Abstract
Objective
To estimate the safety and efficacy of transcatheter arterial embolization (TAE) in the treatment of refractory hematuria of prostatic origin (RHPO).
Methods
This retrospective study included 23 patients who underwent TAE for RHPO between May 2013 and August 2021. Technical and clinical success rates were calculated, and arteriogram findings and complications were detected.
Results
Embolization was performed 24 times in 23 patients. Technical success was achieved in 24/24 (100%) embolization procedures. Contrast agent extravasation was detected during 2 of the 24 angiographic procedures. Bilateral embolization was performed in 23 (95.8%) of the 24 procedures. The clinical success rate was 21/23 (91.3%), and hematuria stopped 1–4 days after TAE. No serious complications were observed.
Conclusion
TAE is a safe and effective minimally invasive technique for treating patients with RHPO.
Keywords: Transcatheter arterial embolization, Hematuria, Benign prostatic hyperplasia
1. Introduction
The causes of hematuria include benign prostatic hyperplasia (BPH), prostate malignancy, iatrogenic bleeding, and radiation therapy.1,2 The incidence of hematuria caused by BPH is 2.5%, while that in patients with post-urinary retention is 2–16%.2 The incidence of hematuria caused by prostate cancer is 0.7%. After radiotherapy, it increases to 5.1%.3 After prostate biopsy, radical prostatectomy, and transurethral resection of the prostate (TURP), its incidence is 0.7–62%, 0.5–1.6%, and 12%, respectively.3, 4, 5, 6
Refractory hematuria of prostatic origin (RHPO) is defined as repeated active prostatic bleeding after conservative treatment including medication, bladder irrigation, and cystoscopy. The overall incidence of RHPO is low; however, it may be life-threatening and affect quality of life. These patients are mainly elderly and often have several comorbidities; therefore, they are not suitable candidates for surgical treatment. Transcatheter arterial embolization (TAE) has been successfully used for the treatment of hemoptysis, gastrointestinal bleeding, trauma bleeding, and obstetric and gynecological bleeding.1, 2, 3, 4, 5, 6, 7 However, owing to the significant anatomical variants of the prostate artery and the low incidence of RHPO, few studies on TAE for its treatment have been reported. This study aimed to assess the safety and efficacy of TAE in the treatment of RHPO and summarize its technical points.
2. Materials and methods
2.1. Study population
This retrospective study was approved by our institutional review board, which waived the requirement for informed consent.
Patients who underwent TAE for RHPO between May 2013 and August 2021 at 3 national hospitals were included. Those with the following were included: (a) refractory gross hematuria after conservative treatment (medication, blood transfusion, bladder irrigation, indwelling catheter, etc.); (b) prostatic hematuria confirmed by computed tomography, magnetic resonance imaging, and/or cystoscopy; (c) complete intraoperative angiography images; and (d) at least one follow-up visit.
The exclusion criteria were as follows: incomplete clinical follow-up data and inability to evaluate the hemostatic effect; and hematuria due to the bladder, kidney, and/or ureter.
2.2. Pre-procedural preparation
For patients without a Foley catheter or indwelling bladder catheterization before TAE, a Foley catheter was placed to avoid acute urinary retention after TAE due to prostate tissue edema and as a landmark to assist in identifying the prostatic arteries. Antibiotics were administered to prevent infection.
2.3. Embolization procedure
The right femoral artery was cannulated using the Seldinger technique under local anesthesia, and a 4-F vascular sheath was placed. First, pelvic angiography was performed to evaluate the iliac vessels with a 4-F pigtail-type catheter (Cordis, Miami, FL, USA). Second, a 4-F Simmons I or RH catheter (Cordis) was used to perform digital subtraction angiography (DSA) at the main internal iliac artery using ipsilateral anterior oblique projection at 30°–40° to evaluate its branches. According to the DSA images, a 1.98–2.7-F microcatheter was used to catheterize the target artery superselectively. Additional angiography, including the bladder, obturator, internal pudendal, inferior epigastric, and external iliac arteries, was performed if necessary. If the pelvic anastomosis and those with adjacent arteries cannot be accurately identified, cone-beam computed tomography should be performed to avoid non-target embolization. The direct sign of hemorrhage was contrast extravasation, while the indirect signs of hemorrhage included pseudoaneurysm, arteriovenous fistula, arterial irregularity and spasm, and neovascularity.
Embolization was performed using polyvinyl alcohol particles (PVA; diameters of 90–180 μm and 300–500 μm; Cook Incorporated, Bloomington, IN, USA), Embosphere (100–300 μm, 300–500 μm; Merit Medical, South Jordan, UT, USA), gel foam particles (350–560 μm, 560–710 μm; Hangzhou Alicon Pharm SCI & TEC Co., Ltd. Hangzhou, China), and 0.018-in pushable microcoils (MicroNester; Cook Incorporated).
First, embolization was performed slowly using 100–300 μm particles/spheres for the distal branches of the arteries and subsequently with 300–500 μm and/or 500–700 μm particles/spheres for the proximal arteries. The endpoint of embolization was the complete occlusion of the target vessel and casting. After TAE, repeat angiography was performed to detect any additional arteries supplying the prostate. If necessary, additional arteries were selected and embolized to ensure complete embolization.
If hematuria is caused by advanced tumor invasion or recurrent bleeding after embolization, angiography of the external iliac, inferior mesenteric, and middle sacral arteries should be performed.
2.4. Post-procedural management
After TAE, acid-suppressing drugs, antibiotics, and nonsteroidal anti-inflammatory drugs were administered for 3–5 days and all patients were monitored for adverse effects. The Foley catheter was removed 2–3 days after the bleeding stopped. The catheter should be reinserted if the patient was unable to urinate spontaneously after the first removal. Additional weekly attempts to remove the catheter were made.
2.5. Follow-up assessment
Technical success was defined as occlusion of the targeted vessels with contrast medium extravasation on arteriography or successful embolization of all angiographically visible arteries supplying the prostate.2,8,9 Clinical success was defined as control or cessation of bleeding post-embolization without recurrent hematuria within 30 days. Post-embolization symptoms and complications were registered and classified according to the Quality Improvement Guidelines for Percutaneous Transcatheter Embolization.10
2.6. Statistical analysis
The statistical analyses were performed using commercial software (SPSS version 18.0 for Windows (SPSS, Chicago, IL, USA). Data are presented as mean ± standard deviation. The paired-samples t-test was used to compare hemoglobin and hepatitis C virus levels pre- and post-TAE. Values of P < 0.05 were considered statistically significant.
3. Results
Based on the inclusion and exclusion criteria, 23 patients (mean age, 74.3 years [range, 55–88 years]) were included in this study. Fourteen patients were diagnosed with BPH, 8 with prostate cancer, and 1 with prostatic sarcoma. Hematuria was caused by radical prostatectomy due to prostate cancer in 1 patient, spontaneous rupture of prostate sarcoma in 1, after urethral dilation due to prostate cancer combined with urethral stricture in 1, and after indwelling catheterization due to BPH in 1. Spontaneous hematuria due to BPH or prostate cancer occurred in the other 19 patients. The mean duration of hematuria was 14.32 ± 16.86 days (range, 1–60 days). Of the 23 patients, 3 underwent indwelling catheterization for cystostomy before hematuria, while the other 20 underwent indwelling catheterization after hemorrhage that was successfully removed 5–7 days post-TAE.
The 23 patients underwent 24 embolization procedures. Technical success was achieved in 24/24 (100%) of the embolization procedures. Active arterial bleeding, such as extravasation of the contrast agent (n = 2), was detected in 23 of the 24 angiographic procedures. The other 22 procedures (91.7% [22/24]) were negative on arteriography, which showed increased and disordered arteries for BPH, multiple and tortuous tumor-feeding arteries for prostate cancer, and hypervascular staining for prostate sarcoma. Bilateral embolization was performed in 23 of 24 (95.8%) procedures. Of the 2 cases with positive angiograms, one was on day 1 after radical prostatectomy for prostate cancer. The responsible artery was the right inferior epigastric artery, and unilateral embolization was performed using 350–560 μm gelatin sponge particles combined with microcoils (Fig. 1). The other was spontaneous bleeding due to BPH; the responsible artery was the left prostatic artery, which underwent bilateral embolized using 100–300 μm PVA (Fig. 2).
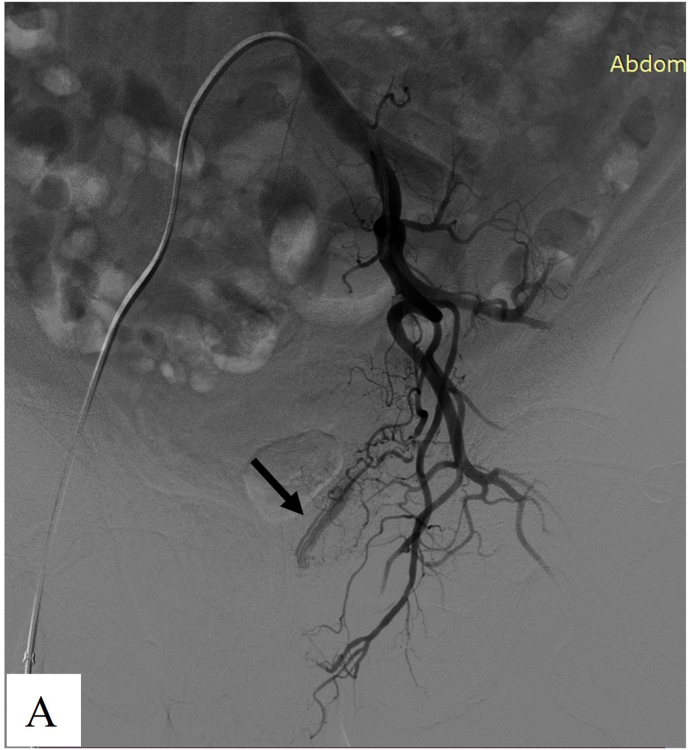
Fig. 1.
Images from a 60-year-old man diagnosed with prostate cancer, he suffered hemoturia on day 1 after radical prostatectomy for prostate cancer. A. Arteriogram showed the responsible artery was the right inferior epigastric artery (arrow). Unilateral embolization was performed using 350–560 μm gelatin sponge particles combined with microcoils. B. Repeat angiography showed the contrast agent extravasation disappeared post-embolization.
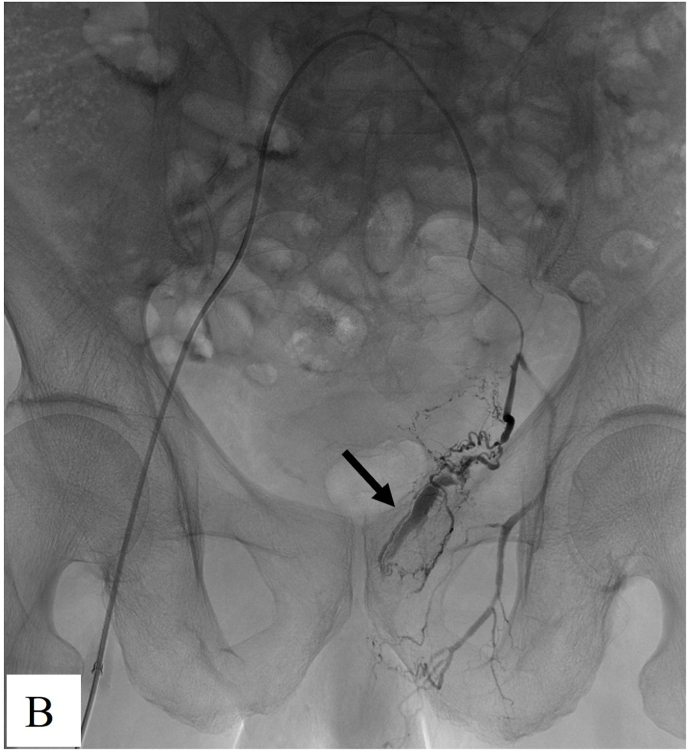
Fig. 2.
Images from a 70-year old BPH man with hematuria. A. The left internal iliac artery arteriography showed active bleeding of prostatic artery (arrow). B. Superselective angiography of prostatic artery showed contrast agent extravasation (arrow). Bilateral embolization was performed using 100–300 μm PVA particles. C. Repeat angiography showed the contrast agent extravasation disappeared post-embolization.
The median follow-up period was 2 months (range, 1–39 months). The clinical success rate was 21/23 (91.3%). For the 21 patients, hematuria was stopped 1–4 days post-TAE without recurrence during 1 month of follow-up. One patient diagnosed with prostate cancer experienced recurrent hematuria 3 months post-TAE and underwent an additional TAE. Of the 2 cases of clinical failure, one involved recurrent hematuria 3 days post-TAE due to over-anticoagulation that ceased after correction, and the patient eventually died of tumor progression 25 days post-TAE. Another patient with prostate cancer experienced decreased hematuria after TAE; however, the intermittent hematuria persisted.
There was no statistically significant difference in hemoglobin (104.69 ± 35.78 vs 103.00 ± 25.38 g/L) or hematocrit (0.31 ± 0.09 vs 0.31 ± 0.08) pre- or 1 week post-TAE (P = 0.705 vs 0.458).
No serious complications were observed. Minor complications included dysuria (n = 2), lower abdominal pain (n = 2), perineal pain (n = 1), voiding irritation (n = 2), and urinary tract infection (n = 1).
4. Discussion
The incidence of RHPO is low; however, most affected patients are elderly and have many complications that make them unsuitable candidates for surgery when conservative treatment fails. Over the past decade, TAE has been used to manage prostatic hematuria with technical and clinical success rates of up to 88–100% and 67–100%, respectively.2,4,6,11, 12, 13, 14 In this study, TAE was a safe, effective, and durable treatment for RHPO secondary to multiple causes with technical and clinical success rates of 100% and 91.3%, respectively. Hematuria stopped in every case within 4 days post-TAE with no serious complications.
The choice of embolic material is very important. Different types and sizes of embolic materials have been used to date for TAE to treat prostatic hematuria, such as 50–700 μm PVA particles, 300–700 μm Embosphere, gelatin sponge particles, 0.035-in coils, 0.018-in platinum microcoils, and a mixture of N-butyl cyanoacrylate (NBCA) and lipiodol.8,11,13,15 The choice of embolic material is mainly dependent on the embolic artery diameter, blood flow velocity, collateral arteries, and catheter tip position.
Unlike TAE for treating hemorrhage of other organs, it has particular characteristics in the treatment of prostatic hemorrhage. Embolization is usually performed only with positive signs of hemorrhage, such as contrast agent extravasation, pseudoaneurysm, arteriovenous fistula, and arterial irregularities and spasm. As for prostatic hemorrhage patients, only 15–30% positive arteriograms were detected previously; in this cohort, only 8.3% were detected. Despite this, bilateral embolization is recommended to completely occlude the prostate arterial blood supply because there are many communication and collateral arteries between the bilateral pelvic arteries and adjacent organs that may cause recurrent bleeding.
Step-by-step embolization is recommended in combination with different embolic material types and sizes. First, distal embolization is performed using small embolic agents (<300 μm); next, proximal embolization is achieved using larger embolic agents (≥300 μm).13,16 Protective embolization of the arteries of important organs, such as the penis, bladder, and rectum, should be performed using microcoils when superselective catheterization fails.13 Additionally, when patients suffer severe hemorrhage and their vital signs are unstable, embolization of the anterior iliac artery using biodegradable embolic materials, such as gelatin sponge particles, can be performed if it is difficult to superselectively catheterize the target arteries.8,13
As for hematuria secondary to TURP and radical prostatectomy, arteriography often shows positive signs, including contrast medium extravasation, pseudoaneurysm, and/or arteriovenous fistula. Complete hemostasis can be achieved only by embolizing the bleeding arteries, under which conditions a mixture of NBCA and lipiodol can be used.17, 18, 19, 20, 21 In this cohort, bleeding of the right inferior epigastric artery was detected and complete hemostasis was achieved after unilateral embolization.
For patients with advanced prostate malignancy, multiple arteries usually supply the tumor, including the prostatic, internal iliac, inferior mesenteric, external iliac, middle sacral, and iliolumbar arteries. Therefore, it is critical to identify and completely embolize all related arteries. Furthermore, for patients with coagulopathy, it is critical to correct hemostatic abnormalities after prostatic artery embolization to reduce hematuria recurrence. In the present study, one patient with clinical failure and another with recurrence were diagnosed with prostate cancer.
This study had several limitations. First, due to its retrospective nature, the baseline of the cohort varied and the sample size was limited. Second, because most patients were treated under emergent conditions, the improvement of lower urinary tract symptoms after TAE could not be evaluated. Finally, long-term follow-up data were lacking.
In conclusion, TAE is a safe and effective minimally invasive technique for treating patients after failure of conservative treatment for prostatic hematuria that avoids surgery in most cases. For patients who require surgical intervention, TAE could establish this opportunity by controlling the hemorrhage and stabilizing the blood circulation.
Declaration of competing interest
The authors declared there was no conflict of interest.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (82072023) and the Fujian Province NaturalScience Fund Project (2020J011096 and 2020J011064).
Contributor Information
Zhuting Fang, Email: 470389481@qq.com.
Maoqiang Wang, Email: wangmq@vip.sina.com.
References
- 1.Pereira K., Halpern J.A., McClure T.D., et al. Role of prostate artery embolization in the management of refractory haematuria of prostatic origin. BJU Int. 2016;118:359–365. doi: 10.1111/bju.13524. [DOI] [PubMed] [Google Scholar]
- 2.Tapping C.R., Macdonald A., Hadi M., et al. Prostatic artery embolization (PAE) for benign prostatic hyperplasia (BPH) with haematuria in the absence of an upper urinary tract pathology. Cardiovasc Intervent Radiol. 2018;41:1160–1164. doi: 10.1007/s00270-018-1941-0. [DOI] [PubMed] [Google Scholar]
- 3.Rastinehad A.R., Ost M.C., VanderBrink B.A., et al. Persistent prostatic hematuria. Nat Clin Pract Urol. 2008;5:159–165. doi: 10.1038/ncpuro1044. [DOI] [PubMed] [Google Scholar]
- 4.Kably I., Pereira K., Chong W., et al. Prostate artery embolization (PAE) in the management of refractory hematuria of prostatic origin secondary to iatrogenic urological trauma: a safe and effective technique. Urology. 2016;88:218–221. doi: 10.1016/j.urology.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Bokhorst L.P., Lepistö I., Kakehi Y., et al. Complications after prostate biopsies in men on active surveillance and its effects on receiving further biopsies in the Prostate cancer Research International: active Surveillance (PRIAS) study. BJU Int. 2016;118:366–371. doi: 10.1111/bju.13410. [DOI] [PubMed] [Google Scholar]
- 6.Quinlan M.R., Bolton D., Casey R.G. The management of rectal bleeding following transrectal prostate biopsy: a review of the current literature. Can Urol Assoc J. 2018;12:E146–E153. doi: 10.5489/cuaj.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong C.W., Park Y.H., Ku J.H., et al. Minimally invasive management of postoperative bleeding after radical prostatectomy: transarterial embolization. J Endourol. 2010;24:1529–1533. doi: 10.1089/end.2009.0686. [DOI] [PubMed] [Google Scholar]
- 8.Loffroy R., Pottecher P., Cherblanc V., et al. Current role of transcatheter arterial embolization for bladder and prostate hemorrhage. Diagn Interv Imaging. 2014;95:1027–1034. doi: 10.1016/j.diii.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Golzarian J., Antunes A.A., Bilhim T., et al. Prostatic artery embolization to treat lower urinary tract symptoms related to benign prostatic hyperplasia and bleeding in patients with prostate cancer: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2014;25:665–674. doi: 10.1016/j.jvir.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Angle J.F., Siddiqi N.H., Wallace M.J., et al. Quality improvement guidelines for percutaneous transcatheter embolization: society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2010;21(10):1479–1486. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen J.W., Shin J.H., Tsao T.F., et al. Prostatic arterial embolization for control of hematuria in patients with advanced prostate cancer. J Vasc Interv Radiol. 2017;28:295–301. doi: 10.1016/j.jvir.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Prasad V., Sacks B.A., Kraus S., et al. Embolotherapy for lower urinary tract hemorrhage. J Vasc Interv Radiol. 2009;20:965–970. doi: 10.1016/j.jvir.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Korkmaz M., Şanal B., Aras B., et al. The short- and long-term effectiveness of transcatheter arterial embolization in patients with intractable hematuria. Diagn Interv Imaging. 2016;97:197–201. doi: 10.1016/j.diii.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Delgal A., Cercueil J.P., Koutlidis N., et al. Outcome of transcatheter arterial embolization for bladder and prostate hemorrhage. J Urol. 2010;183:1947–1953. doi: 10.1016/j.juro.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Cui L., Bai Y., Zhang J., et al. Prostatic artery embolization: progress and prospect. J Interv Med. 2020;3:77–79. doi: 10.1016/j.jimed.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonne L., Gillardin P., De Wever L., et al. Endovascular management of severe arterial haemorrhage after radical prostatectomy: a case series. Cardiovasc Intervent Radiol. 2017;40:1698–1705. doi: 10.1007/s00270-017-1715-0. [DOI] [PubMed] [Google Scholar]
- 17.Beckley I., Patterson B., Hamaday M., et al. Case report: delayed hemorrhage from an accessory internal pudendal artery pseudoaneurysm after robotic radical prostatectomy: successful management with ct angiography and embolization. J Endourol. 2007;21:923–925. doi: 10.1089/end.2006.0419. [DOI] [PubMed] [Google Scholar]
- 18.Lopes R.I., Mitre A.I., Rocha F.T., et al. Case report: late recurrent hematuria following laparoscopic radical prostatectomy may predict internal pudendal artery pseudoaneurysm and arteriovenous fistula. J Endourol. 2009;23:297–299. doi: 10.1089/end.2008.0494. [DOI] [PubMed] [Google Scholar]
- 19.Celtikci P., Ergun O., Tatar I.G., et al. Superselective arterial embolization of pseudoaneurysm and arteriovenous fistula caused by transurethral resection of the prostate. Pol J Radiol. 2014;79:352–355. doi: 10.12659/PJR.890900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asimakopoulos A.D., Dutto L., Preziosi P., et al. Holmium laser enucleation of the prostate and iatrogenic arteriovenous fistula treated by superselective arterial embolization. Case Rep Urol. 2016;2016:4918081. doi: 10.1155/2016/4918081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezaeimehr B., Modanloo M., Davoodi M., et al. Angioembolization of internal pudendal artery for treatment of long lasting gross hematuria after transurethral resection of the prostate. Urol J. 2019;16:517–518. doi: 10.22037/uj.v0i0.4657. [DOI] [PubMed] [Google Scholar]