ABSTRACT
Background
Animal models have demonstrated that maternal nutrition can alter fetal vulnerability to prenatal alcohol exposure (PAE). Few human studies have examined the role of nutrition in fetal alcohol spectrum disorders (FASD).
Objectives
Our objectives were to examine whether fetal vulnerability to PAE-related growth restriction is modified by: 1) rate of gestational weight gain; or prenatal dietary intakes of 2) energy, 3) iron, or 4) choline.
Methods
In a prospective longitudinal birth cohort in Cape Town, South Africa, 118 heavy-drinking and 71 abstaining/light-drinking pregnant women were weighed and interviewed regarding demographics, alcohol, cigarette/other drug use, and diet at prenatal visits. Infant length, weight, and head circumference were measured at 2 wk and 12 mo postpartum.
Results
Heavy-drinking mothers reported a binge pattern of drinking [Mean = 129 mL (∼7.2 drinks)/occasion on 1.3 d/wk). Rate of gestational weight gain and average daily dietary energy, iron, and choline intakes were similar between heavy-drinking women and controls. In regression models adjusting for maternal age, socioeconomic status, cigarette use, and weeks gestation at delivery, PAE [ounces (30 mL) absolute alcohol per day] was related to smaller 2-wk length and head circumference and 12-mo length, weight, and head circumference z-scores (β = −0.43 to −0.67; all P values <0.05). In stratified analyses for each maternal nutritional measure (inadequate compared with adequate weight gain; tertiles for dietary energy, iron, and choline intakes), PAE-related growth restriction was more severe in women with poorer nutrition, with effect modification seen by weight gain, energy, iron, and/or choline for several anthropometric outcomes.
Conclusions
Gestational weight gain and dietary intakes of energy, choline, and iron appeared to modify fetal vulnerability to PAE-related growth restriction. These findings suggest a need for screening programs for pregnant women at higher risk of having a child with FASD to identify alcohol-using women who could benefit from nutritional interventions.
Keywords: fetal alcohol syndrome (FAS), fetal alcohol spectrum disorders (FASD), prenatal alcohol exposure (PAE), dietary energy intake, gestational weight gain, iron deficiency, choline, intrauterine growth restriction, postnatal growth restriction, fetal alcohol growth restriction
Introduction
Fetal alcohol spectrum disorders (FASD) are manifest by growth restriction and a broad range of cognitive and behavioral deficits. FASD comprise the most common preventable cause of neurodevelopmental disabilities worldwide, with prevalence estimates of 1.1–5.0% in the United States and Western Europe (1) and 13.6–20.9% in South Africa (2). Fetal alcohol-related growth restriction is characterized by reductions in length, weight, and head circumference at birth that can persist through adulthood (3–10). We have demonstrated that a child's growth trajectory provides a biological indicator of the severity of FASD-related neurocognitive deficits, with the most affected children exhibiting both fetal and postnatal growth restriction (11).
It has been widely postulated that the nutritional status of women who drink alcohol during pregnancy can affect the severity of alcohol's teratogenic effects, but few human studies have examined the role of maternal nutrition in FASD. In rat models, protein and food restriction have been shown to exacerbate prenatal alcohol exposure (PAE)–related skeletal abnormalities (12) and fetal growth restriction (13). In a prospective longitudinal birth cohort in Detroit, United States (recruited 1986–1989), we found that PAE-related growth restriction through adulthood was markedly more severe in children born to mothers with prepregnancy weight below the cohort median (4). PAE has been associated with alterations in maternal and fetal iron bioavailability and utilization in rats that exacerbate PAE-related growth and neurobehavioral deficits and are ameliorated by maternal iron supplementation (14–16). We have found similar alterations in maternal and infant iron homeostasis in 2 prospective longitudinal birth cohorts in Cape Town, South Africa (one recruited from 1999 to 2001; the other from 2011 to 2015) (3, 17, 18). Animal models and 3 human trials have demonstrated protective effects of the methyl donor nutrient choline on PAE-related growth restriction and/or a range of neurobehavioral deficits (19–27).
In our more recent prospective South African birth cohort (28), we found that diet and body composition were similar between heavy-drinking pregnant women and abstainers/light-drinkers from the same community (29). However, nutrition was very poor in both groups across several dietary and anthropometric measures. In this article we examine the hypothesis that fetal vulnerability to PAE-related growth restriction can be modified by 4 elements of maternal nutrition: 1) rate of gestational weight gain; and dietary intakes of 2) energy (i.e., calories), 3) iron, and 4) choline.
Methods
Sample
Women were recruited from 2 antenatal clinics in Cape Town, South Africa, from 2011 to 2015 to take part in a prospective, longitudinal cohort study examining developmental effects of PAE (28, 29). The analysis plan and primary maternal nutritional measures and growth outcomes examined here comprise planned secondary analyses and were chosen prior to study initiation; sample size was chosen based on the primary outcome in the parent study, eyeblink conditioning (data not yet published). See Supplemental Figure 1 for recruitment and retention flow diagram. In timeline follow-back interviews (30, 31), any woman averaging ≥1.0 oz (30 mL) absolute alcohol (AA)/day (∼1.67 standard drinks) or reporting binge drinking [≥2.0 oz (60 mL) AA/drinking occasion] was invited to participate. Women who abstained or drank only minimally (no binge episodes) were recruited as controls. Maternal exclusion criteria at recruitment were age <18 y, HIV infection, multiple gestation pregnancy, and chronic medical conditions requiring pharmacological treatment. Infants meeting the following criteria (chosen prior to study initiation) were excluded from follow-up due to the potential negative effects of these conditions on growth and development: chromosomal anomalies (none), seizures (none), neural tube defects (none), very low birthweight (<1500 g) obtained from medical records (1 exposed, 2 controls), and extreme prematurity (<32 weeks gestation; 3 controls). The final sample included 118 exposed infants and 71 controls. Consents and interviews were conducted in the mother's preferred language (Afrikaans or English). Approval was obtained from the ethics committees at Wayne State University, University of Cape Town Faculty of Health Sciences, and Columbia University Medical Center. At each study visit, all women reporting alcohol or drug use were informed by our staff about the risks of prenatal alcohol and drug use, encouraged to stop or reduce their alcohol/drug use, and offered a referral to a substance use counseling/intervention program (South African National Council on Alcoholism and Drug Dependence).
Ascertainment of maternal alcohol, cigarette smoking, and drug use
In timeline follow-back interviews (30, 31) at recruitment and 4 and 12 weeks thereafter, women were asked about their alcohol use on a day-by-day basis during the previous 2 weeks, with recall linked to specific daily activities, as well as cigarette smoking and other drug use [cocaine, methamphetamine, opiates, methaqualone, and marijuana; validated by urine ELISA drug testing (32)]. At the recruitment visit, women were asked if there were weeks since conception when they drank smaller or greater quantities and were asked to report drinking for those weeks as well. At the 4- and 12-week visits, women were asked if there were any weeks since their last visit when they drank smaller or greater quantities and were asked to report drinking for those weeks as well. Summary alcohol measures were constructed using data from the 3 prenatal timeline follow-back interviews across pregnancy: ounces AA per day, ounces AA per drinking occasion, and frequency of drinking (1 oz = 30 mL) (33). The predictive validity of this timeline follow-back interview method is supported by consistent findings in 3 longitudinal cohorts in Detroit and Cape Town (4, 11, 30, 34) and validation studies using meconium alcohol biomarkers (35–37). Women who delivered before the 4- or 12-week study visit did not complete those visits; heavy-drinking women and controls did not differ in number of visits (Mean = 2.6 compared with 2.5, respectively; P = 0.110).
Maternal nutritional measures
Dietary interviews and weight measurements were conducted at each prenatal study visit by trained staff blinded to the mother's alcohol and drug use. Second/third-trimester rate of maternal gestational weight gain, which is generally stable over the second and third trimesters, was calculated as the average change in weight between study visits, divided by the number of weeks between visits (29). Because prepregnancy BMI was not known for any woman, the single inadequate gestational weight gain cutoff for second and third trimesters (<0.42 kg/week) was used (38). Average daily energy and iron intakes were calculated from multipass 24-h dietary recall interviews processed with FoodFinder3 (South African Medical Research Council) (29). Because FoodFinder3 does not include choline, average daily choline intake was calculated as the mean of values from a quantitative, choline-indicated FFQ developed and validated for this community (39). Given the propensity of 24-h recall interviews to overestimate the prevalence of values at upper and lower extremes, nutrient intake values were adjusted using the Nutrition Research Council method, which transforms outcome distributions to more closely match those of the general population while preserving cohort means (40). Dietary energy adequacy was calculated as the ratio of a woman's average daily energy intake to her estimated energy requirement, calculated based on her age, height, weight, activity level, and weeks gestation (29, 41, 42).
Infant anthropometry outcomes
Using standard WHO protocols, trained research staff blinded to maternal alcohol and drug use measured infant length, weight, and head circumference at 2 weeks and 12 months postpartum, and midupper arm circumference (MUAC) and triceps and biceps skinfold thickness (TSF and BSF, respectively) at 12 months (interexaminer reliability = 0.90–1.00) (43). WHO age/sex-specific anthropometric z-scores (43) were calculated for all but BSF (for which z-scores are unavailable) as the primary outcomes. Two-week measures were considered a proxy for birth size as a measure of fetal growth; this timepoint was chosen to avoid the impact of any early breastfeeding difficulties.
Demographics and other covariates
Mothers were interviewed regarding demographic background, including age, gravidity, education, and socioeconomic status. Weeks gestation at delivery was determined by early pregnancy ultrasound, if available, or last menstrual period. At 6.5 months postpartum, mothers were interviewed regarding number of weeks the infant was breastfed and if/when the infant was given formula.
Statistical analyses
All statistical analyses were 2-sided, using SPSS (v.24; IBM). All variables were examined for normality of distribution; AA per day was log-transformed due to skewness (>3.0). Missing data were treated with list-wise deletion. (Supplemental Table 1 compares key demographic, exposure, and prenatal nutritional measures between those with and without gestational weight gain and infant outcome measure data, which were all very similar.) The following covariates, which were found to be related to fetal alcohol growth restriction in prior studies (3, 4), were included as potential confounders in multivariable models: maternal age, prenatal cigarettes per day, socioeconomic status, and weeks gestation at delivery. (See Supplemental Table 2 for univariate relations between growth outcomes and demographics and prenatal cigarette/drug use.) Sixty-nine mothers were part of a pilot feasibility randomized controlled trial of prenatal choline supplementation (34 received choline; 35 placebo); the choline supplement and placebo were calorie- and iron-free (44). The 34 mother-infant pairs in the active treatment arm were excluded from analyses examining dietary choline; these infants were included in the other analyses to optimize power to detect effect modification, and choline treatment (Yes/No) was included as a covariate.
Linear regression models were used to examine associations of PAE to infant anthropometric outcomes; multivariable models were used to examine these relations after adjustment for potential confounding variables. Effect modification by each of the maternal nutritional measures was examined in regression models examining the relation of PAE to each anthropometric outcome stratified by the maternal nutritional measure and confirmed in unstratified regression models regressing the anthropometric outcome on PAE, the maternal nutritional measure, and a PAE × maternal nutritional measure interaction term. Given the low power of interaction terms in epidemiological studies, effect modification was inferred when the P value for the interaction term was <0.10, as recommended by McClellan and Judd (45).
Results
The mothers in the cohort were poorly educated and socioeconomically disadvantaged (Table 1). Heavy-drinking mothers were 2.1 years older than controls, had completed 0.7 fewer years of school, and scored 0.4 SD lower on the Hollingshead socioeconomic index (46). On average, women were recruited toward the end of the second trimester, with heavy-drinking pregnant women enrolling 3 weeks earlier than abstainers/light-drinkers. Few women developed medical problems after recruitment (9% gestational hypertension; <5% gestational diabetes or pre-eclampsia), with no differences between drinking groups. Heavy-drinking mothers averaged 4.4 oz (132 mL) AA (the equivalent of ∼7.3 standard drinks) per occasion on 2.2 d/week around time of conception and 4.3 oz (129.0 mL) AA (∼7.2 standard drinks) per occasion on 1.3 days across pregnancy. Binge drinking among drinking mothers was prevalent, with 92% averaging ≥2 oz (60 mL) AA/occasion. All controls abstained from drinking during pregnancy except for 7 women who reported light drinking (1–3 occasions for 4 women, monthly for 2, once weekly for 1; none of the 7 reported any binge drinking) and 1 woman who drank 2.1 oz (63 mL) AA (∼3.5 drinks) on 1 occasion. Although smoking was prevalent, with heavy-drinking women more likely to smoke than controls, number of cigarettes per day was similar and generally low in both groups (86% <0.5 packs/day; 2% ≥1.0 packs/day). Coexposures were prevalent, with 25% of heavy-drinking women reporting marijuana use (compared with 11% of control women) and 12% reporting methamphetamine use (compared with 16% of controls); no woman reported methaqualone, cocaine, or opiate use. Alcohol consumption was not related to weeks gestation at delivery, and most women (88%) had full-term pregnancies. Almost all mothers breastfed; roughly half reported supplementing with formula, with no group differences.
Table 1.
Maternal and infant sample characteristics1
| Controls (n = 71) | Heavy-drinking mothers (n = 118) | ||||
|---|---|---|---|---|---|
| n | Mean ± SD or n (%) | n | Mean ± SD or n (%) | P 2 | |
| Maternal characteristics | |||||
| Maternal age at conception, y | 71 | 25.5 ± 4.9 | 118 | 27.6 ± 6.0 | 0.011 |
| Gravidity: no. live births, % | 71 | 1.5 ± 1.3 | 118 | 1.9 ± 1.6 | 0.146 |
| Education (y of school completed) | 70 | 9.9 ± 1.7 | 118 | 9.2 ± 1.7 | 0.006 |
| Marital status, no. married, % | 71 | 30 (42.3) | 118 | 34 (28.8) | 0.059 |
| Socioeconomic status3 | 71 | 23.0 ± 7.4 | 118 | 20.4 ± 7.1 | 0.014 |
| Wk gestation at enrollment | 71 | 26.1 ± 5.1 | 118 | 23.2 ± 5.7 | <0.001 |
| Gestational hypertension, n (%) | 70 | 6 (8.6) | 116 | 10 (8.6) | 0.991 |
| Gestational diabetes, n (%) | 70 | 3 (4.3) | 116 | 5 (4.3) | 0.994 |
| Pre-eclampsia, n (%) | 70 | 1 (1.4) | 116 | 5 (4.3) | 0.281 |
| Iron/folic acid supplementation, n (%) | 70 | 58 (82.9) | 116 | 99 (85.3) | 0.650 |
| Took supplement on “most days,” n (%) | 56 (96.6) | 99 (96.0) | 0.749 | ||
| Second/third-trimester weight gain rate, kg/wk | 68 | 0.4 ± 0.3 | 111 | 0.4 ± 0.3 | 0.923 |
| Having inadequate weight gain rate,4n % | 34 (50.0) | 65 (58.6) | 0.264 | ||
| Dietary energy intake,5 kcal/d | 71 | 2326.7 ± 894.8 | 118 | 2247.7 ± 806.1 | 0.532 |
| Inadequate intake, 6n (%) | 34 (47.9) | 46 (39.0) | 0.230 | ||
| Iron intake from diet only,5 mg/d | 71 | 12.4 ± 3.7 | 118 | 11.7 ± 2.8 | 0.199 |
| Inadequate intake, 7n (%) | 70 (98.6) | 118 (100.0) | 0.196 | ||
| Choline intake from diet only,8 mg/d | 71 | 311.5 ± 119.3 | 118 | 311.1 ± 130.3 | 0.984 |
| Having inadequate intake,9n (%) | 63 (88.7) | 105 (89.0) | 0.958 | ||
| Iron/folic acid supplementation, n (%) | 70 | 58 (82.9) | 116 | 99 (85.3) | 0.650 |
| Took supplement on “most days,” n (%) | 58 | 56 (96.6) | 99 | 95 (96.2) | 0.852 |
| Alcohol and drug use | |||||
| Alcohol use at conception: | 71 | 118 | |||
| oz AA/d | 0.0 ± 0.1 | 1.6 ± 1.6 | <0.001 | ||
| oz AA/drinking d | 0.1 ± 0.3 | 4.4 ± 2.9 | <0.001 | ||
| Drinking days/wk | 0.0 ± 0.1 | 2.2 ± 1.2 | <0.001 | ||
| Alcohol use across pregnancy: | 71 | 118 | |||
| oz AA/d | 0.0 ± 0.0 | 0.9 ± 1.2 | <0.001 | ||
| oz AA/drinking d | 0.1 ± 0.4 | 4.3 ± 2.4 | <0.001 | ||
| Drinking days/wk | 0.0 ± 0.2 | 1.3 ± 1.0 | <0.001 | ||
| Reporting cigarette smoking, n (%) | 71 | 49 (60.0) | 118 | 101 (85.6) | 0.006 |
| Cigarettes/d among smokers | 5.8 ± 3.8 | 6.7 ± 4.2 | 0.222 | ||
| Reporting marijuana use, n (%) | 71 | 8 (11.3) | 118 | 29 (24.6) | 0.026 |
| Marijuana d/mo among users | 4.0 ± 4.7 | 9.7 ± 9.4 | 0.108 | ||
| Reporting methamphetamine use, n (%) | 80 | 11 (15.5) | 118 | 14 (11.9) | 0.476 |
| Methamphetamine d/mo among users | 10.2 ± 8.9 | 4.4 ± 5.0 | 0.069 | ||
| Infant characteristics | |||||
| Weeks gestation at delivery | 71 | 39.1 ± 1.8 | 118 | 38.8 ± 2.0 | 0.369 |
| Preterm delivery (<37 wk), n (%) | 9 (12.7) | 15 (12.7) | 0.994 | ||
| Infant sex, no. female (%) | 71 | 33 (46.5) | 118 | 66 (55.9) | 0.208 |
| Infant birthweight, g | 70 | 3078.8 ± 534.5 | 118 | 2876.8 ± 570.5 | 0.017 |
| No. low birthweight (<2500 g), % | 8 (11.4) | 31 (26.3) | 0.015 | ||
| No. small for gestational age,10 % | 70 | 13 (18.6) | 118 | 43 (36.4) | 0.010 |
| No. reporting breastfeeding, % | 61 | 60 (98.4) | 103 | 100 (97.1) | 0.609 |
| Weeks breastfed | 60 | 27.4 ± 6.9 | 100 | 27.4 ± 5.5 | 0.991 |
| No. reporting formula feeding, % | 61 | 28 (45.9) | 103 | 57 (55.3) | 0.242 |
| Weeks formula given | 28 | 18.6 ± 8.2 | 57 | 18.9 ± 7.2 | 0.878 |
| 2-wk z-scores11 | |||||
| Length | 61 | −1.3 ± 1.3 | 98 | −1.7 ± 1.4 | 0.053 |
| Weight | 61 | −0.8 ± 1.2 | 98 | −1.3 ± 1.3 | 0.021 |
| Weight-for-length | 61 | 0.1 ± 1.0 | 98 | 0.1 ± 1.1 | 0.702 |
| Head circumference | 61 | −0.4 ± 1.2 | 98 | −0.8 ± 1.4 | 0.040 |
| 12-mo z-scores11 | |||||
| Length | 68 | −0.8 ± 0.9 | 111 | −1.5 ± 1.1 | <0.001 |
| Weight | 69 | 0.1 ± 0.9 | 111 | −0.7 ± 1.2 | <0.001 |
| Weight-for-length | 68 | 0.6 ± 0.9 | 111 | 0.1 ± 1.1 | <0.001 |
| MUAC | 69 | 1.1 ± 0.9 | 111 | 0.6 ± 1.1 | <0.001 |
| Triceps skinfold thickness | 69 | 0.6 ± 1.1 | 111 | 0.0 ± 1.3 | 0.002 |
| Head circumference | 69 | −0.2 ± 0.9 | 111 | −0.8 ± 1.1 | <0.001 |
| 12-mo biceps skinfold thickness, mm | 69 | 6.5 ± 2.1 | 111 | 5.5 ± 1.8 | 0.001 |
AA, absolute alcohol (1 oz AA = 30 mL AA = 1.67 standard drinks); MUAC, midupper arm circumference.
From independent samples t tests for continuous outcomes, χ2 for binary outcomes, and linear-by-linear association for ordinal outcomes.
Score from the Hollingshead scale (46).
Defined as <0.42 kg/wk (38).
From 3 multipass 24-h recall dietary interviews.
Defined as energy intake below the estimated energy requirement based on height, weight, activity level, and weeks gestation (41, 42).
Defined as dietary iron intake <23 mg/d for women aged <19 y; <22 mg/d for ages ≥19 y (59).
From 3 choline-indicated FFQ interviews (39).
Defined as dietary choline intake <450 mg/d (60).
Defined as birthweight <10th percentile for gestational age (61).
Age- and sex-specific z-scores from WHO norms (43).
Maternal nutritional measures
As we have previously reported (29), maternal nutrition was similarly poor between heavy-drinking women and controls, with no group differences (Table 1). At least half of women in both groups had inadequate second/third-trimester rate of gestational weight gain, and 42% of the cohort reported inadequate dietary energy intake, with no group differences. Over 80% of the women reported being prescribed prenatal iron/folic acid supplements, with the vast majority reporting taking the supplements on “most days.” All but 1 woman reported inadequate iron intake (from diet only). Almost 90% reported inadequate choline intake (from diet only), with no group differences.
Relation of prenatal alcohol consumption and nutritional measures to infant anthropometric outcomes
Infants born to heavy-drinking mothers were smaller than infants born to controls across almost every measure at 2 weeks and at 12 months (Table 1). In univariate models (Table 2), average prenatal AA per day was related to smaller infant length, weight, and head circumference z-scores at age 2 weeks, and smaller length, weight, MUAC, TSF, and head circumference z-scores at 12 months. After adjustment for potential confounders, average daily PAE (AA per day) was related to smaller infant length and head circumference at ages 2 weeks and 12 months, and smaller weight, MUAC, and TSF at 12 months. The magnitudes of the associations between PAE and length and weight were larger at age 12 months than at 2 weeks; this pattern was not seen for head circumference. When adjusting for 2-week z-scores, the magnitudes of the associations of PAE to 12-month length and head circumference were reduced by 16% and 49%, respectively, whereas the magnitudes of the associations of PAE to 12-month weight, weight-for-length, and MUAC (adjusted for 2-week weight-for-length z-score) increased (by 16%, 50%, and 44%, respectively).
Table 2.
Relation of prenatal alcohol exposure and maternal nutritional measures to fetal and postnatal growth outcomes1
| AA/d (logged values) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariable adjusting for | Gestational weight gain rate, kg/wk | Dietary energy adequacy2 | Dietary iron,3 mg/d | Dietary choline,4 g/d | |||||||
| Univariate | Multivariable | 2-wk z-score5 | Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | |
| 2-wk z-scores6 (n = 98 exposed, 61 controls) | |||||||||||
| Length | −0.71** (−1.24, −0.18) | −0.51* (−0.99, −0.03) | — | 0.38 (−0.36, 1.13) | 0.31 (−0.32, 0.94) | 0.12 (−0.33, 0.56) | 0.14 (−0.24, 0.53) | 0.05 (−0.02, 0.12) | 0.04 (−0.02, 0.10) | 0.26 (−1.50, 2.02) | 0.43 (−1.08, 1.95) |
| Weight | −0.51* (−1.01, −0.02) | −0.32 (−0.76, 0.12) | — | 0.23 (−0.45, 0.92) | 0.15 (−0.42, 0.71) | 0.10 (−0.31, 0.51) | 0.11 (−0.24, 0.46) | 0.05 (−0.02, 0.11) | 0.04 (−0.02, 0.09) | −0.20 (−1.89, 1.49) | −0.07 (−1.47, 1.33) |
| Weight-for-length | 0.17 (−0.25, 0.59) | 0.26 (−0.20, 0.71) | — | −0.15 (−0.76, 0.46) | −0.23 (−0.86, 0.39) | 0.03 (−0.32, 0.38) | −0.03 (−0.39, 0.33) | 0.01 (−0.05, 0.06) | 0.01 (−0.05, 0.06) | −0.43 (−1.85, 1.00) | −0.41 (−1.83, 1.01) |
| Head circumference | −0.62* (−1.14, −0.11) | −0.54* (−1.00, −0.09) | — | 0.31 (−0.39, 1.01) | 0.26 (−0.32, 0.84) | 0.03 (−0.40, 0.45) | 0.04 (−0.33, 0.41) | 0.05 (−0.02, 0.12) | 0.05† (−0.01, 0.10) | −0.61 (−2.37, 1.16) | −0.43 (−1.90, 1.05) |
| 12-mo z-scores6 (n = 111 exposed, 69 controls) | |||||||||||
| Length | −0.83*** (−1.21, −0.45) | −0.67*** (−1.02, −0.31) | −0.56** (−0.92, −0.20) | 0.51† (−0.04, 1.06) | 0.33 (−0.17, 0.82) | 0.09 (−0.22, 0.40) | −0.07 (−0.36, 0.22) | 0.04 (−0.01, 0.09) | 0.02 (−0.03, 0.06) | −0.32 (−1.67, 1.02) | −0.40 (−1.54, 0.74) |
| Weight | −0.72*** (−1.12, −0.31) | −0.61** (−1.02, −0.20) | −0.71** (−1.15, −0.28) | 0.50† (−0.07, 1.06) | 0.32 (−0.23, 0.86) | −0.03 (−0.35, 0.30) | −0.19 (−0.51, 0.14) | 0.03 (−0.03, 0.08) | 0.01 (−0.04, 0.06) | −0.66 (−2.03, 0.70) | −0.72 (−2.00, 0.56) |
| Weight-for-length | −0.35† (−0.75, 0.05) | −0.32 (−0.74, 0.10) | −0.48* (−0.96, 0.00) | 0.31 (−0.23, 0.85) | 0.21 (−0.34, 0.76) | −0.10 (−0.41, 0.21) | −0.21 (−0.53, 0.12) | 0.01 (−0.04, 0.06) | 0.00 (−0.05, 0.06) | −0.62 (−1.91, 0.68) | −0.63 (−1.93, 0.67) |
| MUAC | −0.48** (−0.86, −0.11) | −0.43* (−0.81, −0.05) | −0.62** (−1.05, −0.20) | 0.47† (−0.05, 0.99) | 0.34 (−0.17, 0.86) | 0.02 (−0.28, 0.32) | −0.10 (−0.41, 0.20) | 0.02 (−0.03, 0.07) | 0.01 (−0.04, 0.06) | −0.67(−1.89, 0.55) | −0.71 (−1.89, 0.47) |
| Triceps skinfold thickness | −0.56* (−1.00, −0.12) | −0.61* (−1.08, −0.14) | −0.67** (−1.18, −0.16) | 0.04 (−0.58, 0.67) | 0.02 (−0.63, 0.67) | −0.21 (−0.56, 0.14) | −0.24 (−0.61, 0.14) | −0.03 (−0.08, 0.03) | 0.04 (−0.01, 0.09) | −1.01 (−2.50, 0.49) | −0.99 (−2.50, 0.52) |
| Head circumference | −0.62*** (−1.00, −0.25) | −0.57** (−0.95, −0.18) | −0.29† (−0.65, 0.06) | 0.51† (−0.02, 1.04) | 0.37 (−0.15, 0.90) | 0.03 (−0.28, 0.33) | −0.11 (−0.42, 0.20) | 0.05† (0.00, 0.10) | 0.04 (−0.01, 0.09) | −0.08 (−1.35, 1.19) | −0.12 (−1.34, 1.10) |
| 12-mo biceps skinfold thickness, mm | −0.70† (−1.42, 0.01) | −0.75† (−1.50, 0.01) | −0.80† (−1.67, 0.06) | 0.27 (−0.78, 1.31) | 0.26 (−0.75, 1.26) | −0.20 (−0.77, 0.36) | −0.21 (−0.81, 0.40) | −0.06 (−0.16, 0.03) | −0.07 (−0.17, 0.03) | −1.06 (−3.50, 1.37) | −1.01 (−3.45, 1.44) |
Values are regression coefficients (95% CI) from linear models regressing infant anthropometric z-scores on a given predictor; multivariable models also include prenatal cigarette use (cigarettes per day), maternal age, household socioeconomic status (46), weeks gestation at delivery, and, for all predictors except for dietary choline, whether the mother received a choline supplement as part of a pilot feasibility study; infants whose mothers received choline treatment (n = 34) were excluded from models including maternal choline intake. †P ≤ 0.10; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. AA, absolute alcohol [oz (30 mL)]; MUAC, midupper arm circumference.
Ratio of average daily energy intake (from 3 multipass 24-h recall dietary interviews) to estimated energy requirement (calculated based on mother's age, height, weight, activity level, and weeks gestation) (41, 42).
From 3 multipass 24-h recall dietary interviews.
From 3 choline-indicated FFQ interviews (39).
Missing for 18 exposed and 10 control infants who were measured at 12 mo but not 2 wk. MUAC and triceps skinfold z-scores and biceps skinfold models are adjusted for 2-wk weight-for-length z-score.
Age- and sex-specific z-scores from WHO norms (43).
Univariate association trends (P < 0.10) were seen between rate of maternal second/third-trimester gestational weight gain and larger 12-month length, weight, MUAC, and head circumference (Table 2). Association trends (P < 0.10) were also seen between maternal dietary iron and larger head circumference at 2 weeks (multivariable model, adjusting for potential confounders) and 12 months (in univariate but not multivariable models). Maternal dietary energy adequacy and choline intake were not related to any anthropometric outcome.
Effect modification by maternal nutritional measures in fetal alcohol growth restriction
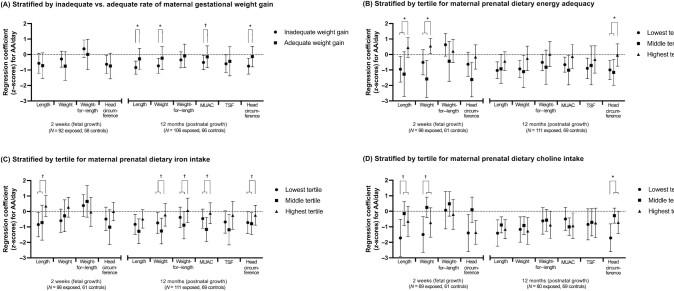
Figure 1 compares the relations of average daily PAE (AA per day) to each growth outcome stratified by maternal nutritional measures (values shown in Supplemental Table 3). Associations between alcohol exposure and 12-month length, weight, MUAC, and head circumference and a trend association with TSF were only apparent in infants born to mothers with inadequate second/third-trimester gestational weight gain rate. Effect modification by weight gain rate was confirmed in regression models with an inadequate weight gain (Yes/No) × alcohol interaction term for 12-month length (interaction term P = 0.025), weight (interaction term P = 0.037), MUAC (interaction term P = 0.081), and head circumference (interaction term P = 0.030).
Figure 1.
Relation of prenatal alcohol exposure to fetal and postnatal growth, stratified by 4 maternal nutritional indicators: (A) gestational weight gain; (B) prenatal dietary energy adequacy; (C) prenatal dietary iron intake; and (D) prenatal dietary choline intake. Y-axis values are raw regression coefficients with 95% CI error bars (in z-scores) for the effect of prenatal alcohol exposure (logged AA/d) on fetal and postnatal anthropometric z-scores (43) from multivariable models adjusted for potential confounders. *,†Significant effect modification (*P < 0.05; †P < 0.10) for interaction terms indicating effect modification by maternal nutrition from unstratified multivariable regression models regressing the anthropometric outcome on AA/d, the maternal nutritional measure, an AA/d × maternal nutritional measure interaction term, and potential confounders. Given the low power of interaction terms in epidemiological studies, effect modification was inferred when the P value for the interaction term was <0.10, as recommended by McClellan and Judd (45). Inadequate gestational weight gain was defined as <0.42 kg/wk (38). Tertile cutpoints: dietary energy adequacy [percentage of estimated energy requirement (41, 42)]: 94.2, 123.4; dietary iron intake (mg/d): 10.5, 13.0; dietary choline intake (mg/d): 243.5, 342.0. AA, absolute alcohol (oz); MUAC, midupper arm circumference; TSF, triceps skinfold thickness.
Associations between PAE and smaller length and head circumference at 2 weeks and length, weight, MUAC, TSF, and head circumference at 12 months were only apparent in infants born to mothers in the lowest or middle tertiles for dietary energy adequacy. The magnitude of the association of PAE to 2-week weight was larger in infants born to mothers in the middle tertile for dietary energy adequacy than the highest tertile. Effect modification by dietary energy adequacy was confirmed in regression models with a dietary energy adequacy × alcohol interaction term for 2-week length (interaction term P = 0.031) and weight (interaction term P = 0.029) and 12-month head circumference (interaction term P = 0.016).
The relations of PAE to 2-week length and 12-month length, weight, weight-for-length, MUAC, TSF, and head circumference were only seen in infants born to mothers in the lowest or middle tertiles for dietary iron intake. Effect modification by dietary iron intake was confirmed in regression models with an iron × alcohol interaction term for 2-week length (interaction term P = 0.077) and 12-month weight (interaction term P = 0.054), weight-for-length (interaction term P = 0.066), MUAC (interaction term P = 0.076), and head circumference (interaction term P = 0.089).
The associations of PAE to 2-week length and weight were only seen in infants born to women in the lowest tertile for dietary choline, and the effect size of PAE-related 12-month head circumference reduction was much larger in women in the lowest tertile than the middle and highest tertiles. Effect modification by dietary choline intake was confirmed in a regression model with a choline × alcohol interaction term for all 3 outcomes (2-week length interaction term P = 0.069; 2-week weight interaction term P = 0.054; 12-month head circumference interaction term P = 0.027).
Table 3 examines the degree to which lower gestational weight gain and energy, iron, and choline intakes were seen in the same mothers. The Cohen κ statistic showed only slight agreement between gestational weight gain and the dietary measure tertiles, and only fair-to-moderate agreement between the 3 dietary measures.
Table 3.
Comparisons of tertile agreement between maternal nutritional measures (n = 189)
| Same tertile n (%) | Different tertile n (%) | Weighted κ1 | |
|---|---|---|---|
| Gestational weight gain rate vs. dietary energy adequacy2 | 71 (39.7) | 108 (60.3) | 0.10 |
| Gestational weight gain rate vs. dietary iron intake3 | 80 (44.7) | 99 (55.3) | 0.17 |
| Gestational weight gain rate vs. dietary choline intake4 | 65 (43.6) | 84 (56.6) | 0.16 |
| Dietary energy adequacy vs. dietary iron intake | 117 (61.9) | 72 (38.1) | 0.43 |
| Dietary energy adequacy vs. dietary choline intake | 90 (57.0) | 68 (43.0) | 0.35 |
| Dietary iron intake vs. dietary choline intake | 101 (63.9) | 57 (36.1) | 0.46 |
0.00 ≤ κ ≤ 0.20 denotes slight agreement; 0.21 ≤ κ ≤ 0.40 denotes fair agreement; 0.41 ≤ κ ≤ 0.60 denotes moderate agreement (62).
Ratio of average daily energy intake (from 3 multipass 24-h recall dietary interviews) to estimated energy requirement (calculated based on mother's age, height, weight, activity level, and weeks gestation) (41, 42).
From 3 multipass 24-h recall dietary interviews.
From 3 choline-indicated FFQ interviews (39).
Discussion
In this prenatally recruited, prospective, longitudinal birth cohort, PAE-related fetal and postnatal growth restriction were more severe in women with poorer nutrition across 4 measures: second/third-trimester gestational weight gain and dietary energy, iron, and choline intakes; in many cases growth effects were evident only in the more poorly nourished mothers. These findings provide evidence that variation in these elements of maternal nutrition across ranges commonly found in the general population can modify fetal vulnerability to PAE. Of note, there was relatively little overlap among these measures in terms of which mothers had poorer nutrition, indicating that these measures assessed specific elements of nutrition rather than serving as proxies for one another. To our knowledge, this is the first observational human study to examine the impact of maternal prenatal nutrition on fetal alcohol-related growth restriction.
Our finding that the magnitudes of the associations between PAE and weight, MUAC, and triceps skinfolds were larger after adjusting for 2-week measures is consistent with our original Cape Town and Detroit birth cohort studies, in which we demonstrated that PAE-related reductions in weight worsen in the first year of life (3, 4). These findings support the hypothesis that different mechanisms underlie pre- and postnatal growth restriction in FASD (9). PAE-related reductions in length were relatively stable from 2 weeks to 12 months, which is consistent with our previous finding that PAE-related height/length reductions are more stable through adolescence than weight reductions (11). Our finding of smaller weight-for-length z-scores in children born to heavy drinkers compared with controls and the associations of PAE to smaller MUAC and triceps skinfolds seen in this study are consistent with our previous finding of PAE-related reductions in percentage body fat, as measured with bioelectric impedance, in our original Cape Town cohort (3).
Effect modification by maternal gestational weight gain could indicate a moderating effect of overall global nutrition. Alternatively, higher gestational weight gain might protect against the teratogenic effects of alcohol consumption by decreasing fetal alcohol exposure, because higher nonfat body mass results in a larger pharmacological volume of distribution and a lower blood alcohol concentration (BAC) for a given quantity of alcohol consumed (47). Although nonnutritional factors can affect gestational weight gain, including illness and pregnancy complications, such issues were rare in our review of antenatal medical records in this cohort, suggesting that the effect modification seen was likely due to nutritional and related physiological factors. BAC is also affected by dietary energy intake. Food digestion induces ethanol metabolism in the liver, thereby decreasing BAC (48). Higher energy intake could thus lead to higher ethanol metabolism and lower levels of fetal exposure for a given quantity of alcohol. Alternatively, dietary energy intake might serve as a summative proxy for groups of micronutrients that have the potential to alter fetal vulnerability but have smaller individual effect sizes.
Maternal iron and choline intake each appeared to modify fetal vulnerability to PAE-related growth restriction. These findings are consistent with animal models and human trials demonstrating protective effects of iron (3, 14–17) and choline (19–23, 27, 49) supplementation and detrimental effects from restriction of these micronutrients. Although the mechanisms underlying effect modification by iron remain unknown, animal and human studies have demonstrated PAE-related alterations in maternal, fetal, and postnatal iron homeostasis, including iron-restricted placental iron transport, which, in animal models, can be exacerbated by poor maternal iron intake and ameliorated by maternal iron supplementation (3, 14–18). Indeed, iron restriction in utero or during infancy leads to growth restriction and deficits in neurobehavioral outcomes that are also seen in FASD, including poorer recognition memory, slower processing speed, and eyeblink conditioning deficits (33, 50–52). The mechanisms underlying the protective effects of choline supplementation are also unknown, but recent studies suggest that choline can ameliorate PAE-induced alterations in epigenetic programming in its role as a methyl donor (53, 54).
Of note, effect modification by all 4 elements of nutrition was stronger for PAE-related reductions in head circumference than for length or weight. Because head circumference is determined by both somatic and brain growth, future studies are needed to examine the degree to which these maternal nutritional elements also modify FASD neurobehavioral deficits. Animal models examining the impact of iron and choline and 2 human trials of maternal choline supplementation have demonstrated protective effects against teratogenic effects of PAE on neurodevelopment (19–23, 26, 27, 49). We have previously demonstrated that fetal alcohol growth restriction serves as a biomarker of the severity of FASD neurocognitive deficits, with the most severe deficits seen in children with both fetal and postnatal growth restriction (11).
This study had limitations common to other observational studies of prenatal exposures. The small sample size, measurement error surrounding estimates of exposures and covariates, model misspecification, and residual confounding might have led to unmeasured bias and Type 1 (false-positive) and Type 2 (false-negative) errors. Although the 24-h dietary recall interviews included any reported alcoholic beverages, we were unable to ascertain dietary intake on Fridays or Saturdays, when women were more likely to drink. We have previously reported that because alcoholic beverages are high in energy content, the lack of differences between heavy-drinking and control women in weight, BMI, and rate of gestational weight gain indicate that heavy-drinking women maintained generally stable daily energy intake on drinking days compared with nondrinking days (i.e., they replaced food calories with alcohol calories when drinking) (29); sensitivity analyses modeling the scenario in which women replaced food with alcohol demonstrated that such replacement did not cause clinically significant differences in average daily micronutrient intake. Because detailed measures of postnatal infant diet throughout the first year of life were not available, we could not fully account for potential confounding by the child's postnatal nutrition. We have previously reported that formula, breast-, and complementary feeding practices through age 6.5 months were very similar between heavy-drinking mothers and controls (55). Given the unusually high prevalence of inadequate gestational weight gain and energy intake in this population, our findings need to be examined in populations with better nutrition. Of note, the prevalence of inadequate dietary iron and choline intake in this cohort was similar to estimates in studies of pregnant women in the United States and Canada (56–58). Because prepregnancy BMI was unavailable, a single cutoff for inadequate gestational weight gain was used. Overweight and obesity are uncommon in this impoverished community; low prepregnancy BMI is more common. Because the cutoff for adequate gestational weight gain for women with low prepregnancy BMI is higher (0.51 kg/week) than the single cutoff used here (0.42 kg/week) (38), we might have underestimated the true prevalence of inadequate gestational weight gain.
In conclusion, to our knowledge this is the first human study to examine the potential for gestational weight gain and dietary intakes of energy, iron, and/or choline in ranges found in the general population to alter fetal vulnerability to PAE-related growth restriction. Effect modification by these elements of nutrition could explain some of the variability in FASD outcomes in children with similar levels of exposure. Combined with evidence from FASD animal models, these findings support the need for additional studies examining potential maternal nutritional interventions across these domains in heavy-drinking pregnant women. Studies examining the mechanisms underlying effect modification by these maternal nutritional elements could elucidate pathophysiological mechanisms in alcohol teratogenesis. Our findings underscore the need for standardized alcohol and nutritional screening as an important part of standard prenatal care and the continuing need for development and implementation studies of psychosocial and biomedical harm reduction interventions for heavy-drinking pregnant women. Furthermore, given that the prenatal nutritional outcomes studied here are measurable in the clinical environment, they could be useful in identifying women with potential increased fetal risk of FASD, for whom intensified alcohol screening and psychosocial/behavioral and/or nutritional interventions could be warranted.
Supplementary Material
ACKNOWLEDGEMENTS
We thank our University of Cape Town and Wayne State University research staffs, including Maggie September, Beverley Arendse, Patricia O'Leary, and Patricia Solomon for their work on subject recruitment and maintenance; Sharmilah Booley and Baheya Najaar for their work in developing the choline-indicated FFQ; Catherine Lewis, Nadine Lindinger, and Stacey Hall, who performed the measurements of the infants; dietary interviewers Catherine Day, Monika Uys, and Nicola Cooper; Lori Bechard for her computation of dietary data; and Renee Sun, for her assistance with data processing. We also thank Susan Fawcus, MD, Head of Department of Obstetrics, Mowbray Maternity Hospital; the nursing and records department staff at the Hanover Park and Retreat Midwife Obstetric Units, Mowbray Maternity Hospital, Somerset Hospital, and Groote Schuur Hospital, where the mothers were recruited and the infants were born. Lastly, we extend our deep appreciation to our Cape Town study participants for their contributions to this study.The authors’ responsibilities were as follows—RCC, SWJ, JLJ, MS, CPD, CDM: conceptualized the study design. SWJ, JLJ, RCC, CDM: oversaw recruitment and assessment of the longitudinal birth cohort, data acquisition and analysis, and interpretation of findings; CDM, EMM: coordinated and supervised data collection; NCD: coordinated data management and assisted with statistical analyses; RCC: conducted the data analyses and wrote the manuscript; SWJ, JLJ, MS, CPD: edited and revised the manuscript; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the NIH/National Institute on Alcohol Abuse and Alcoholism (R01AA016781, R21AA022203, K23AA020516; R01AA027916); the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (K24DK104676; P30DK040561); and the Lycaki-Young Fund (State of Michigan).
CPD is an Editor on The American Journal of Clinical Nutrition and played no role in the Journal's evaluation of the manuscript.
Supplemental Figure 1 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AA, absolute alcohol; BAC, blood alcohol concentration; BSF, biceps skinfold thickness; FAS, fetal alcohol syndrome; FASD, fetal alcohol spectrum disorders; MUAC, midupper arm circumference; PAE, prenatal alcohol exposure; TSF, triceps skinfold thickness.
Contributor Information
R Colin Carter, Institute of Human Nutrition and Departments of Emergency Medicine and Pediatrics, Columbia University Vagelos College of Physicians and Surgeons and NYP Morgan Stanley Children's Hospital, New York, NY, USA.
Marjanne Senekal, Department of Human Biology, University of Cape Town Faculty of Health Sciences, Cape Town, South Africa.
Christopher P Duggan, Division of Gastroenterology, Hepatology, and Nutrition, Boston Children's Hospital, Boston, MA, USA.
Neil C Dodge, Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, USA.
Ernesta M Meintjes, Department of Human Biology, University of Cape Town Faculty of Health Sciences, Cape Town, South Africa.
Christopher D Molteno, Division of Gastroenterology, Hepatology, and Nutrition, Boston Children's Hospital, Boston, MA, USA; Departments of Nutrition and Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Psychiatry and Mental Health, University of Cape Town Faculty of Health Sciences, Cape Town, South Africa.
Joseph L Jacobson, Department of Human Biology, University of Cape Town Faculty of Health Sciences, Cape Town, South Africa; Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, USA.
Sandra W Jacobson, Department of Human Biology, University of Cape Town Faculty of Health Sciences, Cape Town, South Africa; Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, USA; Department of Psychiatry and Mental Health, University of Cape Town Faculty of Health Sciences, Cape Town, South Africa.
Data Availability
Deidentified, individual participant data that underlie the results reported in this article (text, tables, figures, and appendices) and the study protocol, statistical analysis plan, and analytic code will be available for sharing to journal editors for any reason either before or after publication for checking and to researchers who provide a methodologically sound proposal, as determined by the authors of this article. Proposals from researchers should be directed to SWJ (sandra.jacobson@wayne.edu). Data will be stored in a data warehouse at Wayne State University and transmitted electronically in encrypted form to requestors. Data requestors will need to sign a data access agreement prior to access.
References
- 1. May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning Met al. . Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–92. [DOI] [PubMed] [Google Scholar]
- 2. May PA, Blankenship J, Marais A-S, Gossage JP, Kalberg WO, Barnard Ret al. . Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37(5):818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter RC, Jacobson JL, Molteno CD, Jiang H, Meintjes EM, Jacobson SWet al. . Effects of heavy prenatal alcohol exposure and iron deficiency anemia on child growth and body composition through age 9 years. Alcohol Clin Exp Res. 2012;36(11):1973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carter RC, Jacobson JL, Sokol RJ, Avison MJ, Jacobson SW. Fetal alcohol-related growth restriction from birth through young adulthood and moderating effects of maternal prepregnancy weight. Alcohol Clin Exp Res. 2013;37(3):452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor Pet al. . Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 1989;84(3):536–41. [PubMed] [Google Scholar]
- 6. Day NL, Richardson GA, Geva D, Robles N. Alcohol, marijuana, and tobacco: effects of prenatal exposure on offspring growth and morphology at age six. Alcohol Clin Exp Res. 1994;18(4):786–94. [DOI] [PubMed] [Google Scholar]
- 7. Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26(10):1584–91. [DOI] [PubMed] [Google Scholar]
- 8. Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Shankaran S. Effects of alcohol use, smoking, and illicit drug use on fetal growth in black infants. J Pediatr. 1994;124(5):757–64. [DOI] [PubMed] [Google Scholar]
- 9. Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994;18(2):317–23. [DOI] [PubMed] [Google Scholar]
- 10. Lumeng JC, Cabral HJ, Gannon K, Heeren T, Frank DA. Pre-natal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29(4):446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW. Fetal alcohol growth restriction and cognitive impairment. Pediatrics. 2016;138(2):e20160775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weinberg J, D'Alquen G, Bezio S. Interactive effects of ethanol intake and maternal nutritional status on skeletal development of fetal rats. Alcohol. 1990;7(5):383–8. [DOI] [PubMed] [Google Scholar]
- 13. Shankar K, Hidestrand M, Liu X, Xiao R, Skinner CM, Simmen FAet al. . Physiologic and genomic analyses of nutrition-ethanol interactions during gestation: implications for fetal ethanol toxicity. Exp Biol Med. 2006;231(8):1379–97. [DOI] [PubMed] [Google Scholar]
- 14. Huebner SM, Blohowiak SE, Kling PJ, Smith SM. Prenatal alcohol exposure alters fetal iron distribution and elevates hepatic hepcidin in a rat model of fetal alcohol spectrum disorders. J Nutr. 2016;146(6):1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huebner SM, Helfrich KK, Saini N, Blohowiak SE, Cheng AA, Kling PJet al. . Dietary iron fortification normalizes fetal hematology, hepcidin, and iron distribution in a rat model of prenatal alcohol exposure. Alcohol Clin Exp Res. 2018;42(6):1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saini N, Helfrich KK, Kwan STC, Huebner SM, Abazi J, Flentke GRet al. . Alcohol's dysregulation of maternal-fetal IL-6 and p-STAT3 is a function of maternal iron status. Alcohol Clin Exp Res. 2019;43(11):2332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter RC, Jacobson SW, Molteno CD, Jacobson JL. Fetal alcohol exposure, iron-deficiency anemia, and infant growth. Pediatrics. 2007;120(3):559–67. [DOI] [PubMed] [Google Scholar]
- 18. Carter RC, Georgieff MK, Ennis KM, Dodge NC, Wainwright H, Meintjes EMet al. . Prenatal alcohol-related alterations in maternal, placental, neonatal, and infant iron homeostasis. Am J Clin Nutr. 2021;41(12):2114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121(1):120–30. [DOI] [PubMed] [Google Scholar]
- 20. Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26(1):35–45. [DOI] [PubMed] [Google Scholar]
- 21. Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22(5):703–11. [DOI] [PubMed] [Google Scholar]
- 22. Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22(3):619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120(2):482–7. [DOI] [PubMed] [Google Scholar]
- 24. Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IVet al. . Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Matern Child Health J. 2015;19(12):2605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wozniak JR, Fink BA, Fuglestad AJ, Eckerle JK, Boys CJ, Sandness KEet al. . Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J Neurodev Disord. 2020;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warton FL, Molteno CD, Warton CMR, Wintermark P, Lindinger NM, Zollei Let al. . Maternal choline supplementation mitigates alcohol exposure effects on neonatal brain volumes. Alcohol Clin Exp Res. 2021; 45(9):1762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NMet al. . Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2018;42(7):1327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobson SW, Jacobson JL, Molteno CD, Warton CMR, Wintermark P, Hoyme HEet al. . Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res. 2017;41(5):965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter RC, Senekal M, Dodge NC, Bechard L, Meintjes EM, Molteno CDet al. . Maternal alcohol use and nutrition during pregnancy: diet and anthropometry. Alcohol Clin Exp Res. 2017;41(12):2114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–25. [DOI] [PubMed] [Google Scholar]
- 31. Sokol R, Martier S, Ernhart C. Identification of alcohol abuse in the prenatal clinic. In: Chang N, Chao Meditors. Early identification of alcohol abuse. Rockville (MD): Alcohol, Drug Abuse, and Mental Health Administration Research; 1983. [Google Scholar]
- 32. Carter RC, Wainwright H, Molteno CD, Georgieff MK, Dodge NC, Warton Fet al. . Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin Exp Res. 2016;40(4):753–64. [DOI] [PubMed] [Google Scholar]
- 33. Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HEet al. . Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–72. [DOI] [PubMed] [Google Scholar]
- 34. Molteno CD, Jacobson JL, Carter RC, Jacobson SW. Infant symbolic play as an early indicator of fetal alcohol-related deficit. Infancy. 2010;15(6):586–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bearer CF, Jacobson JL, Jacobson SW, Barr D, Croxford J, Molteno CDet al. . Validation of a new biomarker of fetal exposure to alcohol. J Pediatr. 2003;143(4):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Min MO, Singer LT, Minnes S, Wu M, Bearer CF. Association of fatty acid ethyl esters in meconium and cognitive development during childhood and adolescence. J Pediatr. 2015;166(4):1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peterson J, Kirchner HL, Xue W, Minnes S, Singer LT, Bearer CF. Fatty acid ethyl esters in meconium are associated with poorer neurodevelopmental outcomes to two years of age. J Pediatr. 2008;152(6):788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rasmussen KM, Yaktine ALeditors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 39. Carter RC, Jacobson SW, Booley S, Najaar B, Dodge NC, Bechard LJet al. . Development and validation of a quantitative choline food frequency questionnaire for use with drinking and non-drinking pregnant women in Cape Town, South Africa. Nutr J. 2018;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, Midthune Det al. . Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. 2006;106(10):1640–50. [DOI] [PubMed] [Google Scholar]
- 41. Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8(7a):1133–52. [DOI] [PubMed] [Google Scholar]
- 42. Prentice AM, Poppitt SD, Goldberg GR, Murgatroyd PR, Black AE, Coward WA. Energy balance in pregnancy and lactationIn: Lonnerdal Beditor. Nutrient regulation during pregnancy, lactation, and infant growth. New York: Plenum Press; 1994. p.; 11–26. [DOI] [PubMed] [Google Scholar]
- 43. de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25(1 Suppl 1):S15–26. [DOI] [PubMed] [Google Scholar]
- 44. Jacobson SW, Carter RC, Molteno CD, Meintjes EM, Senekal MS, Lindinger NMet al. . Feasibility and acceptability of maternal choline supplementation in heavy drinking pregnant women: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2018;42(7):1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychol Bull. 1993;114(2):376–90. [DOI] [PubMed] [Google Scholar]
- 46. Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–51. [Google Scholar]
- 47. Lands WE. A review of alcohol clearance in humans. Alcohol. 1998;15(2):147–60. [DOI] [PubMed] [Google Scholar]
- 48. Ramchandani VA, Kwo PY, Li TK. Effect of food and food composition on alcohol elimination rates in healthy men and women. J Clin Pharmacol. 2001;41(12):1345–50. [DOI] [PubMed] [Google Scholar]
- 49. Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok Let al. . The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol. 2015;49(7):647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CDet al. . Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35(2):250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53(2):217–23. [DOI] [PubMed] [Google Scholar]
- 52. McEchron MD, Alexander DN, Gilmartin MR, Paronish MD. Perinatal nutritional iron deficiency impairs hippocampus-dependent trace eyeblink conditioning in rats. Dev Neurosci. 2008;30(4):243–54. [DOI] [PubMed] [Google Scholar]
- 53. Otero NKH, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin Exp Res. 2012;36(10):1701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bekdash RA, Zhang C, Sarkar DK. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in β-endorphin-producing POMC neurons of the hypothalamus. Alcohol Clin Exp Res. 2013;37(7):1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Edwards AE, Senekal M, Dodge NC, Meintjes EM, Molteno CD, Jacobson JLet al. . Fetal alcohol growth restriction is not attributable to infant feeding practices in a prospective birth cohort in Cape Town. Curr Dev Nutr. 2021;5(Suppl 2):739. [Google Scholar]
- 56. Jensen H, Batres-Marquez S, Carriquiry A, Schalinske K. Choline in the diets of the U.S. population: NHANES, 2003–2004. FASEB J. 2007;21:6, 219. [Google Scholar]
- 57. Lewis ED, Subhan FB, Bell RC, McCargar LJ, Curtis JM, Jacobs RLet al. . Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br J Nutr. 2014;112(1):112–21. [DOI] [PubMed] [Google Scholar]
- 58. Bailey RL, Pac SG, Fulgoni VL, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw Open. 2019;2(6):e195967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Insitute of Medicine. Choline . In: Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Washington (DC): National Academies Press; 2006. [PubMed] [Google Scholar]
- 60. Institute of Medicine . Iron. In: Dietary reference intakes. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 61. Oken E, Kleinman KP, Rich-Edwards J. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3(6):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watson PF, Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010;73(9):1167–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified, individual participant data that underlie the results reported in this article (text, tables, figures, and appendices) and the study protocol, statistical analysis plan, and analytic code will be available for sharing to journal editors for any reason either before or after publication for checking and to researchers who provide a methodologically sound proposal, as determined by the authors of this article. Proposals from researchers should be directed to SWJ (sandra.jacobson@wayne.edu). Data will be stored in a data warehouse at Wayne State University and transmitted electronically in encrypted form to requestors. Data requestors will need to sign a data access agreement prior to access.