Myelofibrosis (MF) is a chronic myeloproliferative neoplasm characterized by bone marrow fibrosis (BMF), cytopenias, splenomegaly, elevated proinflammatory cytokine levels, debilitating symptom burden (eg, fatigue, pruritus, night sweats, weight loss),1,2 and reduced survival.3
Ruxolitinib is a potent Janus kinase (JAK)1/JAK2 inhibitor approved to treat MF. Panobinostat, a powerful pan-histone deacetylase (HDAC) inhibitor, impaired JAK signaling and effectively reduced splenomegaly and improved BMF in phase 1/2 studies.4 The ruxolitinib/panobinostat combination has demonstrated synergistic activity in preclinical MF models5 and a clinical study.6 Results of the dose escalation and expansion phases of a study assessing ruxolitinib and panobinostat in combination including data from patients who received ≥5 years of treatment (NCT01433445) are presented here.
The key inclusion criteria were as follows: Adults ≥18 years diagnosed with primary MF, postpolycythemia vera MF, or postessential thrombocythemia MF (PPV/PET-MF); palpable splenomegaly ≥5 cm; ineligible/unwilling for stem cell transplantation; classified as intermediate-1/2 or high risk using the international prognostic scoring system (IPSS)/dynamic IPSS criteria; discontinued all noninvestigational drugs for underlying MF disease.
The key exclusion criteria included splenic irradiation within 12 months; active malignancy within previous 3 years; history of clinically significant toxicities with ruxolitinib, panobinostat, or any JAK or HDAC inhibitor; history of platelet dysfunction, bleeding diathesis, or coagulopathy; impaired cardiac function. The expected sample size was 40–58 patients to establish the maximum tolerated dose/recommended phase 2 dose (MTD/RP2D).
We aimed to establish the MTD/RP2D for the ruxolitinib/panobinostat combination (study treatment) in patients with MF. We used a dose-escalation phase (DEC), guided by the Bayesian logistic regression model (BLRM), to establish the MTD/RP2D. A dose-expansion phase (DEP) was used to further explore the safety and efficacy at the achieved MTD/RP2D. Following the BLRM, newly enrolled patients received increasing doses of ruxolitinib (5–15 mg twice daily [BID]) and panobinostat (10–25 mg thrice weekly/every other week [TIW/QOW]), both in 5-mg increments, until MTD/RP2D was achieved.
The study was reviewed and approved by independent ethics committees/local review boards at each participating institution and conducted according to the ethical principles of the Declaration of Helsinki. All participants provided written informed consent before screening.
The primary endpoint was evaluation of dose-limiting toxicities (DLTs) with study treatment in cycle 1 (C1). Adverse events (AEs) were assessed using the Common Terminology Criteria for Adverse Events v4.03.
Efficacy was an exploratory outcome based on spleen palpation in all patients, spleen volume reduction (SVR) using magnetic resonance imaging/computed tomography (MRI/CT), and biomarkers of response at the MTD/RP2D, compared with baseline values.
Biomarkers samples were collected in the DEP to determine whether study treatment impacted JAK2V617F allele burden and cytokine levels as potential markers of response. The mutations involved in the etiology of MF7 were investigated as potential prognostic markers.
JAK2V617F allele burden: 5 mL of blood was collected on day 1 of cycles 1, 2, 4, 7, 10, and 13 for JAK2V617F mutation analysis in exon 14 using real-time quantitative polymerase chain reaction (qPCR) (Epistem Ltd, United Kingdom).
Cytokine analysis: 5 mL of blood was collected on day 1 of cycles 1, 2, 7, and 13, and processed according to the manufacturer’s instructions (Myriad RBM, United States) for protein marker analysis using the Human MAP 2.0 panel.
Next-generation sequencing (NGS): 5 mL of blood was collected at screening for NGS using a 24-gene panel (Genoptix, United States) (Suppl. Table S1).
The adaptive BLRM was used to identify the MTD, incorporating the escalation with overdose control (EWOC) principle. DLTs were reported based on the dose-determining set (all evaluable patients in every dose combination) for all doses including the MTD/RP2D.
Of the 61 patients in the study, 38 and 23 were in the DEC and DEP, respectively (Suppl. Table S2; Suppl. Figure S1).
Three DLTs were observed during DEC (grade 4 thrombocytopenia [n = 1 each with ruxolitinib 10 mg/panobinostat 10 mg and ruxolitinib 15 mg/panobinostat 20 mg cohort]; grade 3 nausea [n = 1, in ruxolitinib 15 mg/panobinostat 25 mg cohort]). Thrombocytopenia and nausea were consistent with the known profiles of both drugs.8 MTD/RP2D was confirmed as ruxolitinib 15 mg (BID)/panobinostat 25 mg (TIW/QOW). Twenty-three patients were further treated at this dose in DEP. When previously administered as single agents in MF patients, the MTD/RP2D was 25 mg bid for ruxolitinib,9 and 40 mg weekly,10 40 mg thrice weekly,8 or 25 mg thrice weekly for panobinostat.4
Median (min-max) duration of study treatment exposure was 139.9 weeks (3.1–387.0) for the MTD/RP2D. The most common all-grade AEs (>50%) regardless of the relationship to the study treatment were anemia (85.3%), diarrhea (76.5%), and thrombocytopenia (52.9%) at the MTD/RP2D (Table 1), which were similar to results from the COMFORT-II study11 and consistent with the mechanism of action of JAK1/2 inhibitors. Nine patients (26.5%) receiving the MTD/RP2D experienced serious AEs (SAE), suspected to be study-drug related, and anemia, sepsis, and pulmonary hypertension (5.9%, n = 2 each) were the most common. Overall, 26 patients discontinued treatment due to AEs, regardless of study-drug relationship, of which 15 patients discontinued at MTD/RP2D. The most frequent AEs (≥5%; all patients versus MTD/RP2D) included anemia (73.8% versus 85.3%), diarrhea (68.9% versus 76.5%), and thrombocytopenia (55.7% versus 52.9%). Four on-treatment deaths were reported. Two patients were in treatment with panobinostat 15 mg/ruxolitinib 15 mg (myocardial infarction, n = 1 and nasal cavity cancer treated ≥5 years, n = 1). One patient died due to progressive MF in treatment group panobinostat 20 mg/ruxolitinib 15 mg, and one death was reported at MTD/RP2D (cardiac arrest). Overall, the safety profile of the combination was consistent with that of the individual drugs. Hemoglobin levels decreased from baseline through week 6 and remained relatively stable throughout the study. Platelet counts in all treated patients decreased from baseline through week 4 and remained relatively stable throughout the study.
Table 1.
AEs by Grade, Regardless of Study-drug Relationship (≥20% of Patients, All Grades, Safety Set)
| Preferred terms, n (%) | All patients escalation phase (N = 38) | Panobinostat 25 mg Ruxolitinib 15 mg expansion phase (N = 23) | Patients treated at MTD/RP2D (N = 34) | Total patients (N = 61) | ||||
|---|---|---|---|---|---|---|---|---|
| All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | |
| Number of subjects with at least one event | 38 (100.0) | 33 (86.8) | 23 (100.0) | 21 (91.3) | 34 (100.0) | 31 (91.2) | 61 (100.0) | 54 (88.5) |
| Anemia | 24 (63.2) | 18 (47.4) | 21 (91.3) | 13 (56.5) | 29 (85.3) | 17 (50.0) | 45 (73.8) | 31 (50.8) |
| Diarrhea | 25 (65.8) | 6 (15.8) | 17 (73.9) | 5 (21.7) | 26 (76.5) | 9 (26.5) | 42 (68.9) | 11 (18.0) |
| Thrombocytopenia | 22 (57.9) | 10 (26.3) | 12 (52.2) | 7 (30.4) | 18 (52.9) | 10 (29.4) | 34 (55.7) | 17 (27.9) |
| Asthenia | 16 (42.1) | 5 (13.2) | 11 (47.8) | 1 (4.3) | 18 (52.9) | 5 (14.7) | 27 (44.3) | 6 (9.8) |
| Cough | 12 (31.6) | 0 (0.0) | 11 (47.8) | 0 (0.0) | 14 (41.2) | 0 (0.0) | 23 (37.7) | 0 (0.0) |
| Edema peripheral | 14 (36.8) | 0 (0.0) | 9 (39.1) | 0 (0.0) | 13 (38.2) | 0 (0.0) | 23 (37.7) | 0 (0.0) |
| Muscle spasms | 14 (36.8) | 0 (0.0) | 8 (34.8) | 0 (0.0) | 13 (38.2) | 0 (0.0) | 22 (36.1) | 0 (0.0) |
| Nausea | 14 (36.8) | 3 (7.9) | 7 (30.4) | 0 (0.0) | 13 (38.2) | 2 (5.9) | 21 (34.4) | 3 (4.9) |
| Headache | 13 (34.2) | 0 (0.0) | 7 (30.4) | 0 (0.0) | 12 (35.3) | 0 (0.0) | 20 (32.8) | 0 (0.0) |
MTD/RP2D = maximum tolerated dose/recommended phase 2 dose.
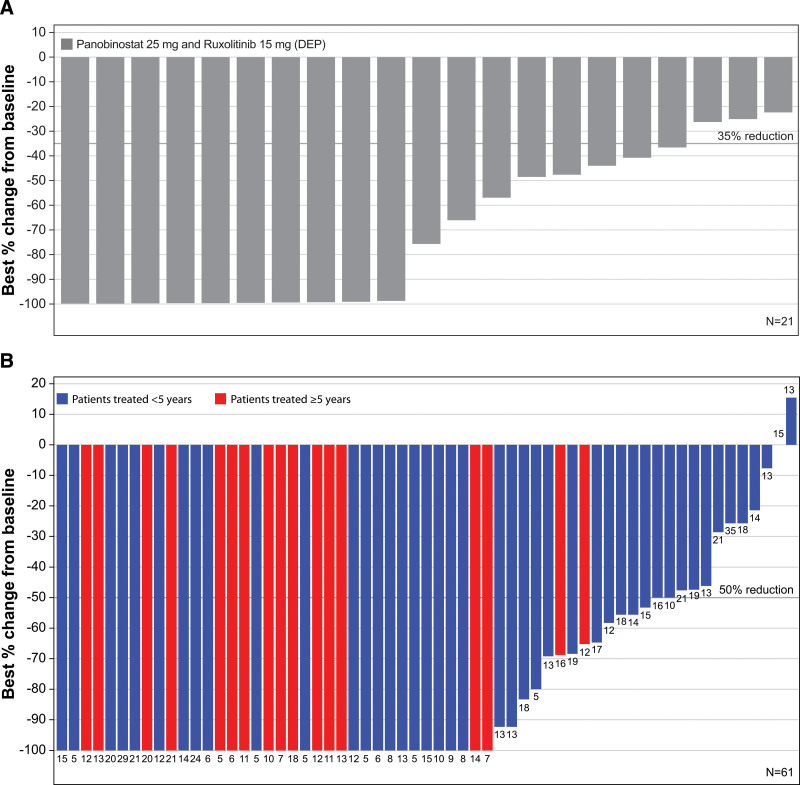
Spleen volume was assessed only in the DEP (n = 23), whereas spleen length was measured in all patients (n = 61). At least 35% SVR was achieved in 39.1% (n = 9/23 [95% CI 19.7, 61.5]) of patients on cycle 4 day 1 (C4D1) compared with baseline; 60.7% (n = 37/61 [95% CI 47.3, 72.9]) and 70.6% (n = 24/34 [95% CI 52.5, 84.9]) of patients achieved at least 50% spleen length reduction (SLR) on C4D1 in all patients and at MTD/RP2D, respectively, compared with baseline. Waterfall plots for best SVR and SLR at any time are presented in Figure 1A and B.
Figure 1.
Best percentage change at any time. (A) Best percentage change in spleen volume for all patients in expansion Phase. Two patients who had no available post-baseline spleen volume assessments are not summarized. Reference line indicates −35% change from baseline. (B) Best percentage change in spleen length (cm) from baseline by group of treatment duration (full analysis set). One patient who had no available post-baseline spleen length assessments was not summarized. Reference line indicates −50% change from baseline. The values on each bar indicates baseline spleen length (cm). DEP = dose-expansion phase.
JAK2V617F mutation data were obtained for 22 patients (17 mutated, 5 wild type). In the mutated population, only 3 patients showed ≥1 log2 fold reduction of JAK2 allele burden in postbaseline assessments compared to baseline levels (data not shown); none of these patients showed SVR >35% and all were on treatment for <5 years. Overall, no correlation between allele burden, SVR, and treatment duration was found.
Selected plasma cytokines (including chemokines and growth factors; Suppl. Table S3) involved in MF and regulated by JAK1/212 and HDAC inhibitors13 were analyzed until C13D1 (Suppl. Figure S2). Proinflammatory cytokine and chemokine levels (eg, interleukin-8 [IL-8], monocyte chemoattract protein-1 [MCP-1], macrophage inflammatory protein-1β [MIP-1β], and macrophage derived chemokine [MDC]) were similar in all patients regardless of whether SVR was achieved. We investigated megakaryocytic markers, including thrombospondin-1, which can lead to fibrosis via activation of TGF-β1 signaling and tissue inhibitor of metalloproteinases (TIMPs), and the simultaneous inhibition of angiogenesis via its receptor CD36 and inhibition of vascular epidermal growth factor (VEGF) signaling4,14; however, levels of TIMP-1 or matrix metalloproteinase (MMP) members and VEGF levels did not differ between the responders and nonresponders.
NGS data were available from 20 patients (Suppl. Figure S3). All patients in the subgroup displayed at least the presence of one high molecular risk (HMR) mutation (EZH2 2/20 [10%], ASXL1 10/20 [50%], SRSF2 18/20 [90%], IDH2 3/20 [15%]),15 identifying the population treated in the DEP as high risk. Overall, a similar mutation profile was observed in patients regardless of the SVR achieved.
In patients treated for ≥5 years (n = 17, median age: 60 years and 8 in MTD/RPIID) in this study, SAEs, regardless of study-drug relationship, were reported in 64.7% (n = 11) of patients, and 11.8% of patients (n = 2) discontinued treatment due to AEs regardless of study-drug relationship (anaplastic astrocytoma and nasal cavity cancer, n = 1 each). In this subgroup of patients, the mean hemoglobin level and mean platelet counts were higher over time than patients that were treated for <5 years. At C4D1, the mean (SD) SVR was 34.4% (n = 4; SD = 28.71) and the mean SLR was 67% (n = 17; SD = 26.37). The mean best spleen length and mean SVR across all time points was slightly higher in patients treated for ≥5 years compared to patients treated <5 years, possibly justifying why patients stayed longer on treatment.
Consistent with other reports,6 the panobinostat/ruxolitinib combination demonstrated the anticipated AEs but was considered tolerable in a small number of patients as showcased by those experiencing clinical benefits after ≥5 years of treatment.4,6,8 Therefore, this combination was effective in some advanced MF patients regardless of their molecular profile, although efficacy was an exploratory endpoint and the overall response rate was not significant to support further development. Other innovative and potentially more tolerable and effective combinations with ruxolitinib should be evaluated.
ACKNOWLEDGMENTS
We thank the patients and their families, investigators, and staff from all participating sites. We thank Haritha Nekkanti of Novartis Healthcare Pvt. Ltd. for providing medical editorial assistance with this article. We thank Sabine Loechner of Novartis Healthcare for contribution to protocol design and significant management and oversight of the trial. We also thank Piia-Piret Eomois of Novartis Pharma AG, who contributed to biomarker analyses and generated outputs.
AUTHOR CONTRIBUTIONS
CH, FHH, AMV, JJK, AH, FP, EC, TK, BM, and VR participated in the conception of the study; acquired and managed patients; reviewed and interpreted data and contributed to the writing of the article. DBL participated in the conception of the study, reviewed and interpreted data, and contributed to the writing of the article. PR, TS, and DG contributed to the design and implementation of the study, analyzed and interpreted the data, and took the lead in writing the article. JE contributed to the implementation of the study and writing of the article.
DISCLOSURES
FHH reports funding and grants from Novartis, Celgene/BMS, and CTI; participated on Advisory Board for, and received consulting fees, honoraria for lectures, and chemical compounds for experimental studies from Novartis and Celgene/BMS; cospeaker for German MPN Study Group; Review Board Member at Krebshilfe e.V.; Elected Review Board Member at DFG. AMV reports receiving honoraria for lectures from Novartis, BMS, GSK, and Abbvie; participated on Advisory Board for Novartis, BMS, Incyte, Blueprint, Roche, and Abbvie. CH reports receiving grants from Novartis and Constellation Pharma; payment or honoraria for lectures from Novartis, BMS, Galecto, Sierra, Roche, AOP, CTI, Jannsen, Abbvie, and Geron; support for attending meeting (ASH 2020) from Celgene; participated as Chair of Data Safety Monitoring Board for Galecto; is an Editor for HemaSphere; and participated on Scientific Board and Financial Board for EHA. FP reports receiving payment or honoraria for lectures from Novartis, Abbvie, BMS, Jannsen, and Amomed; and participated on Data Safety Monitoring Board or advisory board for Abbvie and BMS. VR reports receiving research funding from Astex, GSK, and Epizyme; honoraria from Epizyme; participated on Board or Advisory Committee for AZ, Infinity, MSD, Nanostring, PharmaMar, Roche, Servier, Incyte, Gilead, and BMS; and consultancy for Servier. EC reports receiving honoraria for lectures and support for attending virtual meeting from Novartis; and participated on Advisory Board for Novartis. JJK reports receiving consultancy fees from Novartis and Abbvie; payment or honoraria for lectures from Novartis and AOP Orphan; and participated on Data Safety Monitoring Board or Advisory Board for BMS/Celgene and Incyte. PR is a shareholder of Novartis. All the other authors have no conflicts of interest to disclose.
SOURCE OF FUNDING
This study was supported by research funding from Novartis Pharmaceuticals Corporation.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Keohane C, Radia DH, Harrison CN. Treatment and management of myelofibrosis in the era of JAK inhibitors. Biologics. 2013;7:189–198.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annu Rev Med. 2009;60:233–245.. [DOI] [PubMed] [Google Scholar]
- 3.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901.. [DOI] [PubMed] [Google Scholar]
- 4.Mascarenhas J, Lu M, Li T, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Br J Haematol. 2013;161:68–75.. [DOI] [PubMed] [Google Scholar]
- 5.Evrot E, Ebel N, Romanet V, et al. JAK1/2 and Pan-deacetylase inhibitor combination therapy yields improved efficacy in preclinical mouse models of JAK2V617F-driven disease. Clin Cancer Res. 2013;19:6230–6241.. [DOI] [PubMed] [Google Scholar]
- 6.Mascarenhas J, Marcellino B, Lu M, et al. A phase I study of panobinostat and ruxolitinib in patients with primary myelofibrosis (PMF) and post-polycythemia vera/essential thrombocythemia myelofibrosis (post-PV/ET MF). Leuk Res. 2020;88:106272. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Nicolosi M, Mudireddy M, et al. Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am J Hematol. 2018;93:348–355.. [DOI] [PubMed] [Google Scholar]
- 8.DeAngelo DJ, Mesa RA, Fiskus W, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post–essential thrombocythaemia, and post–polycythaemia vera myelofibrosis. Br J Haematol. 2013;162:326–335.. [DOI] [PubMed] [Google Scholar]
- 9.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeAngelo D, Spencer A, Bhalla K, et al. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia. 2013;27:1628–1636.. [DOI] [PubMed] [Google Scholar]
- 11.Harrison C, Kiladjian J-J, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798.. [DOI] [PubMed] [Google Scholar]
- 12.Dueck AC, Cleeland CS, Dantzer R, et al. Cytokine profile changes in 309 myelofibrosis patients: comparison of JAK1/JAK2 inhibitor therapy vs. placebo—correlative analysis from the comfort-I trial. Blood. 2013;122:4074–4074.. [Google Scholar]
- 13.Gatla HR, Muniraj N, Thevkar P, Yavvari S, Sukhavasi S, Makena MR. Regulation of chemokines and cytokines by histone deacetylases and an update on histone decetylase inhibitors in human diseases. Int J Mol Sci. 2019;20:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondet J, Hussein K, Mossuz P. Circulating cytokine levels as markers of inflammation in Philadelphia negative myeloproliferative neoplasms: diagnostic and prognostic interest. Mediators Inflamm. 2015;2015:670580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vannucchi AM, Lasho T, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.