ABSTRACT
Background
Prostate cancer is the most common noncutaneous cancer in American males. Causal links between dairy, or dietary calcium, and this cancer are considered suggestive but limited.
Objectives
To evaluate these associations in a large North American cohort, including many with no (or very low) dairy intake and much calcium from nondairy sources.
Methods
A prospective cohort study of 28,737 Seventh-day Adventist men in the United States and Canada, of whom 6389 were of black ethnicity. Diet was measured by FFQ, and 275 male participants also provided repeated 24-h dietary recalls as a calibration substudy. Incident cancers were mainly found by matching with cancer registries. Analyses used multivariable proportional hazards regressions and regression calibration for some analyses.
Results
In total, 1254 (190 advanced) incident prostate cancer cases were found during an average 7.8 y of follow-up. Men at the 90th percentile of dairy intake (430 g/d) compared with the 10th percentile (20.2 g/d) had higher prostate cancer risk (HR: 1.27; 95% CI: 1.12, 1.43). Similar findings, comparing the same g/d intakes, were demonstrated for advanced prostate cancers (HR: 1.38; 95% CI: 1.02, 1.88), for nonadvanced cases (HR: 1.27; 95% CI: 1.11, 1.45), in black participants (HR: 1.24; 95% CI: 0.98, 1.58), and when excluding vegan participants (HR: 1.22; 95% CI: 1.03, 1.43). Calibrated dairy (g/d) regressions (all participants and all prostate cancers), adjusting for dietary measurement error, found a HR of 1.75 (95% CI: 1.32, 2.32). Comparing 90th percentile intake to zero intakes (uncalibrated), the HR was 1.62 (95% CI: 1.26, 2.05). There was no evidence of an effect of higher (905 mg/d) compared with lower (349 mg/d) intakes of nondairy calcium (HR: 1.16; 95% CI: 0.94, 1.44).
Conclusions
Men with higher intake of dairy foods, but not nondairy calcium, had a higher risk of prostate cancer compared with men having lower intakes. Associations were nonlinear, suggesting greatest increases in risk at relatively low doses.
Keywords: dairy intake, calcium intake, prostate cancer, Seventh-day Adventists, vegetarians, vegans, regression calibration, cohort study, African American
Introduction
Ecological studies document highly varied prostate cancer incidence and mortality rates between populations (1), suggesting a potentially large impact of environmental factors on the risk of this very common malignancy. However, convincing identification of modifiable risk factors has thus far proven elusive, perhaps in part recently through variable detection of more indolent cancers using Prostate Specific Antigen. Among dietary exposures, calcium and dairy intakes have been hypothesized as potential risk factors. The World Cancer Research Fund (WCRF) and American Institute for Cancer Research recently judged the evidence linking both dairy products and diets high in calcium to an increased risk of prostate cancer to be suggestive but limited (2). A meta-analysis undergirding the WCRF Continuous Update Project report found an adverse risk relation of total dairy product consumption (summary RR: 1.07; 95% CI: 1.02, 1.12 per 400 g/d) (3). Higher risk was also seen with higher intakes of total calcium, dairy calcium, and supplemental calcium (fatal cases only) but not for nondairy calcium intake (3). Calcium and dairy were not adjusted for each other, but the data did not favor dairy calcium or fat as active principles.
Adventist Health Study–2 (AHS-2) is a large and diverse North American cohort, with many participants who adhere to various vegetarian dietary patterns, including vegans who avoid dairy products, lacto-ovo-vegetarians who on average consume about half the typical intake of dairy, and nonvegetarians who eat dairy at typical American levels [∼120 kcal/d; i.e., 200 g/d as milk (4)]. Thus, there is a wide range of intake of dairy products in the AHS-2 cohort, with 11.7% (3370 participants) having very low (≤10 g/d) or no intake (N = 2302 subjects) of this food group (5). This provides the opportunity to examine associations at very low and also more typical intakes. It also allows substantial uncoupling of dairy and calcium, given that many in this population gain most dietary calcium from nondairy sources (principally soy, nuts/seeds, cruciferous vegetables, other green vegetables, legumes, fruit, and fortified cereals). A previous analysis associating vegetarian dietary patterns with prostate cancer risk in this cohort observed a lower risk among vegans compared with nonvegans (6), the absence of dairy products being one of their defining characteristics (7). The AHS-2 cohort also oversampled black participants, who are well known to have a particularly high incidence of prostate cancer (8). Another recent analysis compared multivariable-adjusted prostate cancer incidence rates from AHS-2 to those from the census-based National Longitudinal Mortality Study and its Surveillance, Epidemiology, and End Results substudy (9). Differences were modest and not statistically significant, although detection bias may have increased rates of identified prostate cancer among the relatively well-educated AHS-2 population, as education is a predictor of higher rates of screening for this cancer (10).
We evaluate the risk of prostate cancer outcomes in this population that has a wide range of dairy consumption and in whom much calcium intake is from nondairy sources.
AHS-2 was initiated in 2001, and >96,000 adult (age ≥25 y) Seventh-day Adventist members were enrolled, with baseline dietary data being collected between 2002 and 2007 throughout the United States and Canada. Butler et al. (11) describe cohort formation and characteristics in detail. Loma Linda University's institutional review board approved this study.
Initially included in this analysis were the 33,512 male cohort participants for whom linkage with state/provincial cancer registries was possible. Excluded were those younger than 25 y or missing data for sex (n = 65), improbable response patterns in questionnaire data (e.g., identical high-frequency responses to all questions on a page) (n = 136), >69 missing values in dietary data (n = 710), estimated energy intake <500 kcal/d or >4500 kcal/d (n = 1037), BMI (in kg/m2) <14 or >60 (n = 41), a self-reported history of cancer (except for nonmelanoma skin cancer) (n = 2501), no consent form returned (n = 7), and no date of birth (n = 278). Thus, an analytic sample of 28,737 male participants remains. Of these, 6389 were black. See Supplemental Figure 1.
Methods
Outcome data
The prespecified primary endpoint of these analyses was incident prostate cancer, with secondary endpoints of advanced and nonadvanced variants. Incident cancers were identified via computer-assisted record linkage with state cancer registries. At the time of this analysis, linkage had been achieved for 49 states, Washington, DC, and 3 Canadian provinces. The linkage and follow-up was through 31 December 2010 for 10 states; 31 December 2011 for 28 states and 2 provinces; 31 December 2012 for 7 states, Washington, DC, and 1 province; and 31 December 2013 for 4 states. The procedure for record linkage has been previously described (9, 11).
International Statistical Classification of Diseases, Tenth Edition and International Classification of Diseases for Oncology, Third Edition coding (12) was used to identify cases of incident prostate cancer. The definition applied was prostate cancer, primary site (C61). Carcinomas in situ were not considered cases, nor were tumors with histology codes 9050–9055 (mesothelioma), 9140 (Kaposi sarcoma), and 9590–9992 (lymphoma, myeloma, and leukemia). Cases with regional or metastatic spread or a Gleason score of 4 + 3 or greater were categorized as advanced prostate cancers and localized cases with a Gleason score of 3 + 4 or less as nonadvanced.
In addition to the cancer registry matching, when participants reported a new cancer diagnosis on biennial follow-up questionnaires that was not found in the registry linkage, the participant was telephoned and asked clarifying questions. If still indicated, medical records were requested and reviewed by the principal investigator (GEF) to ascertain whether the self-reported cancer could be verified. This secondary process yielded 5 additional cases.
Dietary data
Study participants were instructed at baseline to evaluate their diets over the previous 1 y, using a detailed, quantitative FFQ with >200 food items. This included 51 items relating to soy intake, 15 items about dairy intake, and questions about meats, nuts/seeds, and other dietary items. Soy items included 40 commercially prepared meat analogues, 15 listed and 2 open-ended soy milk items, and questions about tofu and soybeans, as well as soy isoflavone supplementation. Portion sizes were assessed in relation to a supplied standard size (standard, ≤0.5 standard, ≥1.5 standard). Intakes of calcium and other supplements were assessed in separate questions. For products not listed, study nutritionists and a food technologist created recipes based on food label ingredients. Nutrient composition was calculated using the Nutrition Data System for Research 2008 database (13). Dairy and soy analytic variables include only dairy and soy constituents of mixed foods.
In addition, 275 male participants took part in a calibration/validation substudy, where the reference dietary measure was 6 structured telephone 24-h dietary recalls that represented 2 synthetic weeks. Jaceldo-Siegl et al. (14, 15) provide further details of the methods of dietary measurement using the questionnaire and its validation by repeated 24-h dietary recalls. The energy-adjusted validity correlation for total dairy consumption was 0.86 in white participants and 0.82 in black participants (15), for total calcium was 0.63 for whites and 0.73 for blacks, and for dietary calcium was 0.53 for whites and 0.54 for blacks (14).
Covariate data
The baseline questionnaire also included questions related to demographics, family history, biometrics, prior or current diseases and medications, use of tobacco and alcohol, exercise and other lifestyle factors, and so on, which are the source of covariate data. The following categories and definitions were used for covariates: race (black/nonblack), education (up to high school graduate, trade school/some college/associate degree, bachelor degree or higher), moderate or vigorous exercise (“vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath”) (categories: none, ≤60 min/wk, >60 min/wk), smoking (never, quit ≥1 y ago, current or quit <1 y ago), alcohol (none, less than daily, daily or more use), family history of prostate cancer (yes, no), self-reported history of benign prostatic hyperplasia (yes, no), prostate cancer screening (PSA or digital rectal examination in categories 0–2, 3–4, 5+ y ago, and never), treated for diabetes mellitus within the past year (yes, no), supplemental vitamin D (mcg)/d, dietary energy (continuous kcal/d), and self-reported BMI (in kg/m2; <18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35) and height. Participants self-identified their race/ethnicity in ≥1 of 21 categories. Those self-identifying at least in part as black/African American, West Indian/Caribbean, African, or other black were categorized as black for this analysis and all others as nonblack.
Statistical analysis
Baseline descriptive statistics were calculated according to intakes of total dairy and calcium, adjusted (where appropriate) for age by direct standardization (using the entire analytic sample as the standard population). We used Cox proportional hazards regression to assess the relation between the dietary variables of interest and the risk of prostate cancer, adjusting for plausible confounders; analyses were conducted for all, advanced, and nonadvanced prostate cancers. Attained age was the Cox regression time variable, with left truncation at age of study entry. For dairy intake variables, the analysis was conducted for dairy intake in grams per day and for total energy from dairy products (kcal/d). To evaluate relative effects of full-fat and reduced-fat dairy, a regression model included terms for total dairy and reduced-fat dairy. The coefficient for reduced-fat dairy measures any difference in the effect on risk of prostate cancer comparing the 2 types of dairy product. For calcium intake, the regression was first performed with total calcium as the variable of interest, then with dietary and supplemental calcium, and finally with calcium from dairy sources and calcium from other nondairy sources. Where possible, dietary variables were modeled as both continuous variables with natural log transformation due to right-skewed distributions and as categorical variables in Supplemental Table 1. The categories in the supplemental table are quintiles (calcium variables) or zero intakes plus quintiles of dairy users (6 categories) for dairy variables. Dietary variables were energy adjusted using the residual method (in a race-specific fashion). For total calcium, only the dietary portion was energy adjusted. Hazard ratios from continuous analyses compare relatively extreme intakes as indicated in g/d or kcal/d in the tables and figures.
Covariates were selected in an a priori fashion as plausible confounders. For the main analyses, 2 models were used to show the effect of including additional covariates (see footnotes to tables). Covariates were tested for possible interaction with exposure variables total dairy and total calcium intakes, by using product terms between the exposures and each covariate in regressions. No statistically significant interactions were found. Alcohol use, cigarette smoking, or supplemental vitamin D were not finally included in our models, as there was no evidence of important associations in our data or suggestion of this in literature reporting other data sets.
A sensitivity analysis addressed the question of whether the 8.0% of vegans were sufficiently different in unmeasured ways to bias results with unexpectedly low risks. These analyses excluded vegans. This, however, still included many participants with very low dairy intakes who are nonvegetarians and some lacto-ovo-vegetarians (use dairy and/or eggs >1/mo).
The proportional hazards assumption was evaluated using Schoenfeld residuals, log(-log) plots, and attained-age interaction terms; no statistically significant interactions were found. Residual methods were used to evaluate possible outliers and influential data points (16); no data points required removal. Missing values were imputed using multiple imputation, at the level of individual dietary questions, for the small amount of missing data (3–10%, mean 5% for different questions) in the dietary questionnaire variables and for most covariates; a guided multiple imputation approach was used where possible (17), as we have evidence that many of the missing dietary data are not true zeroes (18). For the analyses distinguishing advanced cancers, competing risk proportional hazards models were employed (19). Calibrated analyses used well-described regression calibration methods (20), with calibration substudy repeated 24-h dietary recalls being the reference values (see Supplemental Document). To check model fit for dairy (Figure 1), we used restricted cubic splines (RMS software) with 5 user-supplied knots. Between knots, curvilinear multivariable regressions independently describe the dairy–prostate cancer associations. Analyses were performed using R version 2.13.1, the Hmisc (21) package for imputation, and RMS (22) for competing risk and spline analyses.
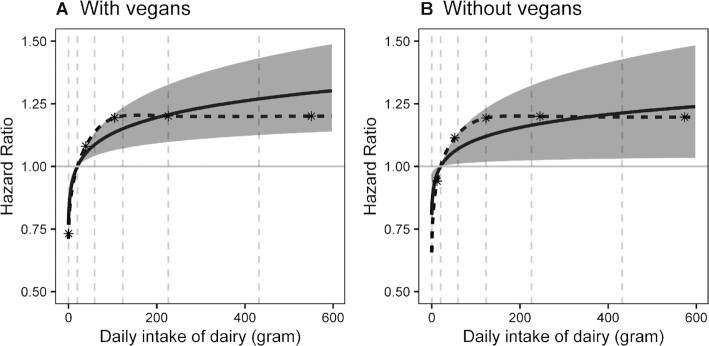
FIGURE 1.
Restricted cubic splined and unsplined multivariable-adjusted associations between dairy consumption (g/d) and risk of prostate cancer (95% confidence bands for unsplined analysis). (A) With vegans included (N = 28,737). (B) With vegans excluded (N = 26,436). Reference value in parts A and B is the 10th percentile of intake of the population without vegans. Cox proportional hazards regressions. The calculation of hazards compares 430 g/d with 20.2 g/d total dairy intakes (90th compared to 10th percentiles of dairy users). Adjusted for age (attained age as time variable), race (black/nonblack), education (up to high school graduate, trade school/some college/associate degree, bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, >60 min/wk), family history of prostate cancer (yes, no), history of benign prostatic hyperplasia (yes, no), prostate cancer screening, treated for diabetes mellitus within the past year (yes, no), height, BMI (in kg/m2; <25, 25–29.9, 30–34.9, ≥35), nondairy and supplemental calcium, and dietary energy (kcal). Also included are red meat, soy, cooked tomatoes, nuts and seeds, and legumes (no soy), measured in grams, as energy-adjusted and log-transformed continuous variables. Spline and unsplined models differ (likelihood ratio test of quadratic and cubic spline terms) using likelihood ratio tests: P = 0.78 (with vegans); P = 0.83 (without vegans). Interrupted lines indicate the 10th, 30th, 50th, 70th, and 90th percentiles of dairy intake in each plot. The asterisks show the positions of spline knots, with these positioned at percentiles 5.0, 27.5, 50.0, 72.5, and 95.0 of intake calculated for population of each plot.
Results
During an average follow-up period of 7.8 y (range: 0.1–11.9 y and 224,130 person-years) among the 28,737 male study participants included in this analysis, there were 1254 total cases of prostate cancer (190 advanced, 1043 nonadvanced, 21 uncategorized), of which 328 were among black participants (41 advanced, 283 nonadvanced, 4 uncategorized). The crude incidence rate of prostate cancer for all participants was 559 cases/100,000 person-years.
Baseline characteristics according to zero intake compared with highest quartile of dairy intake and extreme quintiles of calcium intake are shown in Table 1. Those with the highest intakes of dairy products tended to have a higher BMI and were less likely to be black and to exercise but were somewhat more likely to have screened for prostate cancer; they tended to have higher intakes of energy and most animal-derived nutrients and foods, as well as lower intakes of most mainly vegetable-derived nutrients and foods. Those with the highest intakes of calcium tended to be older, were less likely to be black, and were more likely to have higher educational attainment, to exercise regularly, to have had a family history of prostate cancer, to have undergone recent prostate cancer screening, or to have had benign prostatic hypertrophy; they tended to have higher intakes of energy, isoflavones, α-tocopherol equivalents, vitamin D, selenium, lycopene, and soy but lower intakes of red meat and poultry.
TABLE 1.
Baseline characteristics among 28,737 male Adventist Health Study–2 participants according to extreme quantiles of dairy and calcium intake1
| Dairy | Calcium | ||||
|---|---|---|---|---|---|
| Characteristic | Zero | Fourth quartile | First quintile | Fifth quintile | % Missing2 |
| General demographics | |||||
| Participants | 2306 (8.0) | 6607 (23.0) | 5748 (20.0) | 5747 (20.0) | — |
| Age at baseline, y | 58.54 ± 13.81 | 58.05 ± 14.23 | 56.09 ± 13.53 | 61.73 ± 13.70 | 0.0 |
| Black | 396 (17.2) | 838 (12.7) | 2037 (35.4) | 680 (11.8) | 0.47 |
| BMI, kg/m2 | 23.96 ± 4.04 | 27.63 ± 4.79 | 27.02 ± 5.03 | 26.46 ± 4.50 | 2.4 |
| Height, in. | 69.78 ± 3.06 | 70.08 ± 2.99 | 69.81 ± 3.17 | 69.87 ± 3.00 | 1.8 |
| Educational level | 1.1 | ||||
| High school or below | 412 (17.9) | 1323 (20.0) | 1415 (24.6) | 1015 (17.7) | |
| Some college | 740 (32.1) | 2121 (32.1) | 2027 (35.3) | 1816 (31.6) | |
| Bachelor degree or above | 1154 (50.0) | 3163 (47.9) | 2306 (40.1) | 2916 (50.7) | |
| Health and lifestyle | |||||
| Exercise | 4.1 | ||||
| None | 306 (13.3) | 1278 (19.3) | 1276 (22.2) | 956 (16.6) | |
| Up to 60 min/wk | 746 (32.4) | 2312 (35.0) | 2213 (38.5) | 1747 (30.4) | |
| More than 60 min/wk | 1254 (54.4) | 3017 (45.7) | 2259 (39.3) | 3044 (53.0) | |
| Diabetes mellitus, current | 68 (2.9) | 503 (7.6) | 315 (5.5) | 375 (6.5) | 0.17 |
| Family history of prostate cancer, yes | 201 (8.7) | 651 (9.9) | 490 (8.5) | 602 (10.5) | 0.17 |
| Prostate cancer screening, yes | 0.66 | ||||
| 0–2 y | 907 (39.3) | 3202 (48.5) | 2359 (41.0) | 3319 (57.8) | |
| 3–4 y | 132 (5.7) | 378 (5.7) | 296 (5.1) | 354 (6.2) | |
| ≥5 y | 145 (6.3) | 275 (4.2) | 308 (5.4) | 263 (4.6) | |
| Never | 1122 (48.7) | 2752 (41.7) | 2785 (48.5) | 1811 (31.5) | |
| BPH, yes | 414 (18.0) | 1170 (17.7) | 751 (13.1) | 1365 (23.8) | 0.18 |
| Nutrient intake | |||||
| Energy, kcal/d | 1930.42 ± 724.24 | 2005.06 ± 682.85 | 1975.36 ± 969.12 | 2050.34 ± 676.87 | |
| Total fat, g/d | 58.12 ± 17.85 | 72.59 ± 16.29 | 65.54 ± 19.91 | 68.93 ± 16.75 | |
| Saturated fat, g/d | 9.17 ± 2.81 | 22.14 ± 6.46 | 16.00 ± 6.56 | 17.11 ± 6.43 | |
| Total isoflavone, mg/d | 33.31 ± 27.97 | 8.93 ± 14.17 | 9.08 ± 11.23 | 25.63 ± 31.02 | |
| α-Tocopherol equivalents, mg/d | 112.23 ± 192.27 | 146.60 ± 225.37 | 58.34 ± 130.39 | 292.42 ± 297.45 | |
| Fiber, g/d | 44.74 ± 8.88 | 26.49 ± 7.85 | 29.09 ± 11.00 | 33.50 ± 9.84 | |
| Vitamin D intake, mg/d | 6.68 ± 19.83 | 11.95 ± 21.88 | 3.56 ± 4.19 | 18.12 ± 36.01 | |
| Selenium, mg/d | 132.87 ± 93.03 | 139.27 ± 78.22 | 99.30 ± 52.18 | 183.11 ± 112.38 | |
| α-Linolenic acid, g/d | 1.74 ± 0.77 | 1.57 ± 0.51 | 1.52 ± 0.61 | 1.76 ± 0.66 | |
| Lycopene, mg/d | 6050.84 ± 6307.95 | 5398.15 ± 4718.79 | 4864.27 ± 4957.57 | 6024.02 ± 5609.95 | |
| Calcium, mg/d | 999.50 ± 451.60 | 1321.32 ± 505.78 | 582.46 ± 91.08 | 1860.58 ± 420.94 | |
| Food intake | |||||
| Raw tomato, g/d | 57.85 ± 58.12 | 40.38 ± 43.02 | 39.75 ± 45.55 | 48.34 ± 48.08 | |
| Cooked tomato, g/d | 69.38 ± 81.58 | 59.49 ± 63.38 | 54.78 ± 66.99 | 66.16 ± 71.15 | |
| Total soy, g/d | 207.54 ± 190.36 | 81.43 ± 113.41 | 68.23 ± 80.50 | 201.14 ± 237.48 | |
| Total fruit, g/d | 436.93 ± 250.40 | 225.51 ± 163.25 | 281.18 ± 240.32 | 305.80 ± 205.84 | |
| Cruciferous vegetables, g/d | 39.09 ± 39.76 | 19.99 ± 21.00 | 23.57 ± 23.85 | 27.72 ± 29.00 | |
| Other vegetables, g/d | 128.53 ± 127.95 | 76.59 ± 58.97 | 85.27 ± 81.25 | 94.36 ± 86.40 | |
| Nuts/seeds, g/d | 37.48 ± 26.29 | 18.68 ± 16.47 | 22.40 ± 23.13 | 26.28 ± 21.45 | |
| Legumes, g/d | 64.54 ± 53.47 | 39.87 ± 35.43 | 44.42 ± 44.84 | 48.75 ± 42.57 | |
| Red meat, g/d | 0.00 | 22.21 ± 33.75 | 21.81 ± 40.10 | 11.53 ± 25.77 | |
| Poultry, g/d | 0.00 ± 0.15 | 12.86 ± 18.88 | 12.83 ± 22.35 | 8.17 ± 16.18 | |
| Fish, g/d | 0.10 ± 3.33 | 10.62 ± 15.19 | 10.69 ± 19.05 | 9.48 ± 18.68 | |
| Total dairy, g/d | 0.00 | 448.08 ± 224.95 | 77.81 ± 70.37 | 273.56 ± 307.98 | |
Values are presented as number (%) or mean ± SD. All differences across quintiles of calcium or zeroes and quartiles of dairy have a P value <0.001 except the following: calcium quintiles: height (P = 0.025), diabetes mellitus (P = 0.086), and family history (P = 0.0056); dairy quintiles: family history of prostate cancer (P = 0.60) and BPH (P = 0.36). BPH, benign prostatic hypertrophy.
Percentage of data originally missing before imputation. Dietary data, often coming from many questionnaire items, cannot be summarized in this way. But overall, the mean percent missing for these dietary questions was 3.2% (range: 0.0–14.4%) across different questions.
Table 2 presents results of multivariable-adjusted proportional hazards regression models for prostate cancer where dairy variables are the exposures of interest, these for total prostate cancers (advanced and nonadvanced) and all male participants (black and nonblack). Proportional hazards regression results for dairy products are adjusted for nondairy calcium. Considering total dairy intake, a log-transformed continuous analysis expressed as g/d found that prostate cancer rates were higher among participants at 90th percentile (430 g/d) dairy intakes compared with those at the midpoint of the lowest quintile (20.2 g/d) of dairy consumers (HR: 1.27; 95% CI: 1.12, 1.43; P = 0.00010); a similar analysis expressing dairy intakes as 351 and 35.7 kcal/d found an HR of 1.22 (95% CI: 1.10, 1.34; P < 0.00015). Examining results (Supplemental Table 1) where dairy variables are categorical (zero intakes and 5 quintiles of dairy consumers) shows that the major rise in risk of prostate cancer occurs between the zero intake category and second quintile of the dairy users. Thereafter, this higher risk is maintained with further increases being relatively small. The log-transformed continuous model also displays this nonlinearity, and its fit to the data was very good, as shown by comparison with restricted cubic spline results in Figure 1. The deviation of the more accurate spline from the simple, more easily interpreted, log-transformed linear model is minor and readily explained by chance (P = 0.78 with vegans included and 0.83 when they are excluded).
TABLE 2.
Relative hazard of total incident prostate cancer comparing risk at higher to lower dairy intakes1
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Dairy model | HR | 95% CI | P value | HR | 95% CI | P value |
| Total dairy 430 g/d compared with 20.2 g/d (FFQ values) | ||||||
| All participants | 1.26 | 1.13, 1.40 | 4.11e-5 | 1.27 | 1.12, 1.43 | 0.00010 |
| Excluding vegans2 | 1.18 | 1.01, 1.38 | 0.033 | 1.22 | 1.03,1.43 | 0.018 |
| Dairy energy 351 kcal/d compared with 35.7 kcal/d (FFQ values) | ||||||
| All participants | 1.21 | 1.10, 1.33 | 4.80e-5 | 1.22 | 1.10, 1.34 | 0.00015 |
| Excluding vegans2 | 1.16 | 1.00, 1.35 | 0.055 | 1.19 | 1.01, 1.40 | 0.032 |
| Substituting dairy for isoflavone intakes substituted 107 g/d dairy for 9.72 mg/d isoflavones (medians of FFQ values) | ||||||
| All participants | 1.37 | 1.15, 1.62 | 0.00046 | 1.39 | 1.15, 1.68 | 0.00080 |
| Substitution, excluding vegans2 | 1.26 | 0.99, 1.61 | 0.060 | 1.31 | 1.02, 1.70 | 0.037 |
| Calibrated analyses: total dairy 291 compared with 11.0 g/d (24-h recall values) | ||||||
| All participants | 1.75 | 1.32, 2.32 | 0.00059 | |||
| Calibrated analyses: dairy energy 305 compared with 27.0 kcal/d (24-h recall values) | ||||||
| All participants | 1.57 | 1.24, 1.99 | 0.00072 | |||
From Cox proportional hazard regression models. Model 1: adjusting for age (attained age as time variable), race (black/nonblack), and nondairy calcium. Substitution models require the addition of isoflavones. Model 2: adjusting for age (attained age as time variable); race (black/nonblack); education (up to high school graduate, trade school/some college/associate degree, bachelor degree or higher); moderate or vigorous exercise (none, ≤60 min/wk, >60 min/wk); family history of prostate cancer (yes, no); history of benign prostatic hyperplasia (yes, no); prostate cancer screening; treated for diabetes mellitus within the past year (yes, no); height; BMI (in kg/m2; <25, 25–29.9, 30–34.9, ≥35); dietary energy (kcal); red meat, isoflavones, cooked tomatoes, nuts and seeds, and legumes (nonsoy); and nondairy calcium. All variables are energy adjusted. Dietary variables are log-transformed. P values result from Wald tests. In nonsubstitution models, the midpoint of the upper quintile is compared with the midpoint of the lowest quintile (reference value) of the nonzero dairy intake populations. Substitution models substitute median dairy intake for median isoflavone intake, with these values again taken from dairy and soy consumers, respectively. N = 28,737 when vegans are included and 26,436 when they are excluded.
When vegans are excluded, for comparability, the same g/d (kcal/d) are used in the HR calculations as for analyses that included the vegans.
These dairy model results were adjusted only for nondairy calcium; hence, a dairy effect includes any influence of dairy calcium. Analyses adjusted for total calcium explore possible effects of dairy components aside from calcium content. Results, then, for dairy are slightly stronger, with an HR of 1.30 (95% CI: 1.15, 1.48; P = 4.78e-5) for total dairy (g/d) and 1.25 (95% CI: 1.05, 1.49; P = 0.010) for dairy energy (kcal/d).
Calibrated multivariable associations between total dairy and prostate cancer risk, thus partially correcting effects of measurement errors, were examined using the same covariates as models in the tables. These analyses as usual compare 90th to 10th percentiles of dairy users, now on the dietary recall scale by definition. Again, associations indicated greater risk of prostate cancer in the high (291 g/d) compared with low (11.0 g/d) dairy consumers (HR: 1.75; 95% CI: 1.32, 2.32; P = 0.00059) or in the corresponding kcal/d comparison (305 kcal/d compared with 27.0 kcal/d; HR: 1.57; 95% CI: 1.24, 1.99; P = 0.00072), these being stronger apparent effects, as expected, than those from uncalibrated analyses.
HRs in which calcium variables are the exposure of interest (Table 3) compare the risk of prostate cancer at the 90th to 10th percentiles (e.g., the midpoints of extreme quintiles) of calcium intakes. There was little evidence that those with higher and lower total calcium intakes had different rates of incident prostate cancer (HR: 1.08; 95% CI: 0.92, 1.28; P = 0.34). This was also the case for supplemental calcium and for dietary calcium, when analyzed separately and not adjusted for dairy. Comparing prostate cancer rates for those with the higher (905 mg/d) and lower (349 mg/d) intakes of nondairy dietary calcium also found a relatively small difference in risk of prostate cancer readily compatible with chance (HR: 1.16; 95% CI: 0.94, 1.44; P = 0.17). Similar analyses in which calcium is divided to quintiles are found in Supplemental Table 2. Results are consistent with those from the continuous models.
TABLE 3.
HRs of total incident prostate cancer at varying intakes of calcium variables in all participants (N = 28,737), comparing 90th with 10th percentiles of intake1
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Calcium model | HR | 95% CI | P value | HR | 95% CI | P value |
| Total calcium: 1739 compared with 601 mg/d (FFQ values) | 1.09 | 0.94, 1.27 | 0.22 | 1.08 | 0.92, 1.28 | 0.34 |
| Supplemental calcium1,2: 1000 compared with 0 mg/d (FFQ values) | 1.06 | 0.96, 1.18 | 0.35 | 1.07 | 0.91, 1.26 | 0.40 |
| Dietary calcium1,3: 1216 compared with 560 mg/d (FFQ values) | 1.17 | 1.02, 1.34 | 0.030 | 1.14 | 0.98,1.31 | 0.082 |
| Nondairy calcium1,4: 905 compared with 359 mg/d (FFQ values) | 1.03 | 0.93, 1.14 | 0.61 | 1.16 | 0.94, 1.44 | 0.17 |
From Cox proportional hazard regression models. Model 1: adjusting for age (attained age as time variable) and race (black/nonblack). Model 2: adjusting for age (attained age as time variable), race (black/nonblack), education (up to high school graduate, trade school/some college/associate degree, bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, >60 min/wk), family history of prostate cancer (yes, no), history of benign prostatic hyperplasia (yes, no), prostate cancer screening, treated for diabetes mellitus within the past year (yes, no), height, BMI (in kg/m2; <25, 25–29.9, 30–34.9, ≥35), height, dietary energy (kcal), and isoflavones, α-tocopherol equivalents, fiber, α-linolenic acid, and lycopene. All variables are energy adjusted. Dietary variables are log-transformed. Calcium models are not adjusted for dairy. P values result from Wald tests.
Adjusted for dietary calcium.
Adjusted for supplemental calcium.
Adjusted dairy and supplemental calcium.
The sensitivity analyses that excluded 2301 vegans, whether dairy was measured as g/d or kcal/d, still show HRs with 95% CIs that clearly exclude the null and are in fact very similar to those when vegans were included. With vegans excluded, the estimated HR on the continuous curve for other participants who have very close to zero dairy intake was 0.82 (95% CI: 0.70, 0.97) when compared with participants at the reference intake (the 10th percentile of dairy users). When vegans were included, the measured HR at their zero intake was almost identical at 0.79 (95% CI: 0.70, 0.88). Figure 1B excludes vegans displaying a curve across the range of dairy intakes that is very similar to that of Figure 1A when vegans are included. Thus, vegans do not appear to be unusual in terms of their fit to this model and could also serve as an alternative reference category (zero intake). Then, participants who consumed total dairy at the 90th percentile of dairy users (g/d) had a much higher risk of prostate cancer than nondairy users (HR: 1.62; 95% CI: 1.26, 2.05, P = 0.00010).
Table 4 presents results for dairy variables of multivariable-adjusted competing risks proportional hazards regression models stratified by participant ethnicity (nonblack and black) and by the advanced/nonadvanced status of the prostate cancer. Generally, associations of similar magnitude and statistical significance to those reported above were seen for total dairy intake in advanced and nonadvanced cancers. Analyses confined to black participants, in which power was less, found HRs that also indicated a positive association with dairy consumption. Again, prostate cancer rates were compared between the 90th and the 10th percentiles of intake in all dairy users. The HR for risk of advanced prostate cancer (black and nonblack participants combined) was 1.38 (95% CI: 1.02, 1.88; P = 0.039) when dairy was measured as g/d and 1.26 (95% CI: 0.98, 1.63; P = 0.077) when dairy was measured as kcal/d. The equivalent HRs for risk of advanced prostate cancers in nonblack participants were 1.61 (95% CI: 1.12, 2.31; P = 0.0098, dairy as g/d) and 1.43 (95% CI: 1.05, 1.94; P = 0.024, dairy as kcal/d). For black participants, numbers were too small to support multivariable analysis investigating risk of advanced cases. However, the usual dairy intake comparison, in black participants, again demonstrated a higher risk of all prostate cancers among the high consumers when compared with low dairy consumers (HR: 1.24; 95% CI: 0.98, 1.58; P = 0.078, dairy as g/d; HR: 1.21; 95% CI: 0.99, 1.48; P = 0.065, dairy as kcal/d). A similar comparison for nonadvanced prostate cancers in blacks found HRs of 1.34 (95% CI: 1.03, 1.74; P = 0.031, dairy as g/d) and 1.29 (95% CI: 1.03, 1.62; P = 0.024, dairy as kcal/d).
TABLE 4.
Relative hazards of incident prostate cancer, by tumor classification and race (comparing 90th with 10th percentiles of all dairy users or of calcium variables, respectively)1
| HR (95% CI; P value) | |||
|---|---|---|---|
| Characteristic | All prostate cancers | Advanced cases2 | Nonadvanced cases3 |
| All participants (N = 28,737; all cases = 1254, advanced cases = 190, nonadvanced cases = 1043; 21 uncategorized) | |||
| Total dairy,4,5,6 g/d | 1.27 (1.12. 1.43; 1.0e-4) | 1.38 (1.02, 1.88; 0.039) | 1.27 (1.11, 1.45; 4.0e-4) |
| Dairy energy,4,5,6 kcal/d | 1.22 (1.10, 1.34; 1.5e-4) | 1.26 (0.98, 1.63; 0.077) | 1.22 (1.09, 1.37; 4.0e-4) |
| Total calcium7 | 1.08 (0.92, 1.28; 0.34) | 1.18 (0.78, 1.79; 0.44) | 1.08 (0.91, 1.30; 0.35) |
| Supplemental calcium7,8 | 1.07 (0.91, 1.26; 0.40) | 1.29 (0.86, 1.93; 0.21) | 1.06 (0.89, 1.26; 0.50) |
| Dietary calcium4,7,8 | 1.14 (0.98, 1.31; 0.08) | 1.03 (0.71, 1.49; 0.89) | 1.17 (1.00, 1.36; 0.055) |
| Nondairy calcium4,7,9 | 1.16 (0.94, 1.44; 0.17) | 1.05 (0.53, 2.05; 0.89) | 0.98 (0.74, 1.31; 0.91) |
| Black participants (N = 6389; all cases = 328, advanced cases = 41,10 nonadvanced cases = 283, 4 uncategorized) | |||
| Total dairy,4,5,6 g/d | 1.24 (0.98, 1.58; 0.078) | 10 | 1.34 (1.03, 1.74; 0.031) |
| Dairy energy,4,5,6 kcal/d | 1.21 (0.99, 1.48; 0.065) | 10 | 1.29 (1.03, 1.62; 0.024) |
| Total calcium7 | 1.01 (0.73, 1.40; 0.94) | 10 | 1.05 (0.74, 1.49; 0.79) |
| Supplemental calcium7,8 | 1.05 (0.77, 1.42; 0.77) | 10 | 1.04 (0.75, 1.46; 0.80) |
| Dietary calcium4,7,8 | 1.14 (0.84, 1.54; 0.41) | 10 | 1.25 (0.90, 1.73; 0.19) |
| Nondairy calcium4,7,9 | 0.74 (0.45, 1.24; 0.26) | 10 | 0.70 (0.40, 1.23; 0.21) |
| Nonblack participants (N = 22,348; all cases = 926, advanced cases = 149, nonadvanced cases = 760) | |||
| Total dairy,4,5,6 g/d | 1.26 (1.10, 1.45; 9.3e-4) | 1.61 (1.12, 2.31; 0.0098) | 1.24 (1.06, 1.44; 0.0066) |
| Dairy energy,4,5,6 kcal/d | 1.21 (1.07, 1.35; 0.0015) | 1.43 (1.05, 1.94; 0.024) | 1.19 (1.05, 1.35; 0.0072) |
| Total calcium7 | 1.09 (0.91, 1.31; 0.36) | 1.24 (0.79, 1.97; 0.35) | 1.09 (0.89, 1.34; 0.41) |
| Supplemental calcium7,8 | 1.06 (0.89, 1.27; 0.53) | 1.28 (0.82, 2.01; 0.28) | 1.05 (0.86, 1.28; 0.62) |
| Dietary calcium4,7,8 | 1.13 (0.96, 1.33; 0.15) | 1.12 (0.74, 1.68; 0.59) | 1.14 (0.95, 1.36; 0.16) |
| Nondairy calcium4,7,9 | 1.09 (0.81, 1.47; 0.58) | 1.12 (0.50, 2.40; 0.77) | 1.11 (0.80, 1.55; 0.54) |
Results are those from competing risks Cox proportional hazards regressions. Adjusting for age (attained age as time variable), race (black/nonblack), education (up to high school graduate, trade school/some college/associate degree, bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, >60 min/wk), family history of prostate cancer (yes, no), history of benign prostatic hyperplasia (yes, no), prostate cancer screening, treated for diabetes mellitus within the past year (yes, no), height, BMI (in kg/m2; <24.9, 25–29.9, 30–34.9, ≥35), and dietary energy (kcal). Statistical testing used Wald tests.
Regional or metastatic spread or a Gleason score of 4 + 3 or greater.
Localized cases with a Gleason score of 3 + 4 or less.
Additionally adjusted for supplemental calcium.
Additionally adjusted nondairy calcium.
Dairy models additionally adjusted for red meat, soy, cooked tomatoes, nuts and seeds, and legumes (no soy), measured in grams, as energy-adjusted and log-transformed continuous variables.
Additionally adjusted for total isoflavones, α-tocopherol equivalents, fiber, α-linolenic acid, and lycopene as energy-adjusted and log-transformed continuous variables. Calcium models not adjusted for dairy.
Additionally adjusted for dietary calcium.
Additionally adjusted for dairy calcium.
Too few cases for multivariate analyses in black participants with advanced cancers.
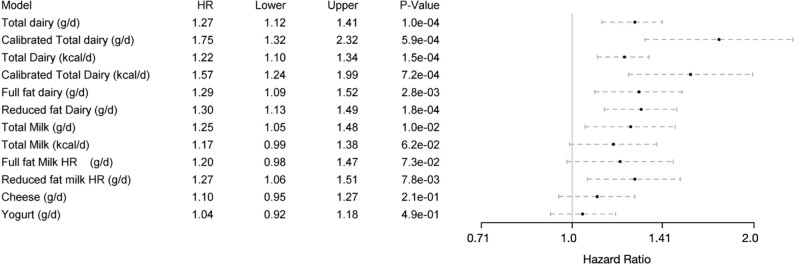
Figure 2 portrays results graphically for some results reported earlier but, in addition, multivariable-adjusted regressions upon regular-fat and low-fat dairy products, as well as categories of dairy foods adjusted for each other. For dairy foods, as usual, we compared 90th with 10th percentiles of intakes of these foods among dairy users. A comparison of higher compared with lower consumers of total milk (345 compared with 6.19 g/d) found a greater risk of prostate cancer with high total milk consumption (HR: 1.25; 95% CI: 1.05, 1.48; P = 0.010) or for the corresponding kcal/d comparison (163 compared with 8.58 kcal/d; HR: 1.17; 95% CI: 0.99, 1.38; P = 0.062), both a little lower than corresponding results for total dairy. Both reduced-fat and full-fat dairy and milk showed results similar to those for total dairy and milk, respectively, with the 95% CIs usually excluding the null. We did not find strong evidence that either cheese or yogurt influenced the risk of prostate cancer. For cheese, comparing 46.1 g/d with 2.03 g/d produced an HR of 1.10 (95% CI: 0.95, 1.27; P = 0.21), and for yogurt, comparing 111 g/d with 1.91 g/d produced an HR of 1.04 (95% CI: 0.92, 1.18; P = 0.49).
FIGURE 2.
Associations of total dairy (calibrated and uncalibrated) and subcategories of dairy foods (uncalibrated) with risk of prostate cancer (N = 28,737 participants and 1254 prostate cancer cases in all analyses). Adjusted for age (attained age as time variable), race (black/nonblack), education (up to high school graduate, trade school/some college/associate degree, bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, >60 min/wk), family history of prostate cancer (yes, no), history of benign prostatic hyperplasia (yes, no), prostate cancer screening, treated for diabetes mellitus within the past year (yes, no), BMI (in kg/m2; <25, 25–30, >30), nondairy and supplemental calcium, and dietary energy (kcal). Also included are red meat, soy, cooked tomatoes, nuts and seeds, and legumes (no soy), measured in grams, as energy-adjusted and log-transformed continuous variables. When exposure variables were milk, cheese, yogurt, they are all included in the models, and hence are adjusted for each other. Statistical testing used Wald tests. Results are presented as Cox proportional hazards regressions and 95% CIs. “Upper” and “lower” columns refer to the upper and lower boundaries of the 95% CI for the HR. Hazard ratios compare risk in higher compared with lower intakes. Comparison points for the hazards are always the 90th and 10th percentiles of values for that dairy product among dairy users, except that for comparability, comparison points for total, full-fat, and reduced-fat dairy and milk always use comparison values for total dairy or milk, respectively. Comparison values on the FFQ scale are as follows: total dairy, full-fat dairy, and reduced-fat dairy, comparison 430 and 20.2 g/d; total dairy, full-fat dairy, and reduced-fat dairy energy, comparison 351 and 35.7 kcal/d. For total milk, full-fat milk, and reduced-fat milk, the comparisons are 345 and 6.19 g/d; the total milk energy comparison is 163 and 8.58 kcal/d. Comparisons are 46.1 and 2.03 g/d for cheese and 111 and 1.91 g/d for yogurt. The comparisons are 291 and 11.0 g/d for calibrated total dairy on the dietary recall scale and 305 and 27.0 kcal/d calibrated dairy energy on the dietary recall scale.
Supplemental Table 3 compares the main dairy model results of these analyses as reported above to those when subjects are restricted to the 78% who had no missing data originally for any of the 15 dairy variables. Results are very similar, perhaps slightly stronger in those with no missing data originally (better educated, younger, fewer black patients) (18). Supplemental Table 4 provides further details of the associations between soy foods and risk of prostate cancer in these data. There is evidence of some confounding, such that adding nondietary and dietary covariates to the model markedly attenuates the initial relatively unadjusted soy association, but leaves the initial dairy association largely intact.
Discussion
Comparing contrasting levels of dairy intake, uncalibrated results find an ∼25% higher risk of prostate cancer with higher consumption of dairy products, comparing the midpoints of the upper and lower quintiles of dairy consumers (430 to 20.2 g/d). After exclusion of vegans, very similar results were found. Compared with the moderate number of zero dairy consumers in this cohort as the reference, high consumers had ∼60% increased risk. These associations were nonlinear on an untransformed scale, with increments in risk being much greater in the lower range of dairy intakes, which were confirmed by spline regressions. Theoretically less biased calibrated analyses found yet much stronger regression slopes, demonstrating the expected bias toward the null (HR: 1.0) of uncalibrated analyses. Low- or regular-fat milk, rather than yogurt or cheese, seemed to provide most of the dairy signal. In contrast, our data provided little evidence of an association between calcium intake and incident prostate cancer. We cannot preclude the possibility that dairy calcium differs qualitatively from other dietary calcium, although our data do not support that.
Several large, well-known cohort studies have explored such associations with risk of prostate cancer, with variable results. For example, the Physicians’ Health Study found that both dairy and calcium intakes associated positively with risk, although these were not mutually adjusted (23). The Prostate, Lung, Colorectal and Ovarian Cancer Screening trial cohort did not find convincing evidence of any association with dairy products (24). The Multiethnic Cohort Study also did not detect any association with calcium intake but did find an increased risk with low-fat or nonfat but not full-fat milk (25). A Japanese cohort (26) and the European Prospective Investigation into Cancer and Nutrition (EPIC) (27) both associated dairy intake with a statistically significant increased risk of prostate cancer. The Aune et al. (3) meta-analysis reported an HR of 1.07 (95% CI: 1.02, 1.12) over a 400-g/d span of dairy intake. This is approximately the same-sized range over which we compared intakes but observed much greater HRs (in the 1.2–1.3 range for uncalibrated analyses). We speculate that meta-analysis populations may provide relatively fewer participants in the low-intake part of the curve associated with a steep increase in risk (see Figure 1) and largely reflect participants in the flatter higher dairy intake ranges. A more recent meta-analysis (28) found a great deal of heterogeneity and was inconclusive. Thus, existing data suggest an adverse effect of dairy consumption but are not convincing. AHS-2 results are consistent with those studies finding a positive association.
One interpretation is that dairy foods (or some closely associated unknown risk factor) are causally related to risk of prostate cancer. However, our (nonsubstitution) regression models should be interpreted as the effect of dairy substituting for similar calories from an average mix of foods not specified in the model. Thus another, perhaps less likely, hypothesis is that the foods dairy substitutes for may be protective, and their absence or reduction increases risk. Foods not included in our covariate list, however, are not currently suspected in any causal role. Analyses substituting dairy for soy did not suggest that soy was such an explanatory food.
We conjecture that our previous report from an older Adventist cohort (29) of a protective association with soy milk consumption was largely due to confounding, in that soy consumers typically avoided dairy products and these were only partially adjusted (full-fat milk only). The multivariable associations between soy food intake and risk of prostate cancer were not close to statistically significant in the current AHS-2 analyses.
Our results may provide some clarification of evidence by separating calcium and dairy intakes. Dairy provides a large proportion of calcium intake of many diets. The AHS-2 population contains a substantial number of vegans and other low-dairy consumers who obtain much of their calcium from other sources, thus avoiding this collinearity. Our results add important weight to the evidence associating dairy products, rather than nondairy calcium, as a possible risk factor for prostate cancer.
This raises the question of which dairy foods or noncalcium component of dairy products might be causally related to prostate cancer and by what possible mechanism(s). Our analyses suggest a possible role for dairy milk, but associations with cheese and yogurt were weaker and not statistically significant. Because dairy foods all begin with the same principal ingredient (i.e., milk), possibly some active principle has been largely destroyed by the processing of cheese and yogurt. Independent associations with dairy macronutrients (results not shown) could not be evaluated, as these were highly correlated (r >0.95) with each other. However, there was little difference in HRs between low-fat and full-fat dairy products or milk, suggesting that any effect is not associated with dairy fat.
Insulin-like growth factors and binding proteins may represent a possible causal mechanism (30) in that higher concentrations of insulin-like growth factor 1 (IGF-1) have been linked with an increased risk of prostate cancer (31, 32). Furthermore, higher dairy protein and dairy calcium intakes have been associated with higher concentrations of IGF-1 in the EPIC study (33–35).
The inclusion of the 8.0% of this population who are vegan, and thus consume no dairy, provides a greater range of dairy consumption but may introduce a concern that the vegans differ in other ways that confound these results, despite the covariate adjustments (e.g., for prostate cancer screening). However, nonvegetarians who happened to be essentially nondairy consumers were not rare, and analyses among the AHS-2 population that excluded vegans produced very similar results.
Very similar reported results from females of this cohort relate dairy milk consumption to risk of breast cancer (36, 37), similar in both the nonlinear form of the association and the approximate magnitude of risk. It seems possible that the same biological mechanisms are at work. The statistically significant but opposite-sense associations from AHS-2, suggesting protection from colorectal cancers associated with dairy consumption, are noteworthy (38) and consistent with a large body of evidence (39), thus illustrating the possible complex effects of dairy on different endpoints.
Strengths of this study include the diverse participants with respect to age, race, geographic location, and socioeconomic status; the absent or low use of tobacco and alcohol; the wide range of dairy intake, including many nonconsumers and low consumers; the high validity of dairy intake measurements; the substantial covariate adjustment; the restricted cubic spline analyses checking the form of the association; the calibrated results partially adjusting for measurement errors; and the guided multiple imputation of missing values. Limitations include limited power for analyses among black participants and for advanced prostate cancers, the single assessment of diet at baseline, and the possibility of unknown and unmeasured confounders. There is no reason to believe that dietary associations with disease should differ in this population, but generalization to other populations, as usual, should be cautious.
In summary, these data from a population with a wide range of dairy and calcium exposure do not clearly support a connection between calcium intake and prostate cancer. However, they do suggest that risk of prostate cancer is causally associated with higher intake of dairy products or some unknown causal factor that is strongly associated with dairy intake.
Supplementary Material
Acknowledgments
We thank the AHS-2 participants, as well as Hanni Bennett, Sonja Hall, and Barbara Burton for their work with the baseline questionnaire data, the calibration substudy data, and data management. Cancer incidence data were provided by the Alabama State Cancer Registry, Alaska Cancer Registry, Arizona Cancer Registry, Arkansas Cancer Registry, California Cancer Registry, Colorado Cancer Registry, Connecticut Tumor Registry, District of Columbia Cancer Registry, Delaware Cancer Registry, Florida Cancer Data System, Georgia Department of Public Health, Hawaii Tumor Registry, Cancer Data Registry of Idaho, Illinois State Cancer Registry, Indiana State Cancer Registry, Iowa Cancer Registry, Kansas Cancer Registry, Kentucky Cancer Registry, Louisiana Tumor Registry, Maryland Cancer Registry, Massachusetts Cancer Registry, Michigan Cancer Surveillance System, Minnesota Cancer Surveillance System, Mississippi Cancer Registry, Missouri Cancer Registry and Research Center, Montana Central Tumor Registry, Nebraska Cancer Registry, Nevada Central Cancer Registry, New Hampshire State Cancer Registry, New Jersey State Cancer Registry, New Mexico Tumor Registry, New York State Cancer Registry, North Carolina Central Cancer Registry, North Dakota Statewide Cancer Registry, Cancer Data Registry of Ohio, Oklahoma Central Cancer Registry, Oregon State Cancer Registry, Pennsylvania Cancer Registry, Rhode Island Cancer Registry, South Carolina Cancer Registry, South Dakota Cancer Registry, Tennessee Cancer Registry, Texas Cancer Registry, Utah Cancer Registry (National Cancer Institute contract HHSN261201300071), Vermont Cancer Registry, Virginia Cancer Registry, Washington State Cancer Registry, West Virginia Cancer Registry, Wisconsin Cancer Reporting System, and Wyoming Cancer Surveillance Program.
The authors’ responsibilities were as follows—GEF and MJO: designed this research; GEF, SFK, LES, and KJ-S: conducted the research; ADM, LES, and JTU: analyzed and managed the data; MJO and GEF: wrote the paper; GEF: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Author disclosures: The authors report no conflicts of interest.
Notes
Supported by the National Cancer Institute at NIH (grant 1U01CA152939), World Cancer Research Fund (grant 2009/93), and Ardmore Institute of Health. The NIH, World Cancer Research Fund, and the Ardmore Institute of Health played no part in the design, implementation, analysis, and interpretation of this data.
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval and payment for associated costs.
Supplemental Figure 1 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHS-2, Adventist Health Study–2; EPIC, European Prospective Investigation into Cancer and Nutrition; IGF-1, insulin-like growth factor 1; WCRF, World Cancer Research Fund.
Contributor Information
Michael J Orlich, Center for Nutrition, Healthy Lifestyle and Disease Prevention, School of Public Health, Loma Linda University, Loma Linda, CA, USA; Department of Preventive Medicine, School of Medicine, Loma Linda University, Loma Linda, CA, USA.
Andrew D Mashchak, Adventist Health Study, Loma Linda University, Loma Linda, CA, USA.
Karen Jaceldo-Siegl, Center for Nutrition, Healthy Lifestyle and Disease Prevention, School of Public Health, Loma Linda University, Loma Linda, CA, USA; Adventist Health Study, Loma Linda University, Loma Linda, CA, USA.
Jason T Utt, Adventist Health Study, Loma Linda University, Loma Linda, CA, USA.
Synnove F Knutsen, Adventist Health Study, Loma Linda University, Loma Linda, CA, USA.
Lars E Sveen, Adventist Health Study, Loma Linda University, Loma Linda, CA, USA.
Gary E Fraser, Center for Nutrition, Healthy Lifestyle and Disease Prevention, School of Public Health, Loma Linda University, Loma Linda, CA, USA; Department of Preventive Medicine, School of Medicine, Loma Linda University, Loma Linda, CA, USA; Adventist Health Study, Loma Linda University, Loma Linda, CA, USA; Department of Medicine, School of Medicine, Loma Linda University, Loma Linda, CA, USA.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. [DOI] [PubMed] [Google Scholar]
- 2. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and prostate cancer. World Cancer Research Fund International . 2018. Available on-line at dietandcancerreport.org. Accessed: 28 April, 2022 [Google Scholar]
- 3. Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, Vatten LJ, Norat T. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101(1):87–117. [DOI] [PubMed] [Google Scholar]
- 4. Quann EE, Fulgoni VL, Auestad N. Consuming the daily recommended amounts of dairy products would reduce the prevalence of inadequate micronutrient intakes in the United States: diet modeling study based on NHANES 2007–2010. Nutr J. 2015;14(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orlich MJ, Jaceldo-Siegl K, Sabate J, Fan J, Singh PN, Fraser GE. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr. 2014;112(10):1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tantamango-Bartley Y, Knutsen SF, Knutsen R, Jacobsen BK, Fan J, Beeson WL, Sabate J, Hadley D, Jaceldo-Siegl K, Penniecook Jet al. Are strict vegetarians protected against prostate cancer?. Am J Clin Nutr. 2016;103(1):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orlich MJ, Fraser GE. Vegetarian diets in the Adventist Health Study 2: a review of initial published findings. Am J Clin Nutr. 2014;100(Suppl 1):353S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Cancer Institute, Surveillance, Epidemiology, and End Results Program . Cancer disparities—cancer stat facts [Internet] [accessed 2021 Sep 20]. Available from:https://seer.cancer.gov/statfacts/html/disparities.html.
- 9. Fraser GE, Cosgrove CM, Mashchak AD, Orlich MJ, Altekruse SF. Lower rates of cancer and all-cause mortality in an Adventist cohort compared with a US Census population. Cancer. 2020;126(5):1102–11. [DOI] [PubMed] [Google Scholar]
- 10. Ogunsanya ME, Jiang S, Thach AV, Bamgbade BA, Brown CM. Predictors of prostate cancer screening using Andersen's behavioral model of health services use. Urol Oncol. 2016;34(12):529.e9–e14. [DOI] [PubMed] [Google Scholar]
- 11. Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, Sabate J, Montgomery S, Haddad E, Preston-Martin Set al. Cohort profile: the Adventist Health Study–2 (AHS-2). Int J Epidemiol. 2008;37(2):260–5. [DOI] [PubMed] [Google Scholar]
- 12. US Centers for Medicare & Medicaid Services . 2014 ICD-10-CM and GEMs. [Internet]. 2014; [cited 2018 Mar 26]. Available from: https://www.cms.gov/medicare/coding/icd10/2014-icd-10cm- and-gems.html. [PubMed] [Google Scholar]
- 13. Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–71. [PubMed] [Google Scholar]
- 14. Jaceldo-Siegl K, Knutsen SF, Sabaté J, Beeson WL, Chan J, Herring RP, Butler TL, Haddad E, Bennett H, Montgomery Set al. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study–2 (AHS-2). Public Health Nutr. 2010;13(6):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaceldo-Siegl K, Fan J, Sabaté J, Knutsen SF, Haddad E, Beeson WL, Herring RP, Butler TL, Bennett H, Fraser GE. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study–2. Public Health Nutr. 2011;14(11):1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hebbali A. Tools for Building OLS Regression Models. The Comprehensive R Archive Network (CRAN) . Available on-line at https://cran.r-project.org/web/packages/olsrr/ index.html. Accessed: 28 April, 2022.
- 17. Fraser G, Yan R. Guided multiple imputation of missing data: using a subsample to strengthen the missing-at-random assumption. Epidemiology. 2007;18(2):246–52. [DOI] [PubMed] [Google Scholar]
- 18. Fraser GE, Yan R, Butler TL, Jaceldo-Siegl K, Beeson WL, Chan J. Missing data in a long food frequency questionnaire: are imputed zeroes correct?. Epidemiology. 2009;20(2):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xue X, Kim MY, Gaudet MM, Park Y, Heo M, Hollenbeck AR, Strickler HD, Gunter MJ. A comparison of the polytomatous logistic regression and joint Cox proportional hazards models for evaluating multiple disease subtypes in prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2013;22(2):275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65(4):1179S–86S. [DOI] [PubMed] [Google Scholar]
- 21. Harrell F. aregImpute: Multiple Imputation using Additive Regression, Bootstrapping, and Predictive Mean Matching. The Comprehensive R Archive Network (CRAN). Available on-line at https://www.rdocumentation.org/ packages/Hmisc/versions/4.6-0/topics/aregImpute. Accessed: 28 April, 2022.
- 22. Harrell FE Jr. Regression modeling strategies. CRAN. 5.1-2 ed. Nashville (TN): Vanderbilt University; 2018. [Google Scholar]
- 23. Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. Am J Clin Nutr. 2001;74(4):549–54. [DOI] [PubMed] [Google Scholar]
- 24. Preble I, Zhang Z, Kopp R, Garzotto M, Bobe G, Shannon J, Takata Y. Dairy product consumption and prostate cancer risk in the United States. Nutrients. 2019;11(7):1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park SY, Murphy SP, Wilkens LR, Stram DO, Henderson BE, Kolonel LN. Calcium, vitamin D, and dairy product intake and prostate cancer risk: the Multiethnic Cohort Study. Am J Epidemiol. 2007;166(11):1259–69. [DOI] [PubMed] [Google Scholar]
- 26. Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS; Japan Public Health Center-Based Prospective Study Group . Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol Biomarkers Prev. 2008;17(4):930–7. [DOI] [PubMed] [Google Scholar]
- 27. Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Tjonneland A, Johnsen NF, Overvad K, Linseisen J, Rohrmann Set al. Animal foods, protein, calcium and prostate cancer risk: the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2008;98(9):1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. López-Plaza B, Bermejo LM, Santurino C, Cavero-Redondo I, Álvarez-Bueno C, Gómez-Candela C. Milk and dairy product consumption and prostate cancer risk and mortality: an overview of systematic reviews and meta-analyses. Adv Nutr. 2019;10(Suppl 2):S212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States). Cancer Causes Control. 1998;9(6):553–7. [DOI] [PubMed] [Google Scholar]
- 30. Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12(2):84–9. [PubMed] [Google Scholar]
- 31. Travis RC, Appleby PN, Martin RM, Holly JMP, Albanes D, Black A, Bueno-de-Mesquita HBA, Chan JM, Chen C, Chirlaque M-Det al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016;76(8):2288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Price AJ, Allen NE, Appleby PN, Crowe FL, Travis RC, Tipper SJ, Overvad K, Gronbaek H, Tjonneland A, Johnsen NFet al. Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, Gronbaek H, Tjonneland A, Halkjaer J, Dossus Let al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1333–40. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46(14):2555–62. [DOI] [PubMed] [Google Scholar]
- 35. Key TJ. Nutrition, hormones and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Recent Results Cancer Res. 2014;202:39–46. [DOI] [PubMed] [Google Scholar]
- 36. Fraser GE, Jaceldo-Siegl K, Orlich M, Mashchak A, Sirirat R, Knutsen S. Dairy, soy, and risk of breast cancer: those confounded milks. Int J Epidemiol. 2020;49(5):1526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penniecook-Sawyers JA, Jaceldo-Siegl K, Beeson L, Knutsen S, Herring P, Fraser GE. Vegetarian dietary patterns and the risk of breast cancer in low-risk population. Br J Nutr. 2016;115(10):1790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tantamango-Bartley Y, Knutsen SF, Jaceldo-Siegl K, Fan J, Mashchak A, Fraser GE. Independent associations of dairy and calcium intakes with colorectal cancers in the Adventist Health Study–2 cohort. Public Health Nutr. 2017;20(14):2577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Cancer Research Fund/American Institute for Cancer Research . Continuous Update Project Expert Report 2018: Meat, fish, and dairy products and risk of cancer. World Cancer Research Fund International. . 2018. Available on-line at dietandcancerreport.org. Accessed: 28 April, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.