for the final macrocyclization step. Here, stoichiometric amounts of bis-alkyne PDI 2a and bis-azide PDI 3a were reacted under high-dilution conditions (0.3 mM), to favor macrocyclization (Figure 2a).55 The desired [1 + 1] macrocycle 1a was isolated in 39% yield following purification by preparative silica thin layer chromatography, with key side products identified as the larger [2 + 2] and [3 + 3] macrocycles (Supporting Information, Section 1). This macrocyclization yield is over four times larger than previous bay connected bis-PDI macrocycles.27,40 Full synthetic procedures and compound characterization data are provided in the Supporting Information, Section 1.
Macrocycle 1a was characterized using 1H and 13C NMR spectroscopy, which, alongside high-resolution mass spectrometry, confirmed a [1 + 1] macrocyclic product (Supporting Information, Section 1). The 1H NMR spectrum in toluene-d8 at room temperature was broad but dramatically sharpened at 100 °C (Figures 2b and S1), as found for a previous bay-strapped PDI macrocycle due to restricted rotations of the linkers and branched undecyl side chains side chains.40 Indeed, even at high temperature, the cyclic framework of 1a is rigid enough to afford diastereotopic splitting of the methylene protons He in the para-xylyl linker56 not found for the acyclic bis-triazole PDI 3a He1,e2 (Figure 2b).
Further structural characterization of this new macrocycle architecture was provided by single-crystal X-ray diffraction (Supporting Information, Section 3). While our attempts to grow crystals of 1a were unsuccessful, an identical macrocycle57 bearing shorter pentyl chains at the imide positions, 1b, yielded purple needle-like crystals by slow diffusion of methanol into a chloroform solution, enabling single-crystal diffraction data to be collected at a synchrotron.58 The crystal structure of 1b reveals a small relative rotation of the PDI units (20°), enabling close contacts between them (3.7 Å), characteristic of strong intramolecular π–π stacking (Figures 2c and S9),59 alongside CHd...O triazole–imide hydrogen bonding. This is in line with density functional theory predictions for the lowest energy conformer (Figures S42 and S43), including its predicted 1H NMR spectrum (Supporting Information, Section 9c), of a version of 1 with methyl groups at the imide position. The PDI–PDI interactions are maintained in toluene-d8 solution. This is because relative to acyclic PDI 3a, the 1H NMR spectrum of macrocycle 1a reveals large upfield shifts of aromatic protons Ha–c, diagnostic of closely stacked π surfaces (Figure 2b), concomitant with a downfield shift of hydrogen-bonded Hd.60 Furthermore, a new through-space NOE between PDI Hc and triazole Hd signals in 1a (Figure S5) is in agreement with their proximity in the crystal structure (Figure 2c).
PDI Chirality
The crystal structure of macrocycle 1b reveals the axial chirality exhibited by each PDI unit, arising from its twisted aromatic framework (dihedral angle = 21°, Figure 2c). The intramolecular π–π stacking is complementary, occurring exclusively between PDIs of the same chirality, such that the unit cell contains only the enantiomers of the macrocycle MM and PP.61 The diastereomer MP is not observed (Supporting Information, Section 3). Importantly, this is also the case in toluene-d8 solution because the 1H NMR spectrum of macrocycle 1a contains only a single set of peaks for the enantiomers MM and PP at 373 K (Figures 2b and S3). Building on previous reports of a preference for PDI–PDI homochirality over heterochirality,31,44 the exclusive formation of PDI enantiomers in solution is unprecedented in solely bay connected bis-PDI macrocycles.27,40
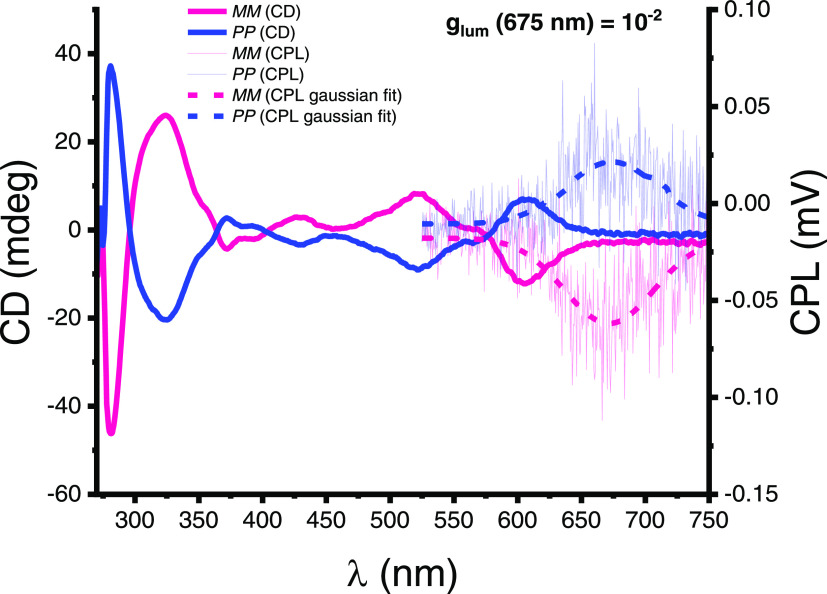
Our next aim was to resolve the enantiomers of macrocycle 1a by chiral high-performance liquid chromatography (HPLC) and characterize them using chiroptical techniques. With toluene as the major eluent, the chromatogram of 1a contained two peaks of equal integration, corresponding to MM and PP (Figure S10). The identity of these enantiomers was confirmed by their opposite circular dichroism (CD) spectra (Figure 3), which is consistent with the single set of signals observed in the 1H NMR spectrum (Figure 2b) as well as the CD spectra of the two enantiomers of a methyl-capped version of 1 predicted by time-dependent density functional theory calculations in toluene (Table S13). We also measured the circularly polarized luminescence of enantiomers MM and PP in toluene (Figure 3 and Supporting Information, Section 5c). Excitingly, macrocycle 1a exhibits one of the highest luminescence dissymmetry factors for a small organic molecule in solution50−53 (glum = 10–2, Table S3) and at wavelengths approaching the near-infrared (λ = 675 nm), useful for advanced security inks62 and multiphoton imaging.63
Figure 3.
Circular dichroism (CD) and circularly polarized luminescence (CPL) spectra of macrocycle 1a enantiomers MM (MM:PP > 99:1 mol %) and PP (MM:PP = 12:88 mol %) in solution (10 μM, toluene, 298 K, λex = 520 nm, full experimental details are in the Supporting Information, Section 5). The enantiomers have been assigned by comparison to their theoretically predicted CD spectra (Supporting Information, Section 9).
We determined the thermodynamics and kinetics of macrocycle racemization in the solvents toluene and dichloromethane by measuring the time dependence of the circular dichroism spectrum decay of an enantiopure solution of 1a (MM:PP > 99:1 mol %) at 298 K (Table 1 and Supporting Information, Section 5b).64 Remarkably, the racemization rate in toluene is more than 400 times slower than in dichloromethane due to a significantly larger interconversion barrier (ΔΔG‡ = 15 kJ mol–1, Figure 4b). Therefore, the MM/PP enantiomer half-life is increased from minutes in chlorinated solvent (t1/2 = 18 min) to days in toluene (t1/2 = 5 days), requiring nearly a month to racemize. To put this in context, the interconversion barrier of 1a in toluene (ΔG‡ = 108 kJ mol–1) is significantly larger than previous dynamically chiral bis-PDI macrocycles employing imide- (53–69 kJ mol–1)31,42 or bay connectivity (86 kJ mol–1).40 Indeed, the barrier is the same as some tetra-ortho-substituted biaryls used for enantioselective catalysis and approaching that required for configurationally stable drugs (ΔG‡ = 114 kJ mol–1).65−67 Importantly for chiroptical materials, the enantiomers of 1a are further stabilized in the solid state (t1/2 ∼ years, Figure S14). The racemization parameters were confirmed by time-course chiral HPLC measurements (Supporting Information, Section 4b).
Table 1. Kinetic and Thermodynamic Parameters for the Racemization of Macrocycle 1aa.
| solvent | t1/2 (h) | ΔG‡ (kJ mol–1, 298 K) |
|---|---|---|
| dichloromethane | 0.3 ± 0.01 | 93 ± 0.1 |
| toluene | 121 ± 10 | 108 ± 0.2 |
Determined by CD spectroscopy using an enantiopure sample of 1a (MM:PP > 99:1 mol %). Full experimental details are in the Supporting Information, Section 5b.
Figure 4.
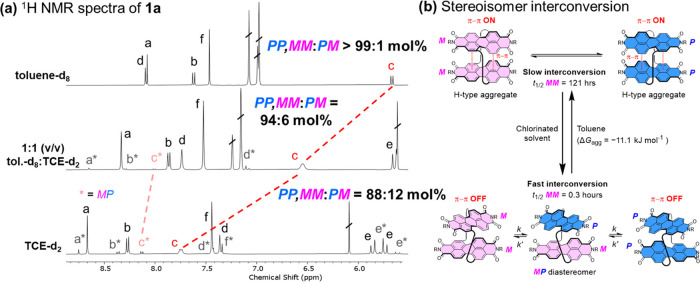
(a) Stacked 1H NMR spectra of macrocycle 1a in different solvents (373 K, 400 MHz, each referenced to the same internal standard, full experimental details are in the Supporting Information, Section 2b). (b) Proposed solvent dependence of macrocycle conformations and stereoisomer interconversion.
To explain the striking difference in enantiomer stabilities, we used 1H NMR spectroscopy in different toluene-d8:1,1,2,2-tetrachloroethane-d2 (TCE-d2) ratios to identify the solvent dependence of macrocycle conformations (Figures 4a and S2).68 In contrast to toluene-d8, the chlorinated solvent disrupts PDI–PDI interactions since Ha–c are shifted downfield (Δδ = 0.7–2.1 ppm) and Hd upfield (0.8 ppm), giving a 1H NMR spectrum that more closely resembles the monomeric bis-triazole PDI 3a (Figures 2b and 4a). The absence of the Hc–Hd NOE for 1a in TCE-d2 (Figure S4) also indicates a new conformation. Indeed, density functional theory calculations suggest new conformations may be adopted in dichloromethane due to their closer relative energies (Supporting Information, Section 9). While there are discrepancies in the energy landscape predicted in dichloromethane, most likely caused by the influence of intermolecular macrocycle–solvent hydrogen bonding not included in our model, there is good agreement between theoretical and experimental 1H NMR and UV–vis spectra (Supporting Information, Section 9b,c) for a distinct homochiral conformation of 1a in a chlorinated solvent. Here, the PDI units are further rotated relative to one another (70°) than in the crystal structure (20°), which reduces their π–π interactions (Figures 4b and S44).69
Interestingly, the 1H NMR spectrum in TCE-d2 also reveals two sets of signals corresponding to two distinct isomers in an 8:1 ratio at 100 °C (Figure 4a), shown to be exchanging by 1H–1H EXSY NMR spectroscopy (Figure S4).70,71 In agreement with previous bis-PDI macrocycles,31,40−42 this strongly suggests the existence of the diastereomer MP, alongside enantiomers MM and PP (Figure 4b). Upon increasing the proportion of toluene-d8, the mole fraction of the major component increases, and its 1H NMR signals converge on the single set of peaks observed in pure toluene-d8 (Figures 4a and S2), meaning high selectivity for the enantiomers over the diastereomer is maintained in chlorinated solvents (MM,PP:MP = 88:12 mol % in TCE-d2 at 373 K). Importantly, the PDI–PDI π–π interactions are stronger in homochiral vs heterochiral conformations because MM,PP undergo the largest solvent-induced perturbations (Δδ = 2.1 ppm for Hc, Figure 4a), while only small shifts are observed for corresponding MP signals (Δδ = 0.1 ppm for Hc*).60
Using dichloromethane as the major eluent, the chromatogram of 1a (Figure S11) contains two major peaks for each enantiomer, MM and PP, alongside a minor third peak for MP (∼10 mol % at 298 K). While it was not possible to isolate the MP diastereomer by HPLC due to poor separation, we used 1H–1H EXSY NMR spectroscopy70,71 in TCE-d2 to show that the interconversion barrier (ΔG‡ = 95 kJ mol–1, 298 K) is close to that for racemization calculated by CD spectroscopy (93 kJ mol–1) and so consistent with the reported mechanism of racemization viaMP (Figure 4b and Supporting Information, Section 2d).40 Indeed, the addition of toluene-d8 decreases the rate of MM/PP loss (Δk = −0.02 s–1) and increases the rate of MP loss (Δk′ = 0.49 s–1).
PDI Photophysics
UV–vis absorption and fluorescence emission spectroscopic studies provided further insight into the macrocycle’s switchable homochiral conformation (Figure 5a and Supporting Information, Section 6).72 Toluene induces the formation of an H-type aggregate in the ground state and a PDI excimer in the excited state of 1a, while in TCE, the PDI–PDI electronic coupling is significantly weaker.73 The main PDI absorption band of 1a in toluene has a 0–1 vibronic peak that is significantly larger than the 0–0 peak (A0–0/A0–1 = 0.58), characteristic of an H-type aggregate (Figures 5a and S23).36 Indeed, this A0–0/A0–1 ratio is one of the lowest of any PDI dimer,28,30,74 indicative of strong PDI–PDI electronic coupling in macrocycle 1a and key to its high glum, since the aggregation of chiral monomers is known to amplify their dissymmetry.52,75 This also implies that strong PDI–PDI π–π stacking is responsible for raising the barrier to macrocycle racemization. By contrast, the UV–vis spectrum of 1a in TCE resembles that of the monomeric bis-triazole PDI 3a (A0–0/A0–1 = 1.19, Figure 5a),76 consistent with the weakly coupled conformation in which H-type aggregation is disrupted (Figure 4b),69 suggested by density functional theory calculations in chlorinated solvents (Figure S44 and Supporting Information, Section 9).72 The UV–vis spectra of 1a follow the Beer–Lambert law in both toluene and TCE, demonstrating that the solvent-mediated π–π stacking observed is intramolecular in origin (Figure S28). The fluorescence spectrum of macrocycle 1a in toluene is indicative of a PDI–PDI excimer,77 since relative to 1a in TCE and acyclic control 3a (Figures S22 and S25), the emission becomes weaker (ΔΦ = 0.42), broader (Δ[FWHM] = 19 nm), and bathochromically shifted (Δλ = 20 nm), with a large Stokes shift (λ = 102 nm). The distinct UV–vis absorption spectra of macrocycle 1a in chlorinated and aromatic solvents enabled us to estimate the strength of intramolecular PDI–PDI π–π interactions. The titration of TCE into a solution of 1a in toluene (at constant concentration) led to the formation of isosbestic points (λ = 505, 523, 577 nm), indicative of conformations that are in equilibrium (Figures 5a and S37).76 Therefore, by applying the method of Moore and Ray,78 used for measuring the solvent-induced folding of aromatic oligomers, the free energy of intramolecular PDI–PDI H-type aggregation for 1a in toluene is estimated to be ΔGagg = −11.1 kJ mol–1 (Supporting information, Section 7). This is over twice as strong as the aggregation of PDIs functionalized with four bulky 4-tert-butylphenoxy groups at the bay positions, as reported using a recent PDI-based macrocycle in toluene.79
Figure 5.
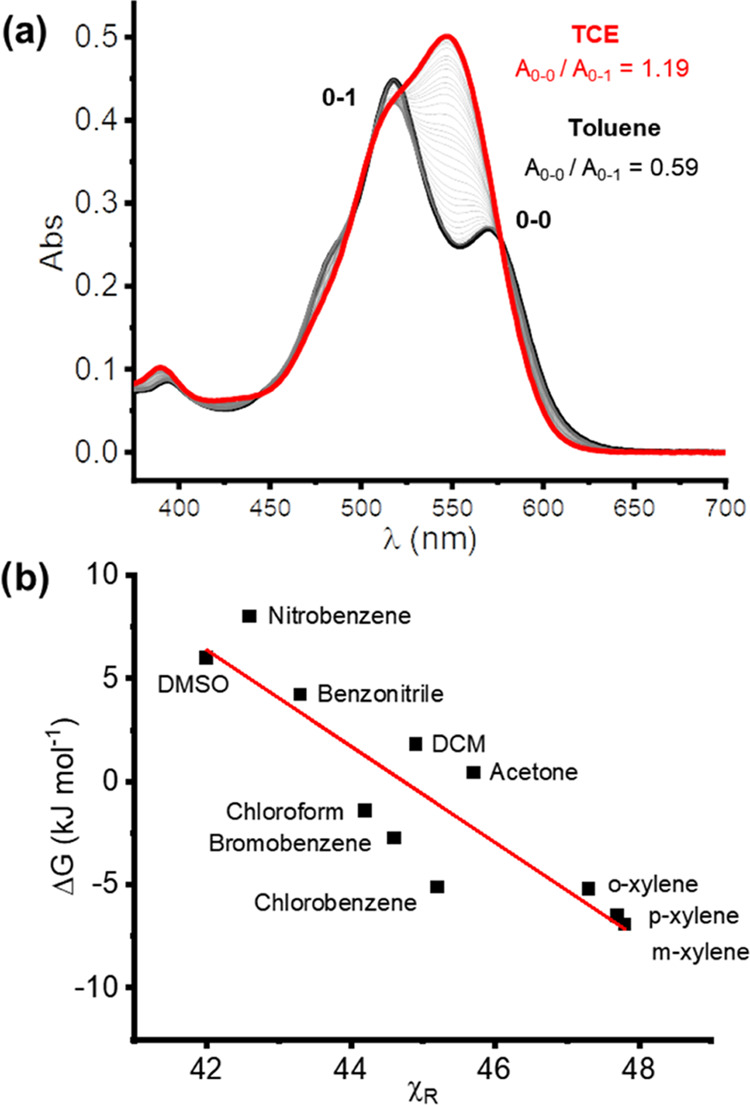
(a) UV–vis absorption spectra of macrocycle 1a (10 μmol) measured in neat toluene (black), TCE (red), and intermediate toluene:TCE solvent ratios (gray). (b) Plot of ΔGagg of 1a vs the solvent scale χr for a range of different solvents (Pearson’s r = 0.9).
The strong PDI–PDI π–π interactions in the H-type aggregate of 1a help to explain the exclusive formation of enantiomers MM,PP and the larger ΔG‡ in toluene (Figure 6). Ideal H-type aggregation requires complementarity between twisted PDI π surfaces and so can only occur in a homochiral conformation. Indeed, density functional theory calculations showed the energy difference between MM/PP and MP decreases in dichloromethane relative to toluene (Supporting Information, Section 9), which will increase the diastereomer population. For bay connected bis-PDI macrocycles, enantiomer interconversion requires PDI units to somersault through the cavity40 and, for 1a, this process will be inhibited by the strong intramolecular π–π stacking interactions in toluene.
Figure 6.
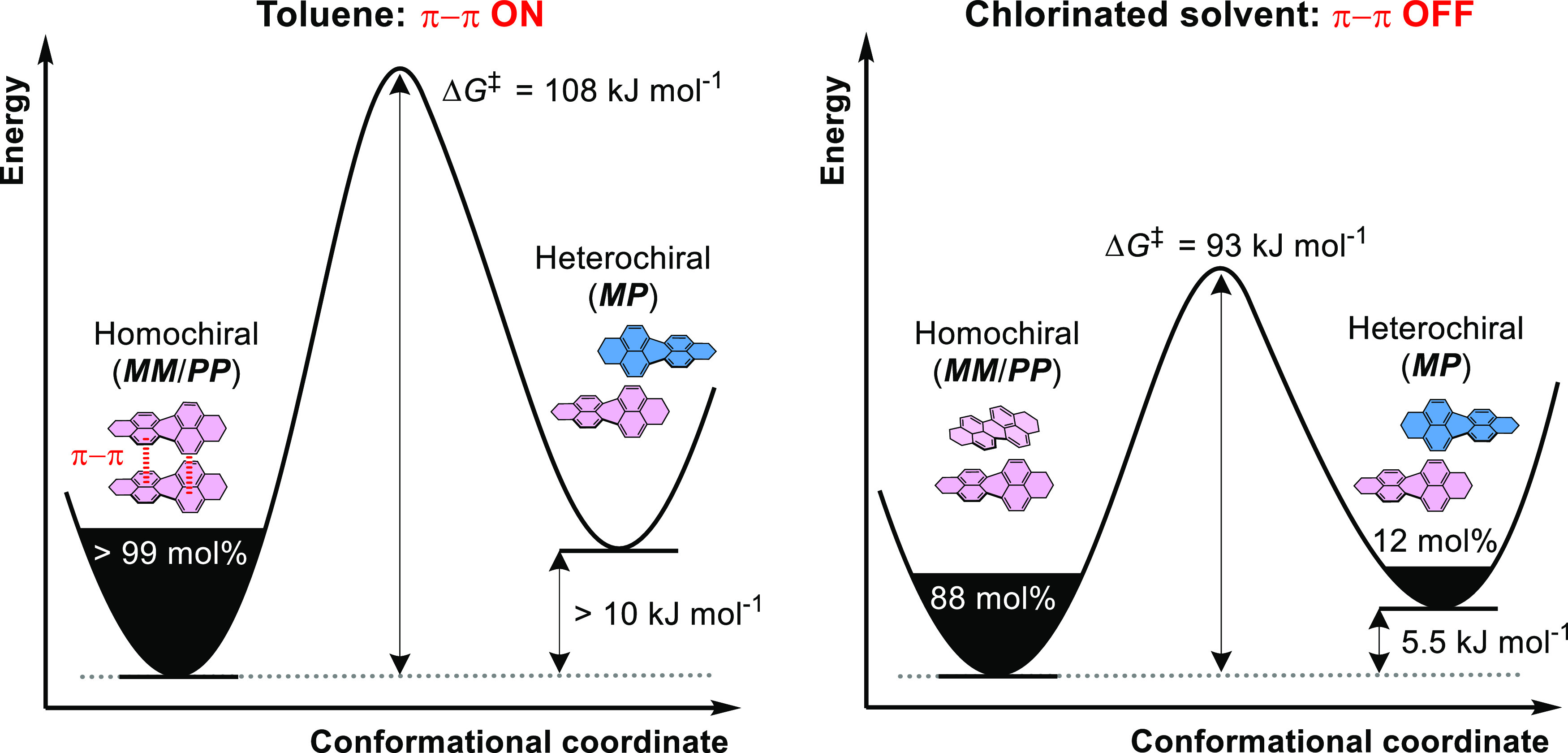
Proposed conformation–energy diagrams for macrocycle 1a in toluene (left) and chlorinated solvent (right) using experimental data from CD spectroscopy and chiral HPLC. All values are at 298 K, except for the populations and free energy difference between MM/PP and MP, which are estimated at 373 K, by 1H NMR spectroscopy.
We performed further UV–vis spectroscopy to understand the important role of solvent on PDI–PDI π–π interactions in macrocycle 1a (Table S5). By correlating ΔGagg with a range of solvent scales, we determined that solvent polarity and polarizability (e.g., the χr scale. Figure 5b)80 are key parameters in dictating their strength while hydrogen bonding is not (Figures S29–S36). Therefore, polar and polarizable chlorinated solvents disrupt the macrocycle’s intramolecular π–π stacking interactions and H-type aggregation because, in contrast to toluene, these solvents better solubilize polycyclic aromatic hydrocarbons.81 This solvent dependence is analogous to that observed in the supramolecular self-assembly of PDI monomers, since both use π–π stacking interactions.82 Molecular recognition studies between 1a and a range of polycyclic aromatic hydrocarbons revealed no evidence for guest binding, most likely due to the macrocycle’s small cavity, apparent from the crystal structure (Figure 2c).83
PDI Electrochemistry
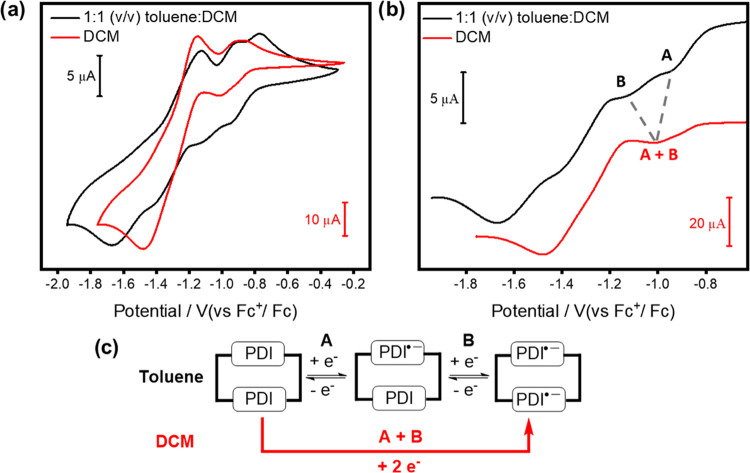
The macrocycle’s solvent-dependent conformation is notable for providing switchable electrochemical properties (Figure 7 and Supporting Information, Section 8). In dichloromethane, cyclic voltammetry of 1a revealed two typical chemically reversible two-electron PDI reduction processes.36,84 However, the addition of toluene splits the first peak into two distinct one-electron reductions, A and B (Figure 7b and Table S6).85 This demonstrates that homochiral intramolecular π–π stacking between PDI units facilitates their strong through-space electronic communication,28,86 which is switchable using a solvent. The H-type aggregate in 1:1 (v/v) toluene/dichloromethane87 also significantly stabilizes the first reduced PDI state (1a•–, E1/2 = −0.84 V) relative to the corresponding species in neat dichloromethane or the acyclic PDI control 3a•– (E1/2 = −0.94 V for both, Table S6).88 To put this in context, the enhancement in electron-accepting power of a PDI unit arising from its H-type homochiral π–π stacking is superior to the covalent addition of two ortho-cyanophenyl electron-withdrawing groups to the perylene core.54 Indeed, the electron deficiency of macrocycle 1a (E1/2 = −0.84 V) is in line with a tetrachloro-substituted PDI (E1/2 = −0.87 V),34 demonstrating its potential as an n-type semiconducting material.
Figure 7.
(a) Full and (b) zoomed-in region of the cyclic voltammograms of macrocycle 1a recorded in dichloromethane and 1:1 (v/v) toluene:dichloromethane (each containing 0.4 M [nBu4N][BF4], full experimental details are provided in the Supporting Information, Section 8). (c) Proposed schematic of the initial redox processes of macrocycle 1a in each solvent.
Summary and Conclusions
In this work, we enhance the through-space π–π electronic communication of two PDI units by tuning their relative chirality (homochiral), orientation (H-type aggregate), and distance (3.7 Å) using a macrocycle. We have developed a novel bis-PDI macrocycle (1), in which complementary π–π interactions optimize the PDI’s chiroptical and electrochemical properties, demonstrated using a range of experimental and computational techniques.
Macrocycle 1 is unique among solely bay connected bis-PDI macrocycles27,40 in exhibiting homochiral π–π stacking exclusively in solution (MM,PP:MP > 99:1 mol % in toluene). The closest analogue exhibits a 72% diastereomeric excess of its homochiral stereoisomers.40 An intramolecular H-type aggregate with strong π–π interactions (ΔGagg = −11.1 kJ mol–1) is key to the chiral complementarity in 1 and arises from a short PDI–PDI separation (3.7 Å), shorter than other bay connected bis-PDI macrocycles.27,40 Homochiral aromatic stacking interactions anchor the PDI units of 1, slowing the racemization rate by >400-fold and increasing the enantiomer half-life from minutes in chlorinated solvents to days in toluene and years in the solid state. As such, stereoisomer interconversion is significantly slower than previous dynamically chiral bis-PDI macrocycles (t1/2 = days vs minutes),31,40−42 with a barrier nearing that required for configurational locking.43 The enantiomers of 1 exhibit the highest circularly polarized luminescence dissymmetry factor of any discrete PDI in solution (glum = 10–2 vs 10–3).45 Indeed, in comparison to other small organic molecules in solution, the circularly polarized luminescence of 1 is the most red-shifted and has one of the highest dissymmetry factors (Table S3).50−53 The PDI electron-accepting ability of 1 is also elevated (ΔE1/2 = 0.1 V) and matches that of a tetrachloro-substituted PDI semiconducting material34 but with a notably higher barrier to stereoisomer interconversion (ΔG‡ = 108 vs 97 kJ mol–1).33 Therefore, this work demonstrates that the ordering of dynamic chiral PDIs using macrocyclic scaffolds can elevate their key chiroptical and electrochemical properties. Efforts to assemble multiple chiral PDIs using supramolecular chemistry are continuing in our laboratory.
Acknowledgments
T.A.B. would like to thank the University of Birmingham and an RSC Research Enablement Grant (E20-4166) for funding. T.A.B. and S.E.P thank Dr. Cécile Le Duff at the University of Birmingham for support with NMR spectroscopy.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c03531.
Author Present Address
∥ School of Medicine, Shanghai University, 99 Shangda Road, Shanghai 200444, China
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Pedersen C. J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. 10.1021/ja00986a052. [DOI] [Google Scholar]
- Steed J. W.; Atwood J. L.. Supramolecular Chemistry, 3rd ed.; Wiley, 2022. [Google Scholar]
- Cram D. J. The Design of Molecular Hosts, Guests, and Their Complexes (Nobel Lecture). Angew. Chem., Int. Ed. 1988, 27, 1009–1020. 10.1002/anie.198810093. [DOI] [PubMed] [Google Scholar]
- Spenst P.; Würthner F. A Perylene Bisimide Cyclophane as a “Turn-On” and “Turn-Off” Fluorescence Probe. Angew. Chem. 2015, 127, 10303–10306. 10.1002/ange.201503542. [DOI] [PubMed] [Google Scholar]
- Yang J.-M.; Yu Y.; Rebek J. Selective Macrocycle Formation in Cavitands. J. Am. Chem. Soc. 2021, 143, 2190–2193. 10.1021/jacs.0c12302. [DOI] [PubMed] [Google Scholar]
- Ball M.; Zhang B.; Zhong Y.; Fowler B.; Xiao S.; Ng F.; Steigerwald M.; Nuckolls C. Conjugated Macrocycles in Organic Electronics. Acc. Chem. Res. 2019, 52, 1068–1078. 10.1021/acs.accounts.9b00017. [DOI] [PubMed] [Google Scholar]
- Leonhardt E. J.; Jasti R. Emerging applications of carbon nanohoops. Nat. Rev. Chem. 2019, 3, 672–686. 10.1038/s41570-019-0140-0. [DOI] [Google Scholar]
- Pérez E. M. Putting Rings around Carbon Nanotubes. Chem. - Eur. J. 2017, 23, 12681–12689. 10.1002/chem.201702992. [DOI] [PubMed] [Google Scholar]
- Shi Q.; Wang X.; Liu B.; Qiao P.; Li J.; Wang L. Macrocyclic host molecules with aromatic building blocks: the state of the art and progress. Chem. Commun. 2021, 57, 12379–12405. 10.1039/D1CC04400A. [DOI] [PubMed] [Google Scholar]
- Blanco-Gómez A.; Fernández-Blanco Á.; Blanco V.; Rodríguez J.; Peinador C.; García M. D. Thinking Outside the “Blue Box”: Induced Fit within a Unique Self-Assembled Polycationic Cyclophane. J. Am. Chem. Soc. 2019, 141, 3959–3964. 10.1021/jacs.8b12599. [DOI] [PubMed] [Google Scholar]
- Dietel E.; Hirsch A.; Eichhorn E.; Rieker A.; Hackbarth S.; Röder B. A macrocyclic [60]fullerene–porphyrin dyad involving π–π stacking interactions. Chem. Commun. 1998, 1981–1982. 10.1039/a804047h. [DOI] [Google Scholar]
- Odell B.; Reddington M. V.; Slawin A. M. Z.; Spencer N.; Stoddart J. F.; Williams D. J. Cyclobis(paraquat-p-phenylene). A Tetracationic Multipurpose Receptor. Angew. Chem., Int. Ed. 1988, 27, 1547–1550. 10.1002/anie.198815471. [DOI] [Google Scholar]
- Fahrenbach A. C.; Sampath S.; Late D. J.; Barnes J. C.; Kleinman S. L.; Valley N.; Hartlieb K. J.; Liu Z.; Dravid V. P.; Schatz G. C.; Van Duyne R. P.; Stoddart J. F. A Semiconducting Organic Radical Cationic Host–Guest Complex. ACS Nano 2012, 6, 9964–9971. 10.1021/nn303553z. [DOI] [PubMed] [Google Scholar]
- Feng L.; Qiu Y.; Guo Q.-H.; Chen Z.; Seale J. S. W.; He K.; Wu H.; Feng Y.; Farha O. K.; Astumian R. D.; Stoddart J. F. Active mechanisorption driven by pumping cassettes. Science 2021, 374, 1215–1221. 10.1126/science.abk1391. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Gsänger S.; Minameyer M. B.; Imaz I.; Maspoch D.; Shyshov O.; Schwer F.; Ribas X.; Drewello T.; Meyer B.; von Delius M. Highly Strained, Radially π-Conjugated Porphyrinylene Nanohoops. J. Am. Chem. Soc. 2019, 141, 18500–18507. 10.1021/jacs.9b08584. [DOI] [PubMed] [Google Scholar]
- Hoffmann M.; Wilson C. J.; Odell B.; Anderson H. L. Template-Directed Synthesis of a π-Conjugated Porphyrin Nanoring. Angew. Chem., Int. Ed. 2007, 46, 3122–3125. 10.1002/anie.200604601. [DOI] [PubMed] [Google Scholar]
- Isla H.; Gallego M.; Pérez E. M.; Viruela R.; Ortí E.; Martín N. A Bis-exTTF Macrocyclic Receptor That Associates C60 with Micromolar Affinity. J. Am. Chem. Soc. 2010, 132, 1772–1773. 10.1021/ja910107m. [DOI] [PubMed] [Google Scholar]
- Nielsen K. A.; Cho W.-S.; Jeppesen J. O.; Lynch V. M.; Becher J.; Sessler J. L. Tetra-TTF Calix[4]pyrrole: A Rationally Designed Receptor for Electron-Deficient Neutral Guests. J. Am. Chem. Soc. 2004, 126, 16296–16297. 10.1021/ja044664a. [DOI] [PubMed] [Google Scholar]
- Spenst P.; Würthner F. Photo- and redoxfunctional cyclophanes, macrocycles, and catenanes based on aromatic bisimides. J. Photochem. Photobiol., C 2017, 31, 114–138. 10.1016/j.jphotochemrev.2017.03.002. [DOI] [Google Scholar]
- Lee S. K.; Zu Y.; Herrmann A.; Geerts Y.; Müllen K.; Bard A. J. Electrochemistry, Spectroscopy and Electrogenerated Chemiluminescence of Perylene, Terrylene, and Quaterrylene Diimides in Aprotic Solution. J. Am. Chem. Soc. 1999, 121, 3513–3520. 10.1021/ja984188m. [DOI] [Google Scholar]
- Greene M.Perylene Pigments. In High Performance Pigments, Smith H. M., Ed.; Wiley, 2001; pp 249–261. [Google Scholar]
- Nowak-Król A.; Würthner F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. 10.1039/C8QO01368C. [DOI] [Google Scholar]
- Struijk C. W.; Sieval A. B.; Dakhorst J. E. J.; van Dijk M.; Kimkes P.; Koehorst R. B. M.; Donker H.; Schaafsma T. J.; Picken S. J.; van de Craats A. M.; Warman J. M.; Zuilhof H.; Sudhölter E. J. R. Liquid Crystalline Perylene Diimides: Architecture and Charge Carrier Mobilities. J. Am. Chem. Soc. 2000, 122, 11057–11066. 10.1021/ja000991g. [DOI] [Google Scholar]
- Kişnişci Z.; Yüksel ÖF.; Kuş M. Optical properties of perylene-monoimide (PMI) and perylene-diimide (PDI) organic semiconductor thin films. Synth. Met. 2014, 194, 193–197. 10.1016/j.synthmet.2014.05.003. [DOI] [Google Scholar]
- Zink-Lorre N.; Font-Sanchis E.; Sastre-Santos Á.; Fernández-Lázaro F. Perylenediimides as more than just non-fullerene acceptors: versatile components in organic, hybrid and perovskite solar cells. Chem. Commun. 2020, 56, 3824–3838. 10.1039/D0CC00337A. [DOI] [PubMed] [Google Scholar]
- Barendt T. A.; Ball M. L.; Xu Q.; Zhang B.; Fowler B.; Schattman A.; Ritter V. C.; Steigerwald M. L.; Nuckolls C. Supramolecular Assemblies for Electronic Materials. Chem. - Eur. J. 2020, 26, 3744–3748. 10.1002/chem.202000514. [DOI] [PubMed] [Google Scholar]
- Türel T.; Bhargava S.; Valiyaveettil S. Tubular Perylene Bisimide Macrocycles for the Recognition of Geometrical Isomers of Azobenzenes. J. Org. Chem. 2020, 85, 3092–3100. 10.1021/acs.joc.9b02972. [DOI] [PubMed] [Google Scholar]
- Schlosser F.; Moos M.; Lambert C.; Würthner F. Redox-switchable Intramolecular π–π-Stacking of Perylene Bisimide Dyes in a Cyclophane. Adv. Mater. 2013, 25, 410–414. 10.1002/adma.201201266. [DOI] [PubMed] [Google Scholar]
- Feng J.; Zhang Y.; Zhao C.; Li R.; Xu W.; Li X.; Jiang J. Cyclophanes of Perylene Tetracarboxylic Diimide with Different Substituents at Bay Positions. Chem. - Eur. J. 2008, 14, 7000–7010. 10.1002/chem.200800136. [DOI] [PubMed] [Google Scholar]
- Langhals H.; Ismael R. Cyclophanes as Model Compounds for Permanent, Dynamic Aggregates – Induced Chirality with Strong CD Effects. Eur. J. Org. Chem. 1998, 9, 1915–1917. . [DOI] [Google Scholar]
- Solymosi I.; Krishna S.; Nuin E.; Maid H.; Scholz B.; Guldi D. M.; Pérez-Ojeda M. E.; Hirsch A. Diastereoselective Formation of Homochiral Flexible Perylene Bisimide Cyclophanes and their Hybrids with Fullerenes. Chem. Sci. 2021, 12, 15491–15502. 10.1039/D1SC04242D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendt T. A.; Ferreira L.; Marques I.; Felix V.; Beer P. D. Anion- and Solvent-Induced Rotary Dynamics and Sensing in a Perylene Diimide [3]Catenane. J. Am. Chem. Soc. 2017, 139, 9026–9037. 10.1021/jacs.7b04295. [DOI] [PubMed] [Google Scholar]
- Osswald P.; Wuerthner F. Effects of Bay Substituents on the Racemization Barriers of Perylene Bisimides: Resolution of Atropo-Enantiomers. J. Am. Chem. Soc. 2007, 129, 14319–14326. 10.1021/ja074508e. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Debije M. G.; Debaerdemaeker T.; Osswald P.; Würthner F. Tetrachloro-substituted Perylene Bisimide Dyes as Promising n-Type Organic Semiconductors: Studies on Structural, Electrochemical and Charge Transport Properties. ChemPhysChem 2004, 5, 137–140. 10.1002/cphc.200300882. [DOI] [PubMed] [Google Scholar]
- Wilson-Kovacs R. S.; Fang X.; Hagemann M. J. L.; Symons H. E.; Faul C. F. J. Design and Control of Perylene Supramolecular Polymers through Imide Substitutions. Chem. - Eur. J. 2022, 28, e202103443 10.1002/chem.202103443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würthner F.; Saha-Moller C. R.; Fimmel B.; Ogi S.; Leowanawat P.; Schmidt D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. 10.1021/acs.chemrev.5b00188. [DOI] [PubMed] [Google Scholar]
- Hecht M.; Würthner F. Supramolecularly Engineered J-Aggregates Based on Perylene Bisimide Dyes. Acc. Chem. Res. 2021, 54, 642–653. 10.1021/acs.accounts.0c00590. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Zhang Z.; Ma L.; Shi R.; Ling S.; Li X.; He G.; Zhang M. Perylene Diimide-Based Multicomponent Metallacages as Photosensitizers for Visible Light-Driven Photocatalytic Oxidation Reaction. CCS Chem. 2021, 3, 3153–3160. 10.31635/ccschem.021.202101382. [DOI] [Google Scholar]
- Hou Y.; Zhang Z.; Lu S.; Yuan J.; Zhu Q.; Chen W.-P.; Ling S.; Li X.; Zheng Y.-Z.; Zhu K.; Zhang M. Highly Emissive Perylene Diimide-Based Metallacages and Their Host–Guest Chemistry for Information Encryption. J. Am. Chem. Soc. 2020, 142, 18763–18768. 10.1021/jacs.0c09904. [DOI] [PubMed] [Google Scholar]
- Ball M.; Fowler B.; Li P.; Joyce L. A.; Li F.; Liu T.; Paley D.; Zhong Y.; Li H.; Xiao S.; Ng F.; Steigerwald M. L.; Nuckolls C. Chiral Conjugated Corrals. J. Am. Chem. Soc. 2015, 137, 9982–9987. 10.1021/jacs.5b05698. [DOI] [PubMed] [Google Scholar]
- Wang W.; Shaller A. D.; Li A. D. Q. Twisted perylene stereodimers reveal chiral molecular assembly codes. J. Am. Chem. Soc. 2008, 130, 8271–8279. 10.1021/ja7111959. [DOI] [PubMed] [Google Scholar]
- Sapotta M.; Spenst P.; Saha-Moeller C. R.; Wuerthner F. Guest-mediated chirality transfer in the host-guest complexes of an atropisomeric perylene bisimide cyclophane host. Org. Chem. Front. 2019, 6, 892–899. 10.1039/C9QO00172G. [DOI] [Google Scholar]
- Weh M.; Rühe J.; Herbert B.; Krause A.-M.; Würthner F. Deracemization of Carbohelicenes by a Chiral Perylene Bisimide Cyclophane Template Catalyst. Angew. Chem., Int. Ed. 2021, 60, 15323–15327. 10.1002/anie.202104591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safont-Sempere M. M.; Osswald P.; Stolte M.; Gruene M.; Renz M.; Kaupp M.; Radacki K.; Braunschweig H.; Wuerthner F. Impact of Molecular Flexibility on Binding Strength and Self-Sorting of Chiral π-Surfaces. J. Am. Chem. Soc. 2011, 133, 9580–9591. 10.1021/ja202696d. [DOI] [PubMed] [Google Scholar]
- Renner R.; Mahlmeister B.; Anhalt O.; Stolte M.; Würthner F. Chiral Perylene Bisimide Dyes by Interlocked Arene Substituents in the Bay Area. Chem. - Eur. J. 2021, 27, 11997–12006. 10.1002/chem.202101877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Song I.; Ahn J.; Han M.; Linares M.; Surin M.; Zhang H.-J.; Oh J. H.; Lin J. π-Extended perylene diimide double-heterohelicenes as ambipolar organic semiconductors for broadband circularly polarized light detection. Nat. Commun. 2021, 12, 142 10.1038/s41467-020-20390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi A.; Kaji D.; Hara N.; Murata R.; Akiyama S.; Harada T.; Sudo A.; Nishikawa H.; Imai Y. Solid-state AIEnh-circularly polarised luminescence of chiral perylene diimide fluorophores. RSC Adv. 2019, 9, 1976–1981. 10.1039/C8RA09785B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reine P.; Ortuño A. M.; Mariz I. F. A.; Ribagorda M.; Cuerva J. M.; Campaña A. G.; Maçôas E.; Miguel D. Simple Perylene Diimide Cyclohexane Derivative With Combined CPL and TPA Properties. Front. Chem. 2020, 8, 306 10.3389/fchem.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.; Han J.; Huo S.; Qu Z.; Jiao T.; Liu M.; Duan P. Proton triggered circularly polarized luminescence in orthogonal- and co-assemblies of chiral gelators with achiral perylene bisimide. Chem. Commun. 2018, 54, 5630–5633. 10.1039/C8CC02777C. [DOI] [PubMed] [Google Scholar]
- Hasegawa M.; Nojima Y.; Mazaki Y. Circularly Polarized Luminescence in Chiral π-Conjugated Macrocycles. ChemPhotoChem 2021, 5, 1042–1058. 10.1002/cptc.202100162. [DOI] [Google Scholar]
- Sánchez-Carnerero E. M.; Agarrabeitia A. R.; Moreno F.; Maroto B. L.; Muller G.; Ortiz M. J.; de la Moya S. Circularly Polarized Luminescence from Simple Organic Molecules. Chem. - Eur. J. 2015, 21, 13488–13500. 10.1002/chem.201501178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.; Xing P. Regulation of Circularly Polarized Luminescence in Multicomponent Supramolecular Coassemblies. ChemPhotoChem 2022, 6, e202100124 10.1002/cptc.202100124. [DOI] [Google Scholar]
- Ma J.-L.; Peng Q.; Zhao C.-H. Circularly Polarized Luminescence Switching in Small Organic Molecules. Chem. - Eur. J. 2019, 25, 15441–15454. 10.1002/chem.201903252. [DOI] [PubMed] [Google Scholar]
- Dey S.; Efimov A.; Lemmetyinen H. Diaryl-Substituted Perylene Bis(imides): Synthesis, Separation, Characterization and Comparison of Electrochemical and Optical Properties of 1,7- and 1,6-Regioisomer. Eur. J. Org. Chem. 2012, 2012, 2367–2374. 10.1002/ejoc.201101825. [DOI] [Google Scholar]
- Schmidt C. D.; Lang N.; Jux N.; Hirsch A. A Facile Route to Water-Soluble Coronenes and Benzo[ghi]perylenes. Chem. - Eur. J. 2011, 17, 5289–5299. 10.1002/chem.201003232. [DOI] [PubMed] [Google Scholar]
- Anamimoghadam O.; Jones L. O.; Cooper J. A.; Beldjoudi Y.; Nguyen M. T.; Liu W.; Krzyaniak M. D.; Pezzato C.; Stern C. L.; Patel H. A.; Wasielewski M. R.; Schatz G. C.; Stoddart J. F. Discrete Open-Shell Tris(bipyridinium radical cationic) Inclusion Complexes in the Solid State. J. Am. Chem. Soc. 2021, 143, 163–175. 10.1021/jacs.0c07148. [DOI] [PubMed] [Google Scholar]
- Apart from their side chains, the bis-PDI macrocycles 1a and 1b are structurally identical due to matching 1H NMR (aromatic signals) and UV–vis spectra (Supporting information Section 1 and Figures S23 and S24).
- Asingle omega sweep data collection was run on a weakly diffracting crystal. The resulting data was truncated to a resolution of 0.84 Å to improve signal to noise.
- Janiak C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc., Dalton Trans. 2000, 3885–3896. 10.1039/b003010o. [DOI] [Google Scholar]
- Shao C.; Grüne M.; Stolte M.; Würthner F. Perylene Bisimide Dimer Aggregates: Fundamental Insights into Self-Assembly by NMR and UV/Vis Spectroscopy. Chem. - Eur. J. 2012, 18, 13665–13677. 10.1002/chem.201201661. [DOI] [PubMed] [Google Scholar]
- Moss G. P. Basic terminology of stereochemistry (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2193–2222. 10.1351/pac199668122193. [DOI] [Google Scholar]
- MacKenzie L. E.; Pal R. Circularly polarized lanthanide luminescence for advanced security inks. Nat. Rev. Chem. 2021, 5, 109–124. 10.1038/s41570-020-00235-4. [DOI] [PubMed] [Google Scholar]
- Stachelek P.; MacKenzie L.; Parker D.; Pal R. Circularly polarised luminescence laser scanning confocal microscopy to study live cell chiral molecular interactions. Nat. Commun. 2022, 13, 553 10.1038/s41467-022-28220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickhaus M.; Jundt L.; Mayor M. Determining Inversion Barriers in Atrop- isomers – A Tutorial for Organic Chemists. CHIMIA Int. J. Chem. 2016, 70, 192. 10.2533/chimia.2016.192. [DOI] [PubMed] [Google Scholar]
- LaPlante S. R.; Edwards P. J.; Fader L. D.; Jakalian A.; Hucke O. Revealing Atropisomer Axial Chirality in Drug Discovery. ChemMedChem 2011, 6, 505–513. 10.1002/cmdc.201000485. [DOI] [PubMed] [Google Scholar]
- Ahmed A.; Bragg R. A.; Clayden J.; Lai L. W.; McCarthy C.; Pink J. H.; Westlund N.; Yasin S. A. Barriers to rotation about the chiral axis of tertiary aromatic amides. Tetrahedron 1998, 54, 13277–13294. 10.1016/S0040-4020(98)00814-X. [DOI] [Google Scholar]
- Leroux F. Atropisomerism, Biphenyls, and Fluorine: A Comparison of Rotational Barriers and Twist Angles. ChemBioChem 2004, 5, 644–649. 10.1002/cbic.200300906. [DOI] [PubMed] [Google Scholar]
- Here the chlorinated solvent TCE is used as a substitute for dichloromethane to enable 1H NMR spectroscopy studies at high temperature.
- Sebastian E.; Philip A. M.; Benny A.; Hariharan M. Null Exciton Splitting in Chromophoric Greek Cross (+) Aggregate. Angew. Chem. 2018, 130, 15922–15927. 10.1002/ange.201810209. [DOI] [PubMed] [Google Scholar]
- Farahani N.; Zhu K.; Loeb S. J. Rigid, Bistable Molecular Shuttles Combining T-shaped Benzimidazolium and Y-shaped Imidazolium Recognition Sites. ChemPhysChem 2016, 17, 1875–1880. 10.1002/cphc.201600128. [DOI] [PubMed] [Google Scholar]
- Perrin C. L.; Dwyer T. J. Application of two-dimensional NMR to kinetics of chemical exchange. Chem. Rev. 1990, 90, 935–967. 10.1021/cr00104a002. [DOI] [Google Scholar]
- The heterochiral conformation of diastereomer MP will only provide a small contribution to the UV–vis absorption spectrum of 1a in TCE because of its low population (10 mol %).
- Kasha M.; Rawls H. R.; Ashraf El-Bayoumi M. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. 10.1351/pac196511030371. [DOI] [Google Scholar]
- Rühe J.; Bialas D.; Spenst P.; Krause A.-M.; Würthner F. Perylene Bisimide Cyclophanes: Structure–Property Relationships upon Variation of the Cavity Size. Org. Mater. 2020, 02, 149–158. 10.1055/s-0040-1709998. [DOI] [Google Scholar]
- Kumar J.; Nakashima T.; Tsumatori H.; Mori M.; Naito M.; Kawai T. Circularly Polarized Luminescence in Supramolecular Assemblies of Chiral Bichromophoric Perylene Bisimides. Chem. - Eur. J. 2013, 19, 14090–14097. 10.1002/chem.201302146. [DOI] [PubMed] [Google Scholar]
- The acyclic bis-triazole PDI control 3a exhibits no solvent dependence to its UV–vis absorption spectra (Figure S25).
- Kim W.; Nowak-Król A.; Hong Y.; Schlosser F.; Würthner F.; Kim D. Solvent-Modulated Charge-Transfer Resonance Enhancement in the Excimer State of a Bay-Substituted Perylene Bisimide Cyclophane. J. Phys. Chem. Lett. 2019, 10, 1919–1927. 10.1021/acs.jpclett.9b00357. [DOI] [PubMed] [Google Scholar]
- Moore J. S.; Ray C. R.. Supramolecular Organization of Foldable Phenylene Ethynylene Oligomers. In Poly(arylene etynylene)s: From Synthesis to Application Weder C., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2005; pp 91–149. [Google Scholar]
- Spenst P.; Young R. M.; Phelan B. T.; Keller M.; Dostál J.; Brixner T.; Wasielewski M. R.; Würthner F. Solvent-Templated Folding of Perylene Bisimide Macrocycles into Coiled Double-String Ropes with Solvent-Sensitive Optical Signatures. J. Am. Chem. Soc. 2017, 139, 2014–2021. 10.1021/jacs.6b11973. [DOI] [PubMed] [Google Scholar]
- Brooker L. G. S.; Craig A. C.; Heseltine D. W.; Jenkins P. W.; Lincoln L. L. Color and Constitution. XIII.1 Merocyanines as Solvent Property Indicators2. J. Am. Chem. Soc. 1965, 87, 2443–2450. 10.1021/ja01089a025. [DOI] [Google Scholar]
- Würthner F. Solvent Effects in Supramolecular Chemistry: Linear Free Energy Relationships for Common Intermolecular Interactions. J. Org. Chem. 2022, 87, 1602–1615. 10.1021/acs.joc.1c00625. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Fimmel B.; Würthner F. Solvent and substituent effects on aggregation constants of perylene bisimide π-stacks – a linear free energy relationship analysis. Org. Biomol. Chem. 2012, 10, 5845–5855. 10.1039/c2ob07131b. [DOI] [PubMed] [Google Scholar]
- Alongside toluene and benzene, we note that the sterically hindered solvent tert-butylbenzene also triggers intramolecular H-aggregation in 1a (Table S5).
- Analogous to the acyclic bis-triazole PDI control 3a (Table S6 and Figure S40).
- The 4e– reduction of 1a in 1:1 (v/v) toluene:dichloromethane is chemically reversible, yet the CV wave appears different on the reverse sweep because in 1a4– the PDI units repel each other and lose through-space communication.
- Yang L.; Langer P.; Davies E. S.; Baldoni M.; Wickham K.; Besley N. A.; Besley E.; Champness N. R. Synthesis and characterisation of rylene diimide dimers using molecular handcuffs. Chem. Sci. 2019, 10, 3723–3732. 10.1039/C9SC00167K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The H-type aggregate in 1:1 (v/v) toluene:dichloromethane was characterised using UV–vis spectroscopy. It was not possible to measure the cyclic voltammogram in toluene due to the poor solubilities of the reduced species in toluene.
- The electrochemistry of 3a is independent of solvent (Table S6 and Figure S40).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.