ABSTRACT
Background
Vitamin D deficiency is frequently found in patients with chronic obstructive pulmonary disease (COPD). Vitamin D has antimicrobial, anti-inflammatory, and immunomodulatory effects. Therefore, supplementation may prevent COPD exacerbations, particularly in deficient patients.
Objectives
We aimed to assess the effect of vitamin D supplementation on exacerbation rate in vitamin D–deficient patients with COPD.
Methods
We performed a multicenter, double-blind, randomized controlled trial. COPD patients with ≥1 exacerbations in the preceding year and a vitamin D deficiency (15–50 nmol/L) were randomly allocated in a 1:1 ratio to receive either 16,800 International Units (IU) vitamin D3 or placebo once a week during 1 y. Primary outcome of the study was exacerbation rate. Secondary outcomes included time to first and second exacerbations, time to first and second hospitalizations, use of antibiotics and corticosteroids, pulmonary function, maximal respiratory mouth pressure, physical performance, skeletal muscle strength, systemic inflammatory markers, nasal microbiota composition, and quality of life.
Results
The intention-to-treat population consisted of 155 participants. Mean ± SD serum 25-hydroxyvitamin D [25(OH)D] concentration after 1 y was 112 ± 34 nmol/L in the vitamin D group, compared with 42 ± 17 nmol/L in the placebo group. Vitamin D supplementation did not affect exacerbation rate [incidence rate ratio (IRR): 0.90; 95% CI: 0.67, 1.21]. In a prespecified subgroup analysis in participants with 25(OH)D concentrations of 15–25 nmol/L (n = 31), no effect of vitamin D supplementation was found (IRR: 0.91; 95% CI: 0.43, 1.93). No relevant differences were found between the intervention and placebo groups in terms of secondary outcomes.
Conclusions
Vitamin D supplementation did not reduce exacerbation rate in COPD patients with a vitamin D deficiency.
This trial was registered at clinicaltrials.gov as NCT02122627.
Keywords: chronic obstructive pulmonary disease, exacerbation rate, muscle strength, physical function, pulmonary function, vitamin D
Introduction
Vitamin D has emerged as a potential therapeutic in patients with chronic obstructive pulmonary disease (COPD). Vitamin D deficiency is highly prevalent in patients with COPD and related to disease severity (1–3). COPD is characterized by a progressive nonreversible airflow limitation caused by an abnormal inflammatory reaction of the lungs to tobacco smoke or other airway irritants. Various factors, such as bacterial and viral infections, can trigger exacerbations of COPD. These exacerbations may result in hospital admissions, a more rapid decline of respiratory function, and decreased quality of life. Vitamin D has been hypothesized to have beneficial effects in patients with COPD through its immunomodulatory effects. Vitamin D stimulates cells of the adaptive immune system to differentiate toward a tolerogenic phenotype and decreases production of proinflammatory mediators (4). Also, vitamin D stimulates phagocytosis and antimicrobial activity of innate immune cells (4). In addition to effects on the immune system, vitamin D affects muscle function. Meta-analyses show a small, but relevant, effect of vitamin D supplementation on skeletal muscle strength in vitamin D–deficient participants and the elderly (5, 6). In patients with COPD, reduced skeletal muscle strength is common and related to disease severity and mortality (7–9). These findings have led to the hypothesis that vitamin D supplementation might be beneficial in patients with COPD, particularly in the prevention of exacerbations.
Four randomized placebo-controlled trials (RCTs) have studied the effects of vitamin D supplementation on exacerbation rate in COPD patients. Except for 1 small pilot trial (10), these studies recruited patients irrespective of baseline vitamin D concentrations (11–13). Three of these trials did not show an overall effect (10–12), and 1 trial did demonstrate a protective effect of vitamin D (13). However, the latter trial contains methodological issues that hamper interpretation and comparison. An individual participant data (IPD) meta-analysis of the first 3 RCTs confirmed the absence of an effect of vitamin D supplementation on exacerbation rate in the overall population. However, it showed a reduction in exacerbation incidence rate of almost 50% compared with placebo in patients with serum 25-hydroxyvitamin D [25(OH)D] concentrations <25 nmol/L, indicating a clinically relevant favorable effect in the vitamin D–deficient individuals (14).
Therefore, our study aimed to assess the effects of vitamin D supplementation on exacerbation rate specifically in COPD patients with a vitamin D deficiency. In addition to exacerbation rate, we assessed the effects of vitamin D supplementation on pulmonary function, respiratory mouth pressure, physical performance, skeletal muscle strength, systemic inflammatory markers, nasal microbiota composition, and quality of life.
Methods
Study design
The study design and outline of this study (NCT02122627) have been described previously (15). We performed a 2-arm parallel double-blind RCT over 1 y. Study visits took place at baseline, 6 mo, and 12 mo after randomization. In addition, telephone contacts took place every 2 mo. All participants gave written informed consent. The study was approved by the central medical ethics committee of the Amsterdam University Medical Center and all institutional review boards of the participating hospitals. The trial was performed in accordance with the Declaration of Helsinki.
Study population
Participants were recruited from 13 different hospitals from different regions in the Netherlands. Patients with a COPD diagnosis confirmed by a pulmonologist were eligible if they were aged 40 y or older, had a vitamin D deficiency [25(OH)D concentration <50 nmol/L], and had a confirmed history of a COPD exacerbation in the last 12 mo before screening. Main exclusion criteria were severe vitamin D deficiency (<15 nmol/L) owing to ethical reasons (16), and use of supplements containing >400 International Units (IU) vitamin D. Supplemental Table 1 provides a complete description of the eligibility criteria. Data were collected by centrally trained research nurses and physicians.
Intervention and randomization
Eligible participants were allocated in a 1:1 ratio to receive either 16,800 IU vitamin D (3 tablets of 5600 IU) or a matching placebo orally once a week for 1 y. Randomization and preparation of study medication were performed by the pharmacy of Amsterdam University Medical Center. For randomization a sequential balancing method was used with study center as the first step in the balancing algorithm, followed by gender, age, and current smoking status. Blinding was continued until after the data analyses.
At every study visit, participants were asked to return their leftover study medication to count the remaining tablets as a measure of compliance. During the study, participants were advised to have a dietary intake of ≥1000 mg Ca or to use calcium supplements if they were not able to reach this minimum intake.
Primary outcome
The primary outcome of this study was exacerbation rate in 1 y. An exacerbation was defined as sustained worsening of respiratory symptoms during 48 h requiring oral corticosteroid and/or antibiotic treatment that was initiated by a physician. Respiratory symptoms included ≥1 of the major Anthonisen criteria: increased dyspnea, sputum volume, and/or purulence (17). During the study, participants kept a diary card to register symptoms and changes in medication use. In addition, during each telephone contact symptoms, hospitalization, and changes in medication use were discussed and recorded. When necessary, information on potential exacerbations and medication use was confirmed through contact with the providing pharmacist, the general practitioner, or the pulmonologist.
Secondary outcomes
Time to first and second exacerbations and time to first and second hospitalizations were used as secondary outcomes. In addition, the use of antibiotics and oral corticosteroids was assessed. At the first and final study visits all participants underwent spirometry measuring postbronchodilator forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (18). In addition, measurements of absolute lung volume, residual volume, functional residual capacity, and total lung capacity were assessed by body plethysmography (19, 20). Respiratory muscle strength was tested by measurement of the maximal inspiratory and expiratory mouth pressures (21).
During every visit participants performed several physical performance tests, including the 6-min walking test, 3-m walking test, chair-stands test, and tandem test. Muscle strength was measured using handgrip strength. Finally, at every study visit they filled in questionnaires on quality of life and physical activity. Details of these procedures and questionnaires have been described previously (15).
Biochemical measurements
Blood samples were obtained at the first and last study visits and stored at −80°C until determination. 25(OH)D concentrations were assessed by isotope dilution/online solid-phase extraction liquid chromatography/tandem mass spectrometry (ID-XLC-MS/MS) at the Endocrine Laboratory of the Amsterdam University Medical Center (22). The limit of quantitation was 4.0 nmol/L; intra-assay CV was <6% and interassay CV was <8% for concentrations between 25 and 180 nmol/L. 25(OH)D2 and 25(OH)D3 were measured separately. Plasma C-reactive protein (CRP), IL-6, and LL-37 concentrations were measured at the Leiden University Medical Center according to the manufacturer's instructions (CRP and IL-6: Meso Scale Diagnostics; LL-37: Hycult Biotech).
Nasal microbiome analysis
During the first and last study visits a nasal swab was obtained from participants at 10 study centers (88 available paired samples). The swabs were obtained and directly stored at −80°C until determination. Microbiome analysis was performed by amplicon sequencing of the 16S ribosomal RNA gene using the V4 hypervariable region on the Illumina MiSeq sequencer (Illumina). The Supplemental Methods provide a detailed description (p. 3).
Statistical analysis
The sample size calculation for this study was based on post hoc analysis of the RCT performed by Lehouck et al. (11), which was the only trial available at the time of design of this study. Based on differences in Poisson means, a sample size of 94 participants/group was needed to demonstrate a difference of 1 exacerbation/patient-year with 80% power at 5% significance. To account for a dropout rate of 20%, 120 participants were needed per study group (15).
Exacerbation rate was analyzed using a negative binomial regression analysis using study duration as an offset variable. All analyses were adjusted for the variables included in the balancing algorithm for randomization, i.e., study center, gender, age, and smoking status. Time to first exacerbation, second exacerbation, and first hospitalization were analyzed using Cox proportional hazard regression analyses. Time-to-event was calculated by subtracting the date of exacerbation or hospitalization from the date of the baseline measurement. For subjects who did not experience an exacerbation or hospitalization, time to study completion, dropout, or loss-to-follow-up was calculated. Use of antibiotics and corticosteroids was analyzed using logistic regression analyses. Other secondary outcomes, i.e., spirometry measures, lung volumes, maximal respiratory mouth pressures, systemic inflammatory markers, physical function, and handgrip strength, were analyzed using linear mixed models, with intervention group as a fixed independent variable, baseline measurement as a fixed covariate, and a random intercept for individuals. Statistical analysis of microbiome data is described in the Supplemental Methods (p. 3).
All analyses were performed using the intention-to-treat population, defined as all randomly assigned participants who received ≥1 dose of study medication. Participants who dropped out of the study or were lost to follow-up were censored from the date of last contact. In addition, a prespecified subgroup analysis was performed in participants with a baseline serum 25(OH)D concentration ≤25 nmol/L. In addition, a per-protocol analysis was performed including participants with good compliance, i.e., 80% of all doses ingested and/or a serum 25(OH)D concentration >60 nmol/L after 12 mo.
All data analyses were performed using Stata Statistical Software version 13.1 (Statacorp).
Results
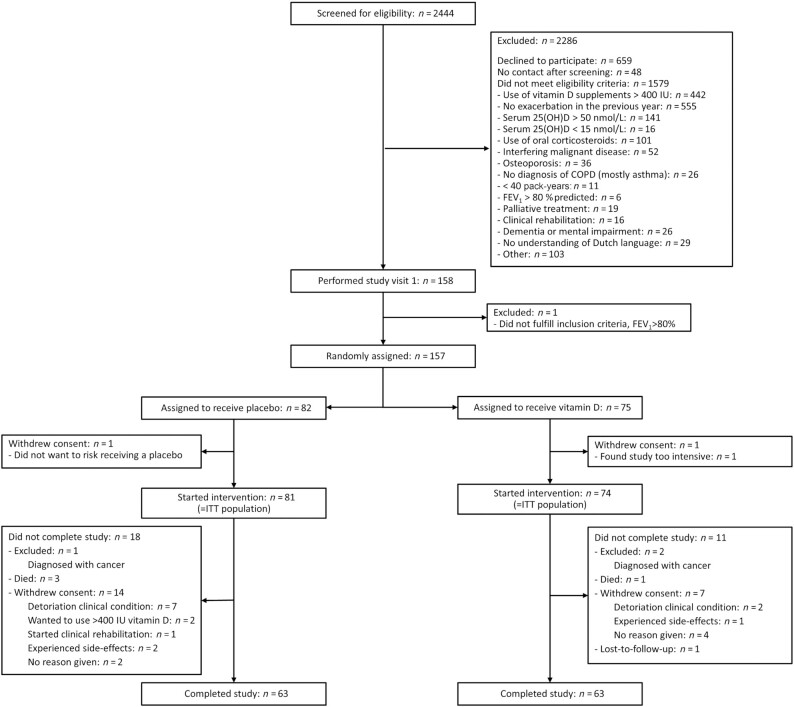
In total, 155 participants were enrolled between 19 January, 2015 and 1 July, 2019 and included in the intention-to-treat analysis (Figure 1). The predefined sample size was not reached despite an extension of the predefined inclusion period of 1.5 y to 3.5 y. Further extension of the inclusion period was limited because of the expiration date of the study medication.
FIGURE 1.
Flow diagram of study participants. This flow diagram depicts the screening and randomization of participants for the study. The numbers of participants excluded because of supplement use or high vitamin D concentrations are not generalizable, because a large part was already prescreened by their physicians at the outpatient clinic or during hospital admission. COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; ITT, intention-to-treat; IU, International Unit; 25(OH)D, 25-hydroxyvitamin D.
Table 1 shows baseline characteristics of the study population. Mean ± SD serum 25(OH)D concentration was 38 ± 15 nmol/L in the vitamin D group and 40 ± 17 nmol/L in the placebo group. Median number of cigarette pack-years was 40 in both groups. Approximately 80% of participants of both groups had COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD)-stage II or III with a mean FEV1 of 50% predicted. No differences were found in baseline characteristics between the 2 groups.
TABLE 1.
Baseline characteristics1
| Placebo group (n = 81) | Vitamin D group (n = 74) | |
|---|---|---|
| Age, y | 67 ± 9 | 65 ± 9 |
| Sex, male | 55 (68) | 46 (62) |
| BMI, kg/m2 | 27.4 ± 5.4 | 28.1 ± 5.1 |
| Smoking | ||
| Current smoker | 23 (28) | 25 (34) |
| Ex-smoker | 58 (72) | 49 (66) |
| Pack-years | 41 [21–55] | 40 [26–55] |
| Ethnic origin, European descent | 73 (90) | 68 (92) |
| GOLD-stage | ||
| I | 6.2 | 5.3 |
| II | 42.0 | 44.0 |
| III | 37.0 | 41.3 |
| IV | 14.8 | 9.3 |
| Spirometry | ||
| FEV1, L | 1.43 ± 0.56 | 1.43 ± 0.54 |
| FEV1, %predicted | 50.3 ± 18.4 | 50.0 ± 16.0 |
| FVC, L | 3.31 ± 0.93 | 3.24 ± 0.93 |
| FVC, %predicted | 89.3 ± 18.1 | 88.9 ± 19.8 |
| FEV1:FVC ratio, % | 43 ± 14 | 45 ± 12 |
| Maximal respiratory mouth pressures | ||
| Maximal inspiratory mouth pressure, kPa | 7.1 [5.1–8.8] | 6.3 [5.3–8.2] |
| Maximal expiratory mouth pressure, kPa | 10.2 [7.0–12.8] | 9.6 [7.4–12.2] |
| Serum 25(OH)D concentration, nmol/L | 40 ± 17 | 38 ± 15 |
| Serum 25(OH)D concentration ≤25 nmol/L | 16 (20.5) | 15 (21.4) |
| Inflammatory markers | ||
| CRP, µg/mL | 5.1 [2.3–9.9] | 5.3 [2.7–10.1] |
| IL-6, pg/mL | 5.3 [4.1–9.4] | 5.8 [3.9–8.6] |
| LL-37, ng/mL | 16.8 [12.3–22.4] | 17.9 [13.0–24.0] |
| Physical function | ||
| 6-min walking test, m | 383 ± 122 | 413 ± 117 |
| Handgrip strength test, kg | 35.3 ± 11.3 | 34.6 ± 10.8 |
| Use of vitamin D supplementation | 10 (12) | 6 (8) |
1Values are n (%), percentages, mean ± SD, or median [IQR]. CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; 25(OH)D, 25-hydroxyvitamin D.
After 1 y, mean ± SD serum 25(OH)D was 112 ± 34 nmol/L in the vitamin D group and 42 ± 17 nmol/L in the placebo group. A total of 187 exacerbations had occurred: 90 in the vitamin D group and 97 in the placebo group (Table 2). Total incidence rate was 1.32/patient-year. No difference in exacerbation rate was found between the intervention and placebo groups [incidence rate ratio (IRR): 0.90; 95% CI: 0.67, 1.21]. Also, no difference was observed in the subgroup of participants with 25(OH)D concentrations <25 nmol/L (n = 31) (IRR: 0.91; 95% CI: 0.43, 1.93). Incidence rate was higher in participants with a higher GOLD-stage; however, the effect of vitamin D supplementation did not differ (Supplemental Table 2).
TABLE 2.
Number of exacerbations of both study groups and IRR1
| Exacerbations, n | Unadjusted incidence rate, per person-year | ||||||
|---|---|---|---|---|---|---|---|
| Total | Placebo group | Vitamin D group | Placebo group | Vitamin D group | Adjusted IRR (95% CI) | P value | |
| Intention-to-treat population (n = 155) | 187 | 97 | 90 | 1.36 | 1.29 | 0.90 (0.67, 1.21) | 0.47 |
| Per-protocol analysis (n = 135) | 158 | 86 | 72 | 1.40 | 1.21 | 0.84 (0.60, 1.17) | 0.30 |
| Subgroup ≤25 nmol/L (n = 31) | 41 | 18 | 23 | 1.29 | 1.60 | 0.91 (0.43, 1.93) | 0.80 |
1Results were based on negative binomial regression analyses. Adjusted analyses were corrected for stratification group and study center. IRR, incidence rate ratio; CI, confidence interval.
No relevant differences were found in time to first and second exacerbations and time to hospitalization (Table 3). Also, no differences were found in the other secondary outcomes: use of antibiotics and oral corticosteroids (Table 4), pulmonary function, maximal respiratory mouth pressure, handgrip strength, 6-min walking test, and systemic inflammatory markers (Table 5). The per-protocol analyses also showed no differences between the groups (Supplemental Tables 3–5).
TABLE 3.
Effect of vitamin D on time to first and second exacerbations and time to first hospitalization1
| HR (95% CI) | P value | |
|---|---|---|
| Time to first exacerbation | ||
| Intention-to-treat population (n = 155) | 1.01 (0.67, 1.54) | 0.93 |
| Subgroup ≤25 nmol/L (n = 31) | 0.55 (0.13, 2.30) | 0.41 |
| Time to second exacerbation | ||
| Intention-to-treat population (n = 155) | 0.80 (0.44, 1.43) | 0.44 |
| Subgroup ≤25 nmol/L (n = 31) | 2.00 (0.24, 16.22) | 0.52 |
| Time to first hospitalization | ||
| Intention-to-treat population (n = 155) | 1.03 (0.51, 2.07) | 0.93 |
| Subgroup ≤25 nmol/L (n = 31) | 1.09 (0.17, 7.01) | 0.92 |
1Results were based on Cox regression analyses. All analyses were adjusted for stratification group and study center. HR, Hazard ratio; CI, confidence interval
TABLE 4.
Effect of vitamin D supplementation on number of exacerbations and number of episodes of antibiotic and corticosteroid use1
| Proportion placebo group | Proportion vitamin D group | OR (95% CI) | P value | |
|---|---|---|---|---|
| Participants with ≥1 exacerbation | ||||
| Intention-to-treat (n = 155) | 50 of 81 (62%) | 52 of 74 (70%) | 1.55 (0.75, 3.17) | 0.23 |
| Subgroup ≤25 nmol/L (n = 31) | 10 of 16 (63%) | 11 of 15 (73%) | 0.64 (0.05, 7.87) | 0.73 |
| Participants with ≥1 episode of antibiotic use | ||||
| Intention-to-treat (n = 155) | 43 of 81 (53%) | 44 of 74 (59%) | 1.27 (0.64, 2.50) | 0.49 |
| Subgroup ≤25 nmol/L (n = 31) | 7 of 16 (44%) | 9 of 15 (60%) | 0.38 (0.02, 6.21) | 0.50 |
| Participants with ≥1 episode of oral corticosteroid use | ||||
| Intention-to-treat (n = 155) | 41 of 81 (51%) | 43 of 74 (58%) | 1.30 (0.66, 2.60) | 0.45 |
| Subgroup ≤25 nmol/L (n = 31) | 7 of 16 (44%) | 10 of 15 (67%) | 2.57 (0.60, 11.06) | 0.20 |
1Results are based on logistic regression analyses. All analyses were adjusted for stratification group and study center. OR, odds ratio; CI, confidence interval
TABLE 5.
Effect of vitamin D supplementation on physical function and inflammatory markers1
| Mean/percentage difference (95% CI) | P value | |
|---|---|---|
| Physical function (n = 155) | ||
| 6-min walking test, m | 34 (−4, 71) | 0.08 |
| Handgrip strength, kg | 1.15 (−1.20, 3.50) | 0.34 |
| Spirometry (n = 154) | ||
| FEV1, %predicted | −0.91 (−6.15, 4.34) | 0.73 |
| FVC, %predicted | −2.52 (−7.72, 2.67) | 0.34 |
| Maximal respiratory mouth pressures (n = 150) | ||
| MIP, kPa | −0.47 (−2.74, 1.79) | 0.68 |
| MEP, kPa | 0.28 (−0.71, 1.27) | 0.58 |
| Inflammatory markers (n = 152) | ||
| CRP, µg/mL | −6.7 (−30.2, 24.1) | 0.63 |
| IL-6, pg/mL | −13.3 (−30.6, 8.4) | 0.21 |
| LL-37, ng/mL | 0.2 (−12.1, 14.3) | 0.97 |
1Values are mean differences between the placebo and intervention groups, and derived from regression coefficients with 95% CIs. A positive value indicates a higher, and a negative value a lower, outcome in the vitamin D group. Coefficients of CRP, IL-6, and LL-37 were back transformed after ln transformation and expressed as percentage differences. All analyses were adjusted for stratification group and study center. CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure.
Explorative analysis of the nasal microbiome data by multidimensional scaling did not reveal clear separation according to visit or treatment (Figure 2). Further statistical analysis at community [using permutational multivariate ANOVA (PERMANOVA)] and species levels (using DEseq2) confirmed that there was no difference between the vitamin D and placebo groups. Also, when analyzed independently of treatment or time variables (baseline or 12-mo visit), no association of microbiota at community or species [amplicon sequence variant (ASV)] levels was observed with other variables, including vitamin D status or FEV1 (data not shown). The only exceptions were an inverse association between ASV0014 (Finegoldia magna) and exacerbations (adjusted P value = 0.0032) (Supplemental Table 6), and an association of ASV0113 (Megasphaera micronuciformis) with BMI (adjusted P value = 0.00122) (Supplemental Table 7). Interestingly, there was also a significant association between sex and ASV0014 (F. magna) (P = 0.021) and ASV0016 (Anaerococcus octavius) (P = 0.023), these being more abundant in male participants (data not shown). No differences were found between serum 25(OH)D2 and 25(OH)D3 concentrations (Supplemental Table 8).
FIGURE 2.
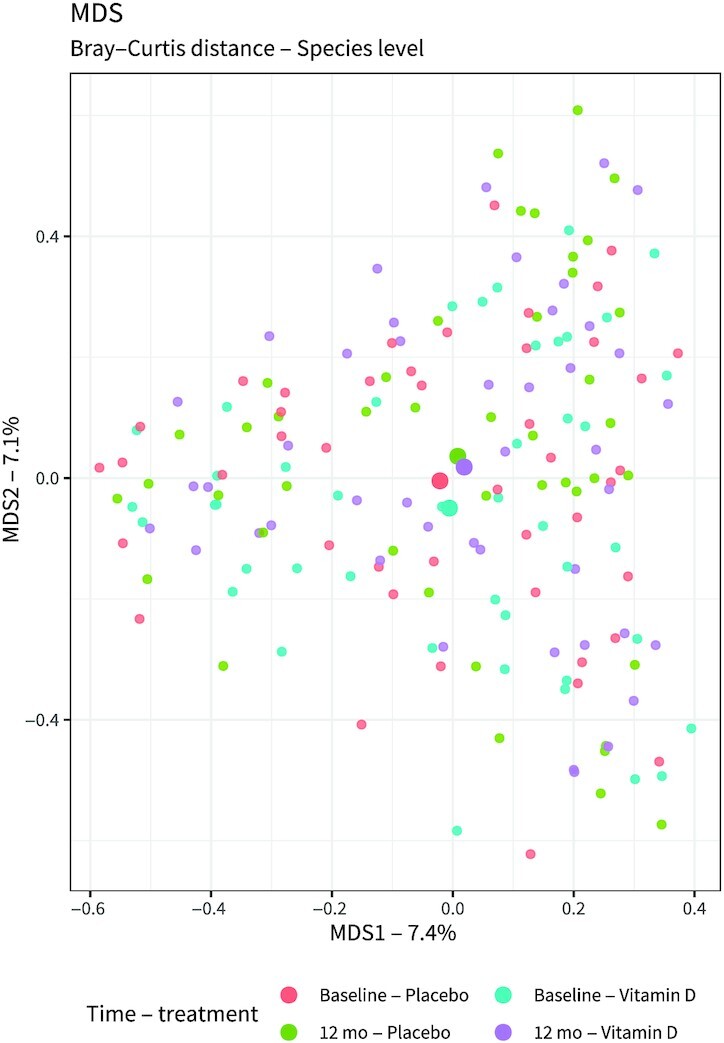
Bray–Curtis distance multidimensional scaling plot showing the nasal microbiota before and after the vitamin D or placebo treatment. Statistical analysis was performed on 88 placebo samples (n = 44, baseline; n = 44, 12-mo) and 88 vitamin D samples (n = 44, baseline; n = 44, 12-mo). No effect of active treatment was observed when taking both sampling points into account (PERMANOVA: R2 = 0.006036, P = 0.837433), nor when only considering the samples collected at the last study visit (PERMANOVA: R2 = 0.0212145, P = 0.538). MDS1: dimension 1, representing 7.4% of total variation; MDS2: dimension 2, representing 7.1% of total variation. PERMANOVA, permutational multivariate ANOVA.
Discussion
This study aimed to investigate the effects of vitamin D supplementation specifically in COPD patients with vitamin D deficiency. Our results do not demonstrate a preventive effect of vitamin D supplementation on number of exacerbations. In addition, vitamin D supplementation did not have a positive effect on any of the secondary outcomes.
Three of 4 previous studies that were published after the initiation of our trial also did not observe an effect of vitamin D supplementation on exacerbation rate (10–12). An IPD meta-analysis did not show an effect in the total population (n = 469) as well as in a subgroup with serum 25(OH)D between 25.0 and 49.9 nmol/L (adjusted IRR: 1.00; 95% CI: 0.77, 1.31; n = 214) (14). The meta-analysis, however, did show a preventive effect in patients with serum 25(OH)D concentrations <25 nmol/L. In a subgroup within our trial including participants with serum 25(OH)D between 15 and 25 nmol/L we did not observe any effect. However, this group only consisted of 31 participants and might have been too small to show an effect.
Several factors could have contributed to these different results. In contrast to other studies (11, 12) we decided not to include participants with serum 25(OH)D concentrations <15 nmol/L. Random assignment to the placebo arm of these participants might have led to other vitamin D–related health issues such as osteomalacia. This resulted in a higher median serum 25(OH)D concentration in the severely deficient group in our study population, than in the population included in the IPD meta-analyses (median [IQR]: 22 nmol/L [18–24 nmol/L] and 19 nmol/L [14–22 nmol/L], respectively, based on DA Jolliffe, AR Martineau, Queen Mary University of London, personal communication, 2020). It seems plausible that effects of vitamin D supplementation are largest in the most deficient participants. Another explanation for different results may be the inclusion of participants with more severe COPD in the other studies, particularly in the first published RCT of Lehouck et al. (11), than in our study. Our study participants had a higher mean FEV1 at baseline than those in the study of Lehouck et al. However, we do not find these explanations to be very likely, because we did not find a difference in effect size in the IPD meta-analysis between participants with GOLD-stage 1–2 as opposed to GOLD-stage 3–4 (14). In addition, in the study of Martineau et al. (12) the exacerbation rate was smaller than in our study (1.04–1.11/patient-year).
The absence of an effect of vitamin D supplementation on exacerbations in our study is in contrast with findings from many observational studies showing associations between (characteristics of) COPD and low vitamin D concentrations (23, 24). Reverse causation (low vitamin D concentrations as a consequence rather than cause of the disease) and (residual) confounding may be explanations for this apparent discrepancy.
Our finding of the absence of an effect of vitamin D supplementation on physical performance is in line with previous clinical trials in COPD patients (10, 25, 26). In the general population, positive effects of vitamin D supplementation on muscle strength are small but present, particularly in older individuals and in persons with 25(OH)D concentrations ˂25 nmol/L (5, 6, 27). The absence of an effect in COPD patients may be related to the fact that other contributory mechanisms such as inactivity and the chronic inflammatory state may override potential small negative effects of vitamin D deficiency on muscle strength (8, 9, 28).
No effect of vitamin D on microbial composition at the nasal mucosa was observed in the subset of patients from whom nasal swabs were collected. We used analysis of the nasal microbiome, instead of the more relevant bronchial airway microbiome, to avoid invasive bronchoscopic procedures. It is important to note that paired-sample analyses have shown important differences between the microbiota of the upper and lower airways (29). Furthermore, whereas studies on the nasal microbiome in COPD have so far not been reported, lung microbiota studies have shown marked differences between COPD patients and (smoking) controls, and a link with exacerbations (30). Because vitamin D treatment is likely to affect both the nasal and bronchial airways, we reasoned that an impact of vitamin D treatment might be detected in nasal samples. Studies on the relation between vitamin D and the airway microbiome are limited, but indicate that vitamin D status may affect the nasopharyngeal microbiome (31). Although we did not find any impact of vitamin D treatment or status on the nasal microbiome, we did make some unrelated observations. The observed association between F. magna and exacerbations is of potential interest, and this species has not previously been specifically linked to airway diseases. F. magna was the second most abundant ASV detected, second to Dolosigranulum pigrum. F. magna has been shown to activate neutrophils and produce several proteases (31), which may be relevant in relation to its link to lung function. It should be considered that because taxonomic assignment was done based on short sequence reads, further confirmation on the taxonomic identity is required.
Various studies have indicated that effects of vitamin D supplementation might be influenced by several factors, including age, BMI, and genetic factors (32, 33). In a previous study in the general population, we found that the relation between vitamin D and pulmonary function was modified by BMI. However, in our study we did not find an effect of BMI. Genetic polymorphisms related to vitamin D metabolism were not assessed in the present study. Another possible explanation is provided by a recent study by Jolliffe et al. (34), suggesting that the effects of vitamin D3 supplementation may not lead to sufficient increases in 25(OH)D3 due to impaired production or sequestration. There are various theoretical explanations for the absence of a sufficient increase in systemic 25(OH)D3 after supplementation, including impaired renal function resulting in reduced 1-α-hydroxylation of vitamin D, but further studies to confirm this and unravel underlying mechanisms are needed. Importantly, in relation to our findings, results from another study from the same authors that focused on the effects of vitamin D supplementation on acute respiratory infections suggested that low daily doses (400–1000 IU) of vitamin D are associated with the strongest protective effects compared with other dosing regimens (35).
Although we did not show a positive effect of vitamin D supplementation on exacerbations, and respiratory and muscle health, this does not imply that vitamin D is an unimportant issue in COPD. Intervention studies have shown that vitamin D deficiency is causally related to low bone density and fractures (4). COPD patients demonstrate a high prevalence of osteoporosis and fractures (36, 37). So, it is justified to supplement all COPD patients with a serum 25(OH)D concentration <50 nmol/L.
This study has several strengths. The most important strength is the RCT methodology and the selection of participants with vitamin D deficiency. Another strength is the application of prospectively completed symptom diaries which allowed us to detect exacerbations that would otherwise not have come to medical attention. In addition, in contrast to other studies we used a weekly oral dosing regimen instead of a bolus regimen (11, 12). With weekly dosing a steady and stable increase in vitamin D concentrations is achieved. A disadvantage of weekly instead of less frequent dosing may be reduced compliance. However, at 88% the compliance of our study population was very good and a large difference in vitamin D status between the intervention and placebo groups was established.
An important limitation of our study is that we did not reach the calculated sample size, although we extended the inclusion period from 1.5 y to 3.5 y. Several factors hampered recruitment. First, fewer COPD patients were vitamin D–deficient than anticipated. A considerable number of participants already used vitamin D supplementation with doses >400 IU. In recent years, attention to prevention of vitamin D deficiency has grown in both the public health domain and specialized health care in the Netherlands, resulting in more widespread use of vitamin D supplements (38). The inclusion of fewer participants than anticipated reduced the power to find a significant effect of the intervention with vitamin D. However, our point estimate of the IRR was close to 1.0, both in the total population as well as in the small subgroup with 25(OH)D concentrations of 15–25 nmol/L. This indicates that it is unlikely that inclusion of the whole calculated sample size would have led to a clinically relevant effect in our study population. Another limitation is the potential overestimation of the exacerbation rate in our sample size calculation. For the calculation, we used data from the subgroup analysis of Lehouck et al. (11) in which the exacerbation rate was 3.45/patient-year in the placebo group compared with 1.84/patient-year in the vitamin D group. The exacerbation rate in our study population was 1.32/patient-year, which is much lower. As previously stated, the point estimate in our study was close to 1, which renders a clinically significant effect unlikely.
To conclude, our study does not show that vitamin D supplementation reduces exacerbation rate in COPD patients with vitamin D deficiency. Although prevention of exacerbations is not a reason for vitamin D screening and supplementation in COPD patients, attention to vitamin D deficiency remains necessary for the prevention of bone adverse effects.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Bernadette Bos-van Noort, Lineke Punselie-Groeneveld, Ans Nicolaas, Ingrid Knufman, Nicolette Pliester, Linda Garms and Saskia van Diepenbeek for their help in setting up and performing this study, and Anne van der Does for helpful discussions on microbiome analysis. A special thanks to Yvonne Heijdra for her continuous support throughout the study. Finally, we thank all research nurses, pulmonary function technicians, and administrative assistants of the Departments of Pulmonology of Amsterdam UMC, Radboud University Medical Center, Leiden Universiy Medical Center, NorthWest Clinics, Onze Lieve Vrouwe Gasthuis, Canisius Wilhelmina Hospital, Amstelland Hospital, Zaans Medical Center, Jeroen Bosch Hospital, Flevoziekenhuis and Sint Franciscus Gasthuis for their help in performing this study.
PRECOVID study group: Martin den Heijer, Renate T de Jongh, Paul Lips and Rachida Rafiq (Department of Internal Medicine and Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands); Floor E Aleva (Department of Pulmonology, Radboud University Medical Center, Nijmegen, Netherlands); André van der Ven (Department of Internal Medicine, Radboud University Medical Center, Nijmegen, Netherlands); Pieter S Hiemstra, Jasmijn A Schrumpf and Annelies M Slats (Department of Pulmonology, Leiden University Medical Center, Leiden, Netherlands); Johannes MA Daniëls (Department of Pulmonology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands); Pierre M Bet (Department of Clinical Pharmacology and Pharmacy, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands); Wim G Boersma (Department of Pulmonology, NorthWest Clinics, Alkmaar, Netherlands); Paul Bresser (Department of Pulmonology, Onze Lieve Vrouwe Gasthuis, Amsterdam, Netherlands); Michiel Spanbroek (Department of Pulmonology, Canisius Wilhelmina Hospital, Nijmegen, Netherlands); Petra Huisman (Department of Pulmonology, Amstelland Hospital, Amstelveen, Netherlands); Serge A van Wolferen (Department of Pulmonology, Zaans Medical Center, Zaandam, Netherlands); Marielle EAC Broeders (Department of Pulmonology, Jeroen Bosch Hospital, Den Bosch, Netherlands); Peter van Hengel (Department of Pulmonology, Flevoziekenhuis, Almere, Netherlands); and Gert-Jan Braunstahl (Department of Pulmonology, Sint Franciscus Gasthuis, Rotterdam, Netherlands).
The authors’ responsibilities were as follows—AJAMvdV, PSH, MdH, and RTdJ: led the funding application of the study; RR, FEA, JAS, PL, PMB, AJAMvdV, PSH, MdH, and RTdJ: were involved in the concept and design of the study; RR: performed the statistical analyses (supervised by RTdJ and MdH) and wrote the first draft of the manuscript; TJvdB: analyzed the data of the microbiome analysis (supervised by BJFK and PSH); and all authors: were involved in the acquisition of data for the study, were involved in the interpretation of the results, revised the manuscript critically, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
The PRECOVID-trial was funded by a Lung Foundation Netherlands grant (project number: 5.1.13.033). The trial was also supported by an unrestricted grant from Almirall.
Supplemental Tables 1–8 and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ASV, amplicon sequence variant; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IPD, individual participant data; IRR, incidence rate ratio; IU, International Unit; RCT, randomized placebo-controlled trial; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Rachida Rafiq, Department of Internal Medicine and Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Floor E Aleva, Department of Pulmonology, Radboud University Medical Center, Nijmegen, Netherlands.
Jasmijn A Schrumpf, Department of Pulmonology, Leiden University Medical Center, Leiden, Netherlands.
Johannes M Daniels, Department of Pulmonology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Pierre M Bet, Department of Clinical Pharmacology and Pharmacy, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Wim G Boersma, Department of Pulmonology, NorthWest Clinics, Alkmaar, Netherlands.
Paul Bresser, Department of Pulmonology, Onze Lieve Vrouwe Gasthuis, Amsterdam, Netherlands.
Michiel Spanbroek, Department of Pulmonology, Canisius Wilhelmina Hospital, Nijmegen, Netherlands.
Paul Lips, Department of Internal Medicine and Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Tim J van den Broek, Microbiology and Systems Biology, TNO, Zeist, Netherlands.
Bart J F Keijser, Microbiology and Systems Biology, TNO, Zeist, Netherlands.
André J A M van der Ven, Department of Internal Medicine, Radboud University Medical Center, Nijmegen, Netherlands.
Pieter S Hiemstra, Department of Pulmonology, Leiden University Medical Center, Leiden, Netherlands.
Martin den Heijer, Department of Internal Medicine and Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Renate T de Jongh, Department of Internal Medicine and Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
PRECOVID-study group, Department of Internal Medicine and Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
PRECOVID-study group:
Martin den Heijer, Renate T de Jongh, Paul Lips, Rachida Rafiq, Floor E Aleva, André van der Ven, Pieter S Hiemstra, Jasmijn A Schrumpf, Annelies M Slats, Johannes M A Daniëls, Pierre M Bet, Wim G Boersma, Paul Bresser, Michiel Spanbroek, Petra Huisman, Serge A van Wolferen, Marielle E A C Broeders, Peter van Hengel, and Gert-Jan Braunstahl
Data Availability
Data described in the article, code book, and analytic code will be made available upon request from the corresponding author pending application and approval.
References
- 1. Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest. 2005;128(6):3792–8. [DOI] [PubMed] [Google Scholar]
- 2. Burkes RM, Ceppe AS, Doerschuk CM, Couper D, Hoffman EA, Comellas APet al. Associations among 25-hydroxyvitamin D levels, lung function, and exacerbation outcomes in COPD: an analysis of the SPIROMICS cohort. Chest. 2020;157(4):856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jolliffe DA, James WY, Hooper RL, Barnes NC, Greiller CL, Islam Ket al. Prevalence, determinants and clinical correlates of vitamin D deficiency in patients with Chronic Obstructive Pulmonary Disease in London, UK. J Steroid Biochem Mol Biol. 2018;175:138–45. [DOI] [PubMed] [Google Scholar]
- 4. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes Bet al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian Jet al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. [DOI] [PubMed] [Google Scholar]
- 6. Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59(12):2291–300. [DOI] [PubMed] [Google Scholar]
- 7. Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré Ret al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson Aet al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36(1):81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJet al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62(2):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rafiq R, Prins HJ, Boersma WG, Daniels JM, den Heijer M, Lips Pet al. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: a pilot trial. Int J Chron Obstruct Pulmon Dis. 2017;12:2583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere Jet al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–14. [DOI] [PubMed] [Google Scholar]
- 12. Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CLet al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120–30. [DOI] [PubMed] [Google Scholar]
- 13. Zendedel A, Gholami M, Anbari K, Ghanadi K, Bachari EC, Azargon A. Effects of vitamin D intake on FEV1 and COPD exacerbation: a randomized clinical trial study. Glob J Health Sci. 2015;7(4):243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RTet al. Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019;74(4):337–45. [DOI] [PubMed] [Google Scholar]
- 15. Rafiq R, Aleva FE, Schrumpf JA, Heijdra YF, Taube C, Daniels JMet al. Prevention of exacerbations in patients with COPD and vitamin D deficiency through vitamin D supplementation (PRECOVID): a study protocol. BMC Pulm Med. 2015;15(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res. 2008;23(11):1859–63. [DOI] [PubMed] [Google Scholar]
- 17. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. [DOI] [PubMed] [Google Scholar]
- 18. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates Aet al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 19. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos Fet al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22. [DOI] [PubMed] [Google Scholar]
- 20. Criee CP, Sorichter S, Smith HJ, Kardos P, Merget R, Heise Det al. Body plethysmography—its principles and clinical use. Respir Med. 2011;105(7):959–71. [DOI] [PubMed] [Google Scholar]
- 21. Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54(10):1348–59. [PubMed] [Google Scholar]
- 22. Dirks NF, Vesper HW, van Herwaarden AE, van den Ouweland JM, Kema IP, Krabbe JGet al. Various calibration procedures result in optimal standardization of routinely used 25(OH)D ID-LC-MS/MS methods. Clin Chim Acta. 2016;462:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afzal S, Lange P, Bojesen SE, Freiberg JJ, Nordestgaard BG. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax. 2014;69(1):24–31. [DOI] [PubMed] [Google Scholar]
- 24. Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert Iet al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–20. [DOI] [PubMed] [Google Scholar]
- 25. Bjerk SM, Edgington BD, Rector TS, Kunisaki KM. Supplemental vitamin D and physical performance in COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2013;8:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hornikx M, Van Remoortel H, Lehouck A, Mathieu C, Maes K, Gayan-Ramirez Get al. Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir Res. 2012;13(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22(3):859–71. [DOI] [PubMed] [Google Scholar]
- 28. Jones SE, Maddocks M, Kon SSC, Canavan JL, Nolan CM, Clark ALet al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–8. [DOI] [PubMed] [Google Scholar]
- 29. Boutin S, Graeber SY, Weitnauer M, Panitz J, Stahl M, Clausznitzer Det al. Comparison of microbiomes from different niches of upper and lower airways in children and adolescents with cystic fibrosis. PLoS One. 2015;10(1):e0116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caverly LJ, Huang YJ, Sze MA. Past, present, and future research on the lung microbiome in inflammatory airway disease. Chest. 2019;156(2):376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toivonen L, Hasegawa K, Ajami NJ, Celedon JC, Mansbach JM, Petrosino JFet al. Circulating 25-hydroxyvitamin D, nasopharyngeal microbiota, and bronchiolitis severity. Pediatr Allergy Immunol. 2018;29(8):877–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Didriksen A, Grimnes G, Hutchinson MS, Kjærgaard M, Svartberg J, Joakimsen RMet al. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur J Endocrinol. 2013;169(5):559–67. [DOI] [PubMed] [Google Scholar]
- 33. Sollid ST, Hutchinson MYS, Fuskevåg OM, Joakimsen RM, Jorde R. Large individual differences in serum 25-hydroxyvitamin D response to vitamin D supplementation: effects of genetic factors, body mass index, and baseline concentration. Results from a randomized controlled trial. Horm Metab Res. 2016;48(1):27–34. [DOI] [PubMed] [Google Scholar]
- 34. Jolliffe DA, Stefanidis C, Wang Z, Kermani NZ, Dimitrov V, White JHet al. Vitamin D metabolism is dysregulated in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202(3):371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jolliffe DA, Camargo CA Jr, Sluyter JD, Aglipay M, Aloia JF, Ganmaa Det al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–92. [DOI] [PubMed] [Google Scholar]
- 36. Graumam RQ, Pinheiro MM, Nery LE, Castro CHM. Increased rate of osteoporosis, low lean mass, and fragility fractures in COPD patients: association with disease severity. Osteoporos Int. 2018;29(6):1457–68. [DOI] [PubMed] [Google Scholar]
- 37. Franco CB, Paz-Filho G, Gomes PE, Nascimento VB, Kulak CA, Boguszewski CLet al. Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int. 2009;20(11):1881–7. [DOI] [PubMed] [Google Scholar]
- 38. Sohl E, van Schoor NM. [Implementation of the Dutch vitamin D supplementation advice: report of an expert meeting]. Ned Tijdschr Geneeskd. 2015;159:A8171. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request from the corresponding author pending application and approval.