Abstract
Based on exposure history and symptom onset of 22 Omicron BA.1 cases in South Korea from November to December 2021, we estimated mean incubation period of 3.5 days (95% CI: 2.5, 3.8), and then compared to that of 6.5 days (95% CI: 5.3, 7.7) for 64 cases during Delta variants' dominance in June 2021. For Omicron BA.1 variants, we found that 95% of symptomatic cases developed clinical conditions within 6.0 days (95% CI: 4.3, 6.6) after exposure. Thus, a shorter quarantine period may be considered based on symptoms, or similarly laboratory testing, when Omicron BA.1 variants are circulating.
Keywords: Incubation period, Omicron BA.1 variant, Delta variant, Quarantine
1. Introduction
Since the end of 2019, COVID-19 continuously posed threat to public health globally [1]. The novel genetic mutations of SARS-CoV-2 have continually challenged the control system for the COVID-19 pandemic, making it critical to monitor key epidemiological parameters for understanding the transmission and clinical characteristics of emerging variants [2,3]. The incubation period is defined as the time interval between exposure and onset of illness for symptomatic infections [4], which is important to inform quarantine policies, to study transmission dynamics of an infectious disease, and to assess the effectiveness of entry screening [5,6]. While estimates of incubation period can be found in literature for various historical SARS-CoV-2 strains [7,8], the knowledge of incubation period for Omicron variants remains largely unassessed.
In this study, we collected information on exposure history and symptom onset of 22 Omicron BA.1 (i.e., B.1.1.529.1) cases in South Korea from November to December 2021, and estimated distribution of incubation period, which was then compared to that of 64 cases during Delta (i.e., B.1.617.2) variants' dominance in June 2021.
2. Methods
2.1. Data collection
Based on the information of COVID-19 cases who tested positive for SARS-CoV-2 previously published [9,10], we extracted exposure history and symptom onset date for patients with this information available. To use for incubation period estimation, we identified 22 cases laboratory-confirmed for Omicron BA.1 variants who were reported in South Korea from November 25 to December 31, 2021, and for comparison, we also included 64 cases reported in June 2021 when the Delta variants were dominant at a prevalence of 68.3% according to GISAID [11]. The exposure history was translated into exposure time window with upper and lower bounds of exposure date, which will be used for the calculation of the likelihood. Among these 86 (64 + 22) patients, all of them have illness onset date observed. Among the 22 identified Omicron BA.1 cases, 21 of them have both lower and upper bounds of exposure date, while 1 only has the upper bound of exposure date, and 12 cases during Delta dominance have both lower and upper bounds but 52 only have the upper bound.
2.2. Statistical analysis
Log-normal, gamma, and Weibull were among the most common distributions applied to estimate the incubation period [6]. The gamma distribution has a more concise mathematical expression compared to the other two distributions, hence less computational power is required to estimate the parameters. In this study, two different Gamma distributions were adopted to govern the distributions of incubation period for Omicron BA.1 cases and cases during Delta variants' dominance, respectively. For the samples with both lower and upper bounds of exposure date, i.e., with exposure window, we calculated the likelihood with interval censoring [6]. For the remaining samples only with upper bound of exposure date observed, we assumed an exponential distribution indexed by this upper bound backwardly, and calculated the likelihood with convolution between Gamma distribution of incubation period of the assumed exponential distribution [12,13]. We assumed the exponential infectiousness distribution has a mean of 3.7 days, which corresponded to the mean infectious period estimated in previous research [14]. We calculated the maximum likelihood estimators of mean and standard deviation of the Gamma distributions. To evaluate the statistical uncertainty, we used a parametric bootstrap with 1000 iterations of resampling to obtain 95% confidence intervals (CI) for each parameter. Limiting the dataset to those with exposure window observed, i.e., with both lower and upper bounds, we repeated the estimation with only 21 samples for Omicron BA.1, and 12 samples for Delta dominance period, respectively.
Sensitivity analysis was conducted by assuming shorter and longer versions of the exponential-distributed exposure window with 2.8 and 4.6 days to repeat the estimation. Additionally, to relax the exponential assumption for the missing exposure window, we assumed the exposure windows of those samples only with upper bound of exposure date observed following an empirical distribution from the samples with both lower and upper bounds of exposure date observed.
3. Results and discussion
For the 22 cases infected by Omicron BA.1 variants, the estimated mean incubation period was 3.5 days (95% CI: 2.5, 3.8), and SD was 1.4 days (95% CI: 1.0, 1.5), see Fig. 1. We found that 50%, 95% or 99% of symptomatic cases may present clinical conditions within 3.3 days (95% CI: 2.4, 3.7), 6.0 days (95% CI: 4.3, 6.6) or 7.4 days (95% CI: 5.3, 8.2) after exposure, respectively. When limiting dataset to the 21 samples with exposure window observed, the mean incubation period decreased was estimated at 3.2 days (95% CI: 2.3, 3.8), see Table 1.
Fig. 1.
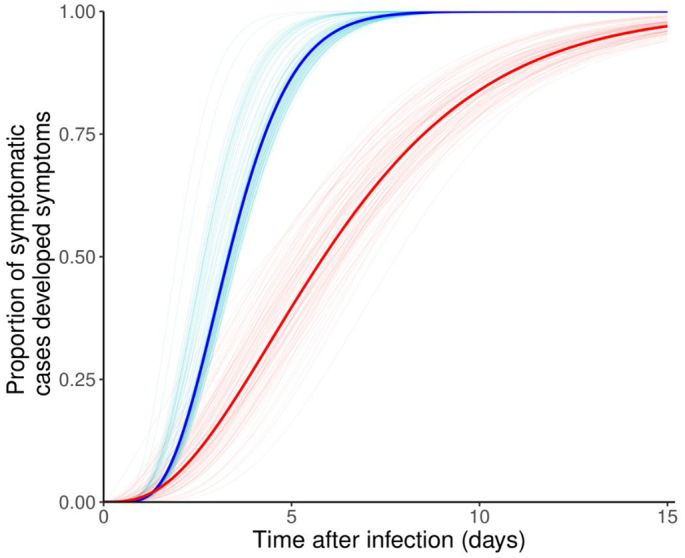
Estimated cumulative distributions of incubation period for Omicron BA.1 variants (in blue), and for cases during Delta dominance (in red). The statistical uncertainty was illustrated by 100 bootstrap estimates, which were curves in light colors, and the mean estimates were the bold curves in dark colors. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Summary of incubation period estimates (unit: day) for cases infected by Omicron BA.1 variants and cases during Delta predominance period in South Korea.
| Type of SARS-CoV-2 strain | sample& |
estimate (95%CI) |
||||
|---|---|---|---|---|---|---|
| type of sample | sample size | mean | median | 95-th percentile | SD | |
| Omicron BA.1 | all samples | n = 22 | 3.5 (2.5, 3.8) | 3.3 (2.4, 3.7) | 6.0 (4.3, 6.6) | 1.4 (1.0, 1.5) |
| with exposure window | n = 21 | 3.2 (2.3, 3.8) | 3.1 (2.2, 3.6) | 5.5 (4.0, 6.6) | 1.3 (0.9, 1.5) | |
| those during Delta dominance$ | all samples | n = 64 | 6.5 (5.3, 7.7) | 5.9 (4.4, 7.1) | 13.6 (11.1, 15.9) | 3.7 (2.9, 4.6) |
| with exposure window | n = 12 | 8.7 (6.0, 11.6) | 8.1 (5.5, 11.0) | 16.0 (10.5, 21.0) | 3.8 (2.4, 5.6) | |
Notes
$ These cases were collected in June 2021 when the Delta variants were dominant at a prevalence of 68.3% in South Korea according to GISAID [11].
& The samples “with exposure window” are those with both lower and upper bounds of exposure date observed, whereas “all samples” included the samples with exposure window and sample with only upper bound of exposure date observed.
By contrast, for the 64 cases identified during Delta dominance, the estimated mean incubation period was 6.5 days (95% CI: 5.3, 7.7), and SD was 3.7 days (95% CI: 2.9, 4.6). We found that 50%, or 95% of symptomatic cases may present clinical conditions within 5.9 days (95% CI: 4.4, 7.1), or 13.6 days (95% CI: 11.1, 15.9) after exposure, respectively.
For the sensitivity analysis, we found that the estimates with either shorter or longer version of exposure bound are consistent with main results in similar scales, which suggested the robustness of our findings, see Supplementary Materials. By using empirical distribution for those with missing exposure window, we found that the estimates were largely in line with the main results.
The mean and percentiles of incubation period of Omicron BA.1 variants were found considerably shorter than those of cases during Delta dominance period, as well as previous estimates based on other historical SARS-CoV-2 strains [15,8]. Given the pre-symptomatic transmission feature of SARS-CoV-2 infection [16], a shorter incubation period indicated the Omicron BA.1 cases are likely to have a relatively higher rate at which they become new sources of infection to other susceptible individuals. Theoretical study also suggests that the generation time may be shortened with a short latent period [17], which is roundly equal to or less than the incubation period, and thus the Omicron BA.1 variants may lead to a lower period doubling time for epidemic curve regarding advantageous transmissibility in natural population and escape feature against herd immunity [18,19].
Linking our findings to the disease control measures, some countries and regions have been using quarantine and entry screening as control measures against COVID-19. The initial quarantine periods were 14 days, and then extended to 21 days in some areas [20]. Although a longer quarantine period may lower the risk of disease spread in community, people under quarantine or isolation were at risk of adverse mental health outcomes suggested by synthesized evidence [21], especially when the containment duration is longer than one week. Considering the latent period was typically shorter than incubation period [5], our estimates of the 99-th percentile at 7.2 days suggested a 7-day quarantine with PCR tests could be sufficient to detect around 99% of infections of Omicron BA.1 variants, and PCR tests have been confirmed effective to filter asymptomatic patients before they have onset of illness [[22], [23]].
There are some limitations in this study. First, for cases collected during Delta dominance period, we could not confirm these cases were infected by Delta variants due to the lack of genetic sequencing data. We could only conclude that the Delta variants were dominant at a prevalence of 68.3% in June 2021 in South Korea. Second, we adopted a Gamma distribution to govern the observed incubation period distribution, where symptoms were assumed to start immediately after infection. This may not be biologically reasonable, where a certain but minor lag may exist for patients to develop symptoms. Third, the exposure windows and illness onset time for patients can only be accurate to days. Therefore, a maximum of one-day error may exist in our determination of the intervals of exposure and symptom onset. Last, our estimate may be subjected to reporting and recall biases. It is suggested to further explore the heterogeneity of the incubation period among different SARS-CoV-2 Omicron variants, in order to adjust the disease control measures.
Ethics approval and consent to participate
The COVID-19 cases surveillance data used in this study were collected via the public domains, and thus neither ethical approval nor individual consent was not applicable. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Availability of materials
The surveillance data of COVID-19 cases used in this work were publicly available in previous studies [9,10].
Consent for publication
Not applicable.
Funding
The work was partially supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (HKU C7123-20G).
Author's contributions
All authors conceived the study, carried out the analysis, wrote the draft, revised the manuscript critically, and approved it for publishing
CRediT authorship contribution statement
Yanwen Liu: Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Project administration. Shi Zhao: Conceptualization, Methodology, Validation, Resources, Writing – review & editing. Sukhyun Ryu: Resources, Writing – review & editing. Jinjun Ran: Writing – review & editing. Junhua Fan: Writing – review & editing. Daihai He: Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
All authors declared no competing interests. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100425.
Contributor Information
Yanwen Liu, Email: yanwen.liu@connect.polyu.hk.
Shi Zhao, Email: zhaoshi.cmsa@gmail.com.
Sukhyun Ryu, Email: gentryu@onehealth.or.kr.
Jinjun Ran, Email: jinjunr@sjtu.edu.cn.
Junhua Fan, Email: fanjunhua@scdc.sh.cn.
Daihai He, Email: daihai.he@polyu.edu.hk.
Appendix A. Supplementary data
Technical Details
References
- 1.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Tropical Med. Int. Health. 2020;25(3):278. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homma Y., Katsuta T., Oka H., Inoue K., Toyoshima C., Iwaki H., Yamashita Y., Shinomiya H. The incubation period of the SARS-CoV-2 B1. 1.7 variant is shorter than that of other strains. J. Inf. Secur. 2021;83(2):e15–e17. doi: 10.1016/j.jinf.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraemer M.U., Pybus O.G., Fraser C., Cauchemez S., Rambaut A., Cowling B.J. Monitoring key epidemiological parameters of SARS-CoV-2 transmission. Nat. Med. 2021;27(11):1854–1855. doi: 10.1038/s41591-021-01545-w. [DOI] [PubMed] [Google Scholar]
- 4.Nelson K.E., Williams C.M. Jones & Bartlett Publishers; 2014. Infectious Disease Epidemiology: Theory and Practice. [Google Scholar]
- 5.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xin H., Wong J.Y., Murphy C., Yeung A., Taslim Ali S., Wu P., Cowling B.J. The incubation period distribution of coronavirus disease 2019: a systematic review and meta-analysis. Clin. Infect. Dis. 2021;73(12):2344–2352. doi: 10.1093/cid/ciab501. [DOI] [PubMed] [Google Scholar]
- 7.McAloon C., Collins Á., Hunt K., Barber A., Byrne A.W., Butler F., Casey M., Griffin J., Lane E., McEvoy D. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin H., Li Y., Wu P., Li Z., Lau E.H., Qin Y., Wang L., Cowling B.J., Tsang T., Li Z. Estimating the latent period of coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2021;74(9):1678–1681. doi: 10.1093/cid/ciab746. [DOI] [PubMed] [Google Scholar]
- 9.Kim D., Ali S.T., Kim S., Jo J., Lim J.-S., Lee S., Ryu S. Estimation of serial interval and reproduction number to quantify the transmissibility of SARS-CoV-2 Omicron variant in South Korea. Viruses. 2022;14(3):533. doi: 10.3390/v14030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu S., Kim D., Lim J.-S., Ali S.T., Cowling B.J. Serial interval and transmission dynamics during SARS-CoV-2 delta variant predominance, South Korea. Emerg. Infect. Dis. 2022;28(2):407. doi: 10.3201/eid2802.211774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data–from vision to reality. Eurosurveillance. 2017;22(13):30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Eurosurveillance. 2020;25(5):2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao S., Gao D., Zhuang Z., Chong M.K., Cai Y., Ran J., Cao P., Wang K., Lou Y., Wang W. Estimating the serial interval of the novel coronavirus disease (COVID-19): a statistical analysis using the public data in Hong Kong from January 16 to February 15, 2020. Front. Phys. 2020;8:347. [Google Scholar]
- 14.Hart W.S., Miller E., Andrews N.J., Waight P., Maini P.K., Funk S., Thompson R.N. Generation time of the alpha and delta SARS-CoV-2 variants: an epidemiological analysis. Lancet Infect. Dis. 2022;22(5):603–610. doi: 10.1016/S1473-3099(22)00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang M., Xin H., Yuan J., Ali S.T., Liang Z., Zhang J., Hu T., Lau E.H., Zhang Y., Zhang M. Transmission dynamics and epidemiological characteristics of SARS-CoV-2 Delta variant infections in Guangdong, China, May to June 2021. Eurosurveillance. 2022;27(10):2100815. doi: 10.2807/1560-7917.ES.2022.27.10.2100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X., Lau E.H., Wu P., Deng X., Wang J., Hao X.…Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 17.Svensson Å. A note on generation times in epidemic models. Math. Biosci. 2007;208(1):300–311. doi: 10.1016/j.mbs.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Eggink D., Andeweg S.P., Vennema H., van Maarseveen N., Vermaas K., Vlaemynck B.…Knol M.J. Increased risk of infection with SARS-CoV-2 Omicron BA. 1 compared with Delta in vaccinated and previously infected individuals, the Netherlands, 22 November 2021 to 19 January 2022. Eurosurveillance. 2022;27(4):2101196. doi: 10.2807/1560-7917.ES.2022.27.4.2101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J., Collier A.R.Y., Rowe M., Mardas F., Ventura J.D., Wan H.…Hachmann N.P. Neutralization of the SARS-CoV-2 Omicron BA. 1 and BA. 2 variants. N. Engl. J. Med. 2022;386(16):1579–1580. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson M.A., Wolford H., Paul P., Diaz P.S., Chen T.-H., Brown C.M., Cetron M.S., Alvarado-Ramy F. Reducing travel-related SARS-CoV-2 transmission with layered mitigation measures: symptom monitoring, quarantine, and testing. BMC Med. 2021;19(1):1–13. doi: 10.1186/s12916-021-01975-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henssler J., Stock F., van Bohemen J., Walter H., Heinz A., Brandt L. Mental health effects of infection containment strategies: quarantine and isolation—a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2021;271(2):223–234. doi: 10.1007/s00406-020-01196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang A., Tong Z.-D., Wang H.-L., Dai Y.-X., Li K.-F., Liu J.-N., Wu W.-J., Yuan C., Yu M.-L., Li P. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26(6):1337. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treibel T.A., Manisty C., Burton M., McKnight Á., Lambourne J., Augusto J.B., Couto-Parada X., Cutino-Moguel T., Noursadeghi M., Moon J.C. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395(10237):1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical Details


