Abstract
Lactic acidosis is the most common anion gap metabolic acidosis in critically ill patients. Type B lactic acidosis is most commonly seen with hematological malignancies, especially lymphomas. It is considered an oncological emergency and is associated with high mortality and poor outcomes if not treated promptly. Here, we present the case of a 48-year-old male who developed Type B lactic acidosis secondary to newly diagnosed diffuse large B-cell lymphoma. This case highlights the importance of including Type B lactic acidosis in the differential diagnosis in a patient with unexplained lactic acidosis and hypoglycemia with otherwise vague symptoms and the need for a thorough search for quick diagnosis and early management.
Keywords: oncological emergency, warburg effect, dlbcl, type b lactic acidosis, lactic acidosis
Introduction
Lactic acidosis is the most common anion gap metabolic acidosis in critically ill patients [1]. It occurs when the production of lactic acid exceeds clearance [2]. Most of the lactate (80-90%) is cleared by the liver through gluconeogenesis while the remaining is cleared by the kidneys [3,4].
Type B lactic acidosis is most commonly seen with hematological malignancies, especially lymphomas. It is considered an oncological emergency and is associated with high mortality and poor outcomes if not treated promptly [2].
Here, we present the case of a 48-year-old male who developed Type B lactic acidosis from newly diagnosed diffuse large B-cell lymphoma (DLBCL).
Case presentation
A 48-year-old Hispanic man presented with a three-day history of left-sided headache, left jaw pain and difficulty speaking, and tongue deviation to left. He sustained a fall six months back without loss of consciousness but noticed some weakness in his right leg and tongue, which resolved in a few hours, and did not seek medical attention. He endorsed about 30 lbs weight loss within a six-month period.
On admission, the patient had a blood pressure of 118/76 mmHg, a pulse rate of 98 beats/minute, respiratory rate of breaths 14/minute, and oxygen saturation of 98% on room air and was afebrile. Physical examination was significant for palpable non-tender supraclavicular, axillary, posterior cervical, and bilateral inguinal lymphadenopathy and decreased breath sounds at the left lung base associated with a dull note on percussion. A detailed neurological examination revealed decreased left eye abduction and decreased light reflex in the left eye and deviation of the tongue to the left. The remainder of the examination was unremarkable.
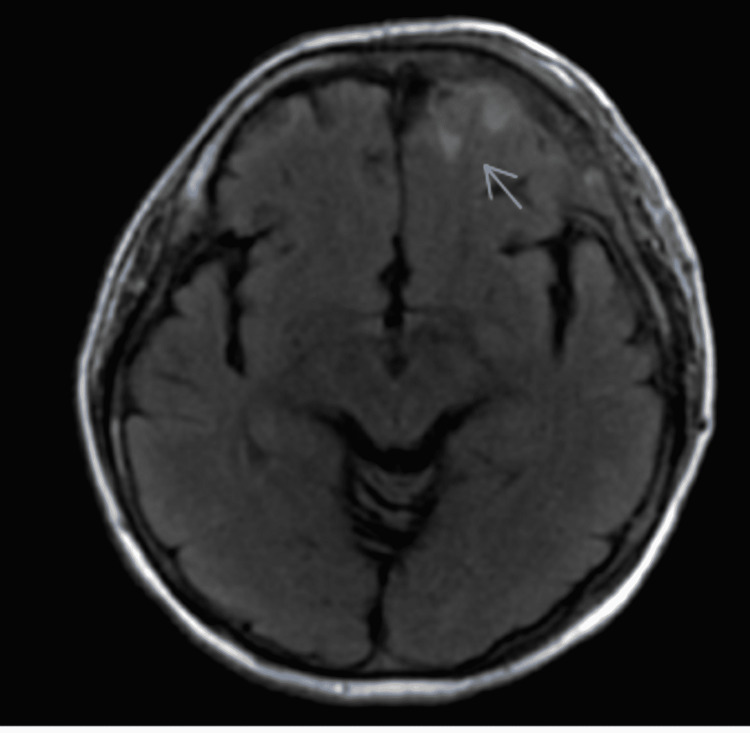
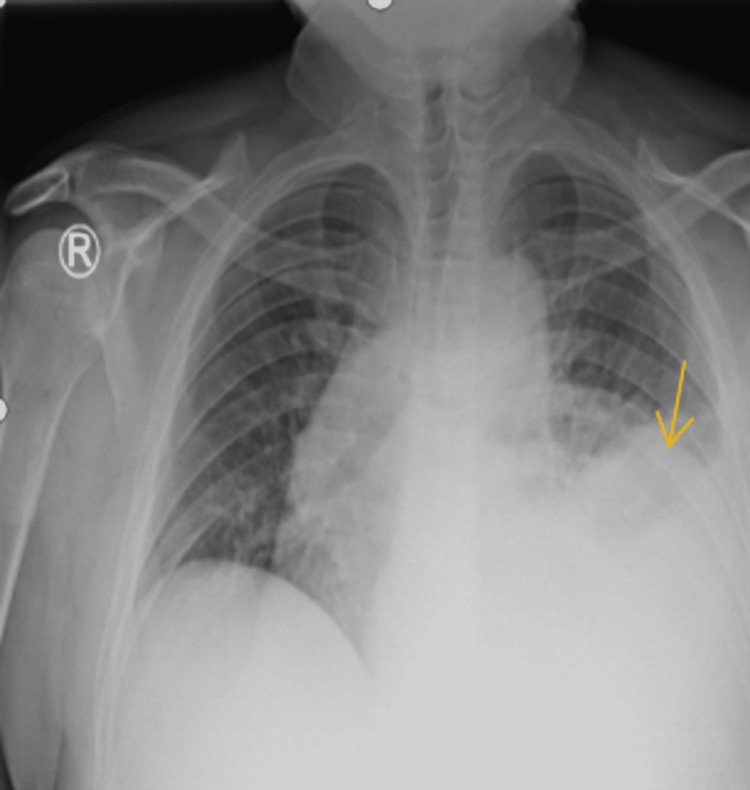
An initial computed tomography (CT) of the head demonstrated a sub-acute on chronic left subdural hematoma associated with a left frontal contusion. Magnetic resonance imaging (MRI) of the brain with angiography demonstrated a multifocal patchy enhancing marrow replacing the process of the calvarium representing osseous metastatic disease and associated soft tissue extension beyond the osseous margins (Figure 1). Pachymeningeal thickening and enhancement at the left frontal convexity; and a small T2/fluid-attenuated inversion recovery (FLAIR) signal abnormality at the left frontal lobe cortical region indicated potential sequelae of parenchymal extension of disease. The presence of generalized lymphadenopathy and radiological evidence of a probable metastatic disease prompted a thorough evaluation. His chest X-ray showed hilar fullness with left-sided pleural effusion, as shown in Figure 2.
Figure 1. MRI showing a small hyperintense lesion in the left frontal cortical region.
MRI: magnetic resonance imaging
Figure 2. Chest X-ray showing left pleural effusion.
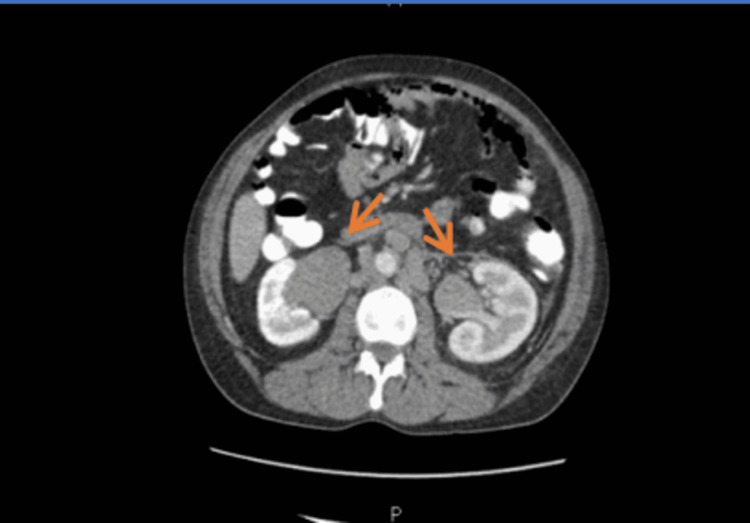
CT thorax, abdomen, and pelvis with contrast showed a large left pleural effusion with pleural thickening, mediastinal, bilateral hilar, axillary, cervical, and abdominal lymphadenopathy along with enlarged spleen with innumerable hypoattenuating lesions and bilateral moderate hydronephrosis due to compression of ureters from lymph nodes (Figure 3).
Figure 3. CT abdomen showing bilateral lymphadenopathy with hydroureteronephrosis (arrows).
CT: computed tomography
His initial blood work revealed microcytic anemia (hemoglobin = 8.7 g/dL), thrombocytosis (495 K/mm3), elevated erythrocyte sedimentation rate (ESR) (78 mm/hour), and lactate dehydrogenase (LDH) levels (471 U/L), acute kidney injury (AKI) with a serum creatinine of 1.9 mg/dL, and mild transaminitis. Peripheral smear was suggestive of anisocytosis, poikilocytosis, polychromasia, ovalocytes, burr cells, schistocytes, target cells, and teardrop cells.
Given the patient’s large left pleural effusion, 1,500 mL of dark yellow-colored fluid was drained. The pleural fluid analysis showed an exudative effusion with lymphocyte predominance (70%) with 3% atypical cells.
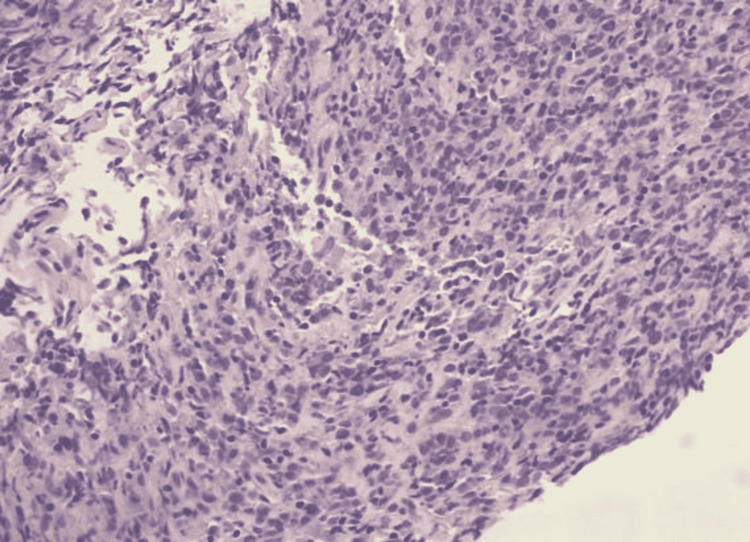
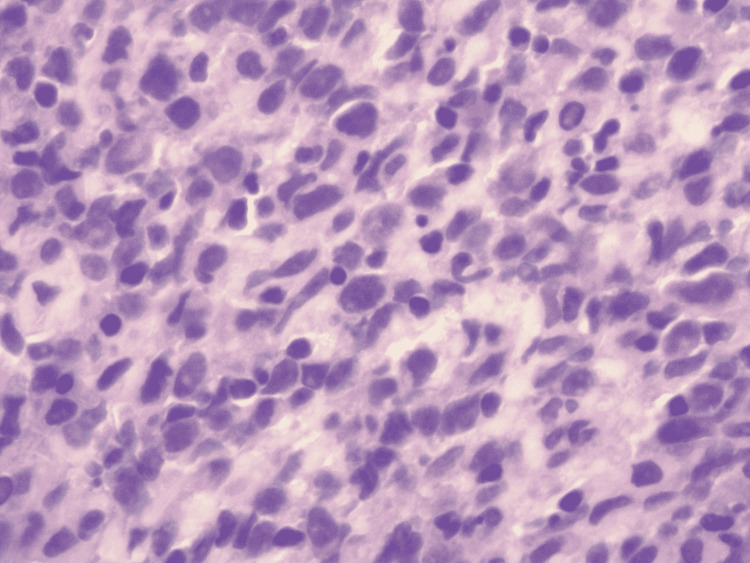
The patient underwent a left axillary lymph node biopsy, bone scan, and serum protein electrophoresis (SPEP) for malignancy workup. A bone scan showed marrow infiltration at multiple levels; however, no overt lesions were noticed. SPEP had a normal pattern and no monoclonal pattern was seen. Left axillary lymph node biopsy revealed a low-level atypical B-cell population with immunohistochemistry positive for CD20, CD79a, PAX5, and sBCL2 dim and bright consistent with DLBCL (Figures 4, 5).
Figure 4. Lymph node core biopsy showing lymphoid tissue with mixed small and intermediate-sized lymphocytes.
Figure 5. Atypical large lymphocytes showing nucleomegaly with inconspicuous nucleoli.
The patient had an extensive hospital course complicated with hypercalcemia and worsening transaminitis and recurrent left-sided malignant pleural effusion. He also developed high anion gap metabolic acidosis secondary to lactic acidosis and hypoglycemia despite adequate oral intake. Workup for viral hepatitis was negative and abdominal ultrasonogram showed no abnormalities.
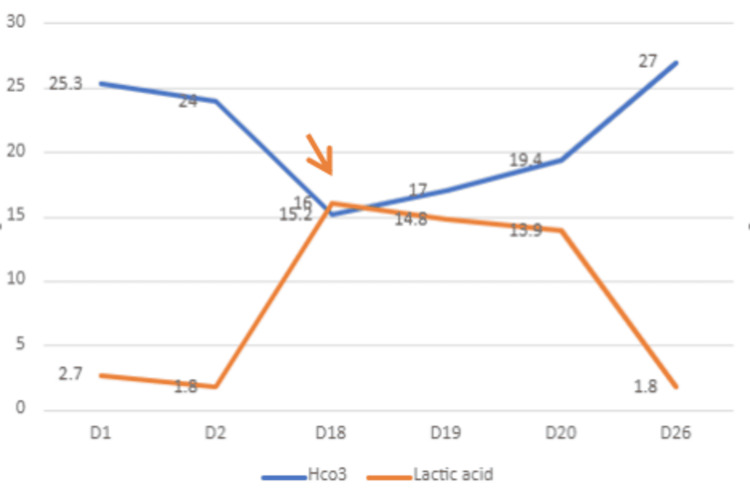
His lactic acidosis worsened with a peak level of 16, as depicted in Figure 6. He was hemodynamically stable with a blood pressure of 112/68 mmHg, a heart rate of 98 beats/minute, and his oxygen saturation was 98% on room air despite high lactate and severe acidosis. His blood glucose levels were noted to be low at 38 mg/dL. He was started on intravenous (IV) fluids and one dose of zoledronic acid for hypercalcemia. He was also started on an IV sodium bicarbonate drip and a high dose of IV thiamine. He was started on emergent hemodialysis (HD) along with chemotherapy with an R-CHOP regimen (rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride (doxorubicin hydrochloride), vincristine (Oncovin), and prednisone), which led to improvement in the patient’s Type B lactic acidosis over the course of a few days.
Figure 6. The trend of lactic acid and HCO3 levels over the course of hospital stay with an arrow indicating initiation of hemodialysis.
Discussion
Luft et al. defined lactic acidosis as lactate levels of more than 5 mmol/L associated with metabolic acidosis with a pH of less than 7.35 [5]. There are two types of lactic acidosis based on pathophysiology: Type A, which is the most common type and is due to poor tissue oxygenation and hypoperfusion, and Type B, which is seen in conditions with normal oxygenation and perfusion and is usually a result of drugs or toxin that interfere with normal cellular metabolism or due to nutritional deficiency [6-8].
Type B lactic acidosis is a dreaded complication associated with solid (15%) and hematological malignancies (85%) [9]. A retrospective study by Friedenberg et al. showed that type B lactic acidosis is most commonly associated with lymphomas, multiple myeloma, and leukemia and is associated with high mortality (>90%) and has a very poor prognosis [10,11].
The underlying mechanism for type B lactic acidosis in hematological malignancies can be multifactorial but can be explained by the “Warburg” effect also termed aerobic glycolysis first described by Otto Warburg. The mechanism involves tumor cells having very high glucose uptake compared to non-cancerous cells, yet preferentially producing lactate via anaerobic metabolism despite normoxic and normotensive conditions [12].
Our patient initially presented with subdural hematoma, significant weight loss, and generalized lymphadenopathy. His laboratory results were significant for microcytic anemia, elevated LDH levels, and transaminitis. Acute kidney injury is presumed secondary to obstructive uropathy caused by adenopathy and radiological evidence of a large pleural effusion, metastatic disease in the marrow and intracranial metastatic lesion, and generalized lymphadenopathy and splenomegaly. Initial differential diagnoses included lymphoma, leukemia, and multiple myeloma. Biopsy of lymph node clinched the diagnosis of DLBCL. He did not have any hypoxia and was hemodynamically stable when he developed lactic acidosis, warranting us to think in terms of type B lactic acidosis. A search for infectious etiology was ruled as blood cultures were negative.
A brief review by Claudino et al. in a case report suggested the likely cause for hypoglycemia is a direct result of excess consumption of glucose by tumor cells [13]. However, the treatment should not be focused on supplementing excess glucose as it could worsen the lactic acidosis from over-consumption by tumor cells, a phenomenon described as hyper-Warburgism [14].
As a high tumor burden is causing lactic acidosis, the mainstay of treatment is cytoreductive chemotherapy, as demonstrated by Chan et al. [15] in their retrospective literature review of patients with lymphoma, who developed Type B lactic acidosis and by Silos et al. [11], in their review of literature of patients with hematological malignancies, who developed Type B lactic acidosis. However, it did not cause resolution in all the cases, as reported by Kestler et al. [16]. Ruiz et al. suggest that treatment modalities such as hemodialysis, thiamine, and sodium bicarbonate infusion should be considered while a response to chemotherapy ensues but their efficacy is controversial [9]. Our patient received three cycles of hemodialysis, thiamine, and sodium bicarbonate infusion, while he was started on chemotherapy, following which the patient’s lactic acidosis improved.
Conclusions
Type B lactic acidosis is an oncological emergency that warrants prompt diagnosis and aggressive treatment due to high mortality and poor prognosis. Currently, the most common treatment strategy is effective cytoreductive chemotherapy to reduce the high tumor burden that precipitated lactic acidosis. However, even with aggressive chemotherapy, mortality rates are high. Hence, unexplained lactic acidosis and hypoglycemia with otherwise vague symptoms should prompt a thorough search for quick diagnosis and early management.
Acknowledgments
Raghavendra Sanivarapu MD and Pratap Kumar Upadrista MD contributed equally to the work and should be considered co-first authors.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Machine learning consensus clustering approach for patients with lactic acidosis in intensive care units. Pattharanitima P, Thongprayoon C, Petnak T, et al. J Pers Med. 2021;11:1132. doi: 10.3390/jpm11111132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Type B lactic acidosis associated with diffuse large B-cell lymphoma and the Warburg effect. Wang C, Lv Z, Zhang Y. J Int Med Res. 2022;50:3000605211067749. doi: 10.1177/03000605211067749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etiology and therapeutic approach to elevated lactate levels. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Mayo Clin Proc. 2013;88:1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lactate as a hemodynamic marker in the critically ill. Fuller BM, Dellinger RP. Curr Opin Crit Care. 2012;18:267–272. doi: 10.1097/MCC.0b013e3283532b8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Definition of clinically relevant lactic acidosis in patients with internal diseases. Luft D, Deichsel G, Schmülling RM, Stein W, Eggstein M. Am J Clin Pathol. 1983;80:484–489. doi: 10.1093/ajcp/80.4.484. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and biochemical aspects of lactic acidosis. Baron DN. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC476665/ J Clin Pathol. 1977;30:92. [Google Scholar]
- 7.Fauci AS, Kasper DL, Longo DL, et al. Harrison’s internal medicine, 17th edition. New York: McGraw-Hill Companies, Inc.; 2004. [Google Scholar]
- 8.Lactic acidosis. Kraut JA, Madias NE. N Engl J Med. 2014;371:2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 9.Type B lactic acidosis secondary to malignancy: case report, review of published cases, insights into pathogenesis, and prospects for therapy. Ruiz JP, Singh AK, Hart P. ScientificWorldJournal. 2011;11:1316–1324. doi: 10.1100/tsw.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Friedenberg AS, Brandoff DE, Schiffman FJ. Medicine (Baltimore) 2007;86:225–232. doi: 10.1097/MD.0b013e318125759a. [DOI] [PubMed] [Google Scholar]
- 11.Lactic acidosis: a metabolic complication of hematologic malignancies: case report and review of the literature. Sillos EM, Shenep JL, Burghen GA, Pui CH, Behm FG, Sandlund JT. Cancer. 2001;92:2237–2246. doi: 10.1002/1097-0142(20011101)92:9<2237::aid-cncr1569>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.On the origin of cancer cells. Warburg O. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 13.Type B lactic acidosis: a rare but life threatening hematologic emergency. A case illustration and brief review. Claudino WM, Dias A, Tse W, Sharma VR. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4497494/ Am J Blood Res. 2015;5:25–29. [PMC free article] [PubMed] [Google Scholar]
- 14."Hyper-warburgism," a cause of asymptomatic hypoglycemia with lactic acidosis in a patient with non-Hodgkin's lymphoma. Elhomsy GC, Eranki V, Albert SG, Fesler MJ, Parker SM, Michael AG, Griffing GT. J Clin Endocrinol Metab. 2012;97:4311–4316. doi: 10.1210/jc.2012-2327. [DOI] [PubMed] [Google Scholar]
- 15.Severe lactic acidosis in a patient with B-cell lymphoma: a case report and review of the literature. Chan FH, Carl D, Lyckholm LJ. https://doi.org/10.1155/2009/534561. Case Rep Med. 2009;2009:534561. doi: 10.1155/2009/534561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hepatic non-Hodgkin's lymphoma with lactic acidosis in HIV-infected patients: report of 2 cases. Kestler MH, Gardner EM, Cohn DL. J Int Assoc Physicians AIDS Care (Chic) 2010;9:301–305. doi: 10.1177/1545109710377803. [DOI] [PubMed] [Google Scholar]