Abstract
Caused by the new SARS-CoV-2 coronavirus, COVID-19 (coronavirus disease 2019) evolves with clinical symptoms that vary widely in severity, from mild symptoms to critical conditions, which can even result in the patient's death. A critical aspect related to an individual response to SARS-CoV-2 infection is the competence of the immune system, and it is well known that several trace elements are essential for an adequate immune response and have anti-inflammatory and antioxidant properties that are of particular importance in fighting infection. Thus, it is widely accepted that adequate trace element status can reduce the risk of SARS-CoV-2 infection and disease severity. In this study, we evaluated the serum levels of Cu, Zn, Se, Fe, I and Mg in patients (n = 210) with clinical conditions of different severity (“mild”, “moderate”, “severe” and “exitus letalis”, i.e., patients who eventually died). The results showed significant differences between the four groups for Cu, Zn, Se and Fe, in particular a significant trend of Zn and Se serum levels to be decreased and Cu to be increased with the severity of symptoms. For Mg and I, no differences were observed, but I levels were shown to be increased in all groups.
Keywords: Trace elements, Cu, Zn, Se, Fe, I, Mg, SARS-CoV-2 infection, COVID-19 severity
1. Introduction
The emergence of the new coronavirus SARS-CoV-2 in late 2019 put the world under threat of a new pandemic. In humans, the virus causes the so-called coronavirus disease (COVID-19), whose most common symptoms are fever, chills, new or worsening cough, fatigue, myalgia, headache, gastrointestinal symptoms (nausea, vomiting, diarrhea) and loss of smell or taste [1], [2]. The elderly population, due to the higher prevalence of underlying diseases, is at increased risk of developing severe forms of COVID-19 and higher mortality and fatality rates [3]. To date, COVID-19 has caused the death of more than 5 million people worldwide [4]. With the number of infections and deaths from COVID-19 still growing exponentially in many regions of the world, although several vaccines are already available, it is imperative to try to understand the effects of SARS-CoV-2 in the human body and to look for potential non-pharmacological and pharmacological approaches to prevent and treat this pandemic [5]. A very important aspect related to the response to SARS-CoV-2 infection is the competence of the immune system, whose main role is to protect the individual from pathogenic microorganisms. The different interaction between the virus and the individual’s immune system results in a much different clinical picture in patients with COVID-19. Although adaptive immune responses are essential for the neutralization of SARS-CoV-2, innate immune cells, particularly NK cells, T lymphocytes and macrophages, innate, may also play a role in disease progression [6], [7], [8]. Several minerals and trace elements (e.g., magnesium, iron, zinc, copper, selenium) are essential for an adequate immune response [9] and have anti-inflammatory and antioxidant properties that may be of great importance for the competent response of patients to COVID-19 [10], [11], [12]. Thus, it is assumed that an adequate physiological status of trace elements with a significant role in the immune response reduces the potential risk of SARS-CoV-2 infection and the disease severity [13]; and it is considered of utmost importance to control the nutritional status of COVID-19 patients, in which possible deficiencies must be promptly corrected, especially in the elderly and patients with comorbidities [14], [15]. Patients with COVID-19 must therefore receive an adequate supply of trace elements to prevent possible complications of the disease, which can result in serious and even fatal consequences [16].
Thus, the assessment of trace element status in COVID-19 patients with different degrees of disease severity can provide important information. In particular, such studies can help to determine the real impact of eventual trace element deficiencies on the susceptibility to the disease and demonstrate their potential as an index to assess the severity of the clinical picture and to predict clinical outcomes. Additionally, they can provide scientific support for dietary supplementation interventions. In this context, we conducted a study on the trace element status of COVID-19 patients (n = 210) grouped according to the degree of disease severity: “mild” (n = 51), “moderate” (n = 54), “severe” (n = 53) and “exitus letalis” (n = 52), the latter corresponding to patients who ended up dying from the disease. Using ICP-MS analytical methodologies, concentrations of magnesium (Mg), iron (Fe), copper (Cu), zinc (Zn), selenium (Se) and iodine (I) were determined in serum samples collected at the time of hospital admission, after confirmation of SARS-CoV-2 infection through a real time reverse transcription-polymerase chain reaction (RT-PCR) test.
2. Materials and methods
2.1. Study population
This study was conducted with SARS-CoV-2 positive patients (RT-PCR positive test) recruited at the time of admission to the General Hospital Tešanj, Bosnia and Herzegovina, between January and July 2021. Based on clinical signs and symptoms, laboratory and radiological (imaging) findings, oxygen saturation values, and general condition [17], patients (n = 210) were classified into four different groups: “mild”, “moderate” and “severe” clinical picture, and “exitus letalis” patients (i.e., patients whose disease led to death). The research was carried out in accordance with the ethical principles outlined in the Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. The study protocol was reviewed and approved by the Ethics Committee of the General Hospital Tešanj (01–4–18/21, 11th January 2021) and all participants gave their written informed consent to take part in the study.
2.2. Samples collection
Blood specimens were collected from subjects by standard venipuncture. After coagulation at room temperature for ∼30 min, serum was separated by centrifugation at 3000 g for 15 min and stored at − 20 ℃ until analysis.
2.3. Samples analysis
Serum samples were analyzed at the Laboratory of Applied Chemistry (Trace Element Analysis Unit), Faculty of Pharmacy, University of Porto, Portugal, using duly validated inductively coupled plasma mass-spectrometry (ICP-MS) analytical procedures. The instrument was an iCAP™ Q (Thermo Fisher Scientific, Bremen, Germany), equipped with a Meinhard® (Golden, CO) TQ+ quartz concentric nebulizer, a Peltier cooled, high purity quartz, baffled cyclonic spray chamber and a demountable quartz torch with a 2.5 mm i.d. quartz injector. The interface consisted of two (sampler and skimmer) Ni cones. High-purity argon (99.9997%) supplied by Gasin (Leça da Palmeira, Portugal) was used as the nebulizer and plasma gas. Before each analytical run, the instrument was tuned for maximum sensitivity and signal stability and for minimal formation of oxides and double-charged ions. The main parameters of ICP-MS operation were as follows: nebulizer gas flow, 1.17 L/min; auxiliary gas flow, 0.79 L/min; plasma gas flow, 14.0 L/min; power of radio frequency generator, 1550 W; dwell time, 10 ms.
Ultrapure water (resistivity > 18.2 MΩ.cm at 25 °C), obtained with an Arium® pro water purification system (Sartorius, Gottingen, Germany) and high purity reagents were always used. All laboratory ware (tubes, volumetric flasks) was made of plastic and was properly decontaminated by immersion for at least 24 h in a 10% (v/v) HNO3 bath, followed by abundant rinsing with ultrapure water.
For the determination of Mg, Cu, Zn, Fe and Se a procedure based on Goullé et al. was used [18]. Briefly, serum samples were diluted 1:10 with a diluent solution containing 0.65 % v/v HNO3 (from Nitric Acid 67–69 %, Trace Metal™, Fisher Scientific, Leicestershire, UK), 0.01% v/v Triton X-100 (Sigma-Aldrich, St. Louis, MO), 0.5% v/v butanol (> 99.0 %, Sigma-Aldrich, St. Louis, MO) and internal standards (IS) at 10 µg/L (added using TraceCERT® “Periodic table mix 3 for ICP”, Sigma-Aldrich, Buchs, Switzerland). For Se, a 5-point calibration curve (25, 50, 100, 250 and 500 µg/L) was generated with standard solutions prepared by adequate dilution of “Periodic table mix 1” (TraceCERT®, Sigma-Aldrich). For Cu, Zn, Fe and Mg, five-point calibration curves (500, 1000, 1500, 2000 and 5000 µg/L for Cu, Zn and Fe; 5 times these concentrations for Mg) were generated with mixed calibration solutions prepared by adequate dilution of single-element standard stock solutions (Certipur® Cu standard solution, Merck, Darmstadt, Germany; Zn standard for AAS, SCP Science, Baie-D’Urfe, Quebec, Canada; TraceCERT® Fe standard for AAS, Fluka, Buchs, Switzerland; Mg standard for AAS, Fluka) in 2% HNO3. These calibration solutions were then diluted 1:10 with the diluent solution, as for the samples. The elemental isotopes 25Mg, 57Fe, 65Cu, 66Zn and 82Se were measured for analytical determinations and the elemental isotopes 45Sc, 89Y, 141Pr and 159Tb were monitored as IS.
For the determination of iodine, a procedure based on CDC (Center for Disease Control and Prevention) Laboratory Procedure Manual was used [19]. Briefly, serum samples were diluted 1:10 with a diluent solution containing 1% (v/v) tetramethylammonium hydroxide (TMAH; 25% m/m, Sigma-Aldrich, St. Louis, MO), 0.01% v/v Triton X-100, and 10 µg/L Te (IS) (Sigma-Aldrich). A five-point calibration curve (20, 50, 100, 200 and 500 µg/L) was generated with calibration standards prepared by adequate dilution of an iodine standard stock solution (TraceCERT®, Sigma-Aldrich) in water. These calibration solutions were then diluted 1:10 with the diluent solution, as for the samples. The elemental isotopes measured in the ICP-MS analysis were 127I and 125Te (IS).
After thorough homogenization in a vortex mixer, diluted samples and calibration standards were presented to ICP-MS instrument using a CETAC ASX-520 autosampler (Teledyne CETAC Technologies, Omaha, NE).
Samples were analyzed in random order to avoid sequence (series) effects. For analytical quality assurance, repeated analysis (at the beginning, middle and end of the analytical run) of Seronorm™ Trace Elements Serum L-1 and L-2 (obtained from SERO AS, Billingstad, Norway) was performed. As shown in S9 (Supplementary Materials), the results obtained in the internal Quality Control were well within the certified ranges.
2.4. Statistical analysis
Statistical analysis was performed using SPSS Statistics v.26.0 (IBM Corporation, NY). The normality of data distribution was evaluated by the Shapiro-Wilk and Kolmogorov-Smirnov tests. Variables not normally distributed were Ln transformed, after which normally distributed data were obtained. The significance of differences in trace elements concentrations between groups was estimated using the ANOVA (analysis of variance) test. Bonferroni test was performed for post hoc analysis. Student’s t-test was used to assess the difference between genders, while Spearman's rho correlation was applied to assess monotonic relationships between variables in each group. P-values less than 0.05 were considered statistically significant. The results are presented as mean ± standard deviation (SD).
3. Results
Table 1 shows the descriptive statistics of demographic data of the subjects included in the study, divided into 4 groups according to the severity of the clinical picture.
Table 1.
Descriptive statistics of patients’ demographic data.
| MILD | MODERATE | SEVERE | EX. LETALIS | |
|---|---|---|---|---|
| TOTAL PATIENTS (N = 210) | 51 | 54 | 53 | 52 |
| Age (mean ± SD) | 45.2 ± 19.4 | 62.8 ± 9.9 | 61.4 ± 11.8 | 72.5 ± 8.9 |
| Men (N = 125) | 31 | 25 | 35 | 34 |
| Age (mean ± SD) | 46.2 ± 18.8 | 61.1 ± 9.4 | 61.9 ± 11.0 | 72.4 ± 7.9 |
| Women (N = 85) | 20 | 29 | 18 | 18 |
| Age (mean ± SD) | 43.6 ± 20.7 | 64.3 ± 10.3 | 60.5 ± 13.5 | 72.6 ± 10.8 |
| Male/female ratio | 1.55 | 0.86 | 1.94 | 1.89 |
The trace elements levels (µg/L) in serum samples from COVID-19 patients grouped according to severity of clinical picture are shown in Table 2. Most of the trace elements determined differed significantly between “mild”, “moderate”, “severe” and “ex. letalis” groups. Cu levels were highest in the “moderate” group, while the lowest values were found in the “mild” group, and the results showed a statistically significant difference between the “mild” and “moderate”, “mild” and “severe”, and “mild” and “ex. letalis” groups. Zn levels were highest in the “mild” group, while the lowest were found in the “ex. letalis” group. The results showed a statistically significant difference between the “mild” and “severe”, “mild” and “ex. letalis”, “moderate” and “ex. letalis”, and “severe” and “ex. letalis” groups. Accordingly, the Cu/Zn ratio was highest in the “ex. letalis” group, while the lowest value was found in patients with “mild” clinical picture, and a statistically significant difference was observed in the Cu/Zn ratio between all groups, except between the “moderate” and “severe” groups. For Se, the serum levels were also highest in the “mild” group, while the lowest values were observed in the “ex. letalis” group. A statistically significant difference also existed between all groups, except between the “moderate” and “severe” groups. Iron levels were highest in the “mild” group, and lowest in the “ex. letalis” group. The difference in Fe levels was statistically significant only between the “mild” and “ex. letalis” groups. Finally, no statistically significant differences were found for I and Mg between all disease severity groups.
Table 2.
Trace element levels (µg/L; mean ± SD) in serum samples from COVID-19 patients according to severity of disease.
| Element | MILD (N = 51) | MODERATE (N = 54) | SEVERE (N = 53) | EX. LETALIS (N = 52) | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | 1186 ± 283 | 1475 ± 281 | 1383 ± 308 | 1412 ± 336 | < 0.001 | 0.003 | 0.001 | 0.667 | 1.000 | 1.000 |
| Zn | 814 ± 164 | 758 ± 124 | 679 ± 195 | 574 ± 218 | 0.694 | 0.002 | < 0.001 | 0.162 | < 0.001 | 0.024 |
| Cu/Zn ratio | 1.55 ± 0.56 | 1.99 ± 0.66 | 2.28 ± 1.23 | 3.09 ± 2.85 | 0.001 | < 0.001 | < 0.001 | 1.000 | 0.001 | 0.025 |
| Se | 85.9 ± 18.0 | 75.0 ± 16.1 | 74.7 ± 18.0 | 64.7 ± 19.8 | 0.033 | 0.022 | < 0.001 | 1.000 | 0.006 | 0.015 |
| Fe | 1234 ± 531 | 1156 ± 492 | 1110 ± 622 | 943 ± 530 | 1.000 | 0.688 | 0.010 | 1.000 | 0.055 | 0.651 |
| I | 93.9 ± 18.9 | 102.9 ± 24.4 | 98.1 ± 23.7 | 104.0 ± 35.6 | 0.546 | 1.000 | 1.000 | 1.000 | 1.000 | 0.748 |
| Mg | 19771 ± 1942 | 20151 ± 3112 | 19078 ± 2803 | 18890 ± 3382 | 1.000 | 0.934 | 0.333 | 0.347 | 0.097 | 1.000 |
P1-P6 are the p-values for the differences in trace elements levels between groups. P1 - difference between “mild” and “moderate” groups; P2 - difference between “mild” and “severe” groups; P3 - difference between “mild” and “ex. letalis” groups; P4 - difference between “moderate” and “severe” groups; P5 - difference between “moderate” and “ex. letalis” groups; P6 - difference between “severe” and “ex. letalis” groups. Differences were tested using the ANOVA test. Bonferroni test was performed for post hoc analysis.
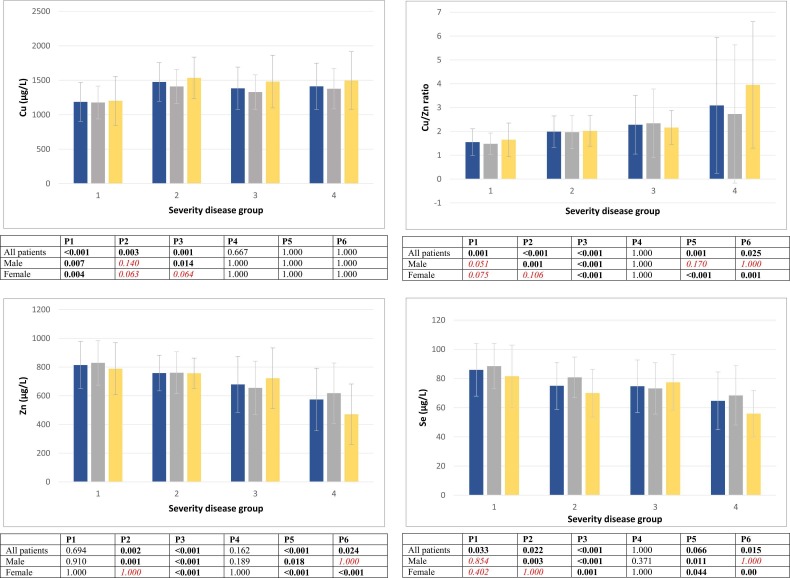
Trace elements levels (µg/L) in the serum of COVID-19 patients, grouped according to the severity of the clinical picture, were then analyzed separately after partitioning by sex. The results for Cu, Zn, Cu/Zn ratio and Se are graphically summarized in Fig. 1. After this sex partitioning, the difference between some disease severity groups lost statistical significance: a) In males, the difference between “mild” and “severe” groups for Cu; between “severe” and “ex. letalis” groups for Zn; between “mild” and “moderate”, “moderate” and “ex. letalis”, and “severe” and “ex. letalis” groups for Cu/Zn ratio; and between “mild” and “moderate”, and “severe” and “ex. letalis” groups for Se; b) In females, the difference between “mild” and “severe”, and “mild” and “ex. letalis” groups for Cu; between “mild” and “severe” groups for Zn; and between “mild” and “moderate”, and “mild” and “severe” groups for both Cu/Zn ratio and Se.
Fig. 1.
Trace element levels (µg/L; mean ± SD) in serum samples from COVID-19 patients according to severity of disease. All patients - left bar; Male - center bar; Female - right bar. P1-P6 are the p-values for the differences in trace elements levels between groups. P1 - difference between “mild” and “moderate” groups; P2 - difference between “mild” and “severe” groups; P3 - difference between “mild” and “ex. letalis” groups; P4 - difference between “moderate” and “severe” groups; P5 - difference between “moderate” and “ex. letalis” groups; P6 - difference between “severe” and “ex. letalis” groups. Differences were tested using the ANOVA test. Bonferroni test was performed for post hoc analysis. Differences that lost statistical significance after sex partition are highlighted in italics.
Table S1, S2, S3 and S4, included in Supplementary Materials, show the comparison between the trace element levels in male and female subjects of the different disease severity groups. There was no statistically significant difference in trace element levels between men and women in the “mild” group (Table S1). Statistically significant differences between men and women were observed for Se and I levels in the “moderate” group (Table S2), for Mg levels in the “severe” group (Table S3), and for Zn, Se and I levels in the “ex. letalis” group (Table S4). Tables S5, S6, S7 and S8, also presented in Supplementary Materials, show the correlations between the determined trace elements levels, age and C-reactive protein (CRP) in the different disease severity groups.
4. Discussion
4.1. Age and gender
Since the onset of the COVID-19 pandemic, it has been known that the risk of infection, hospitalization and death is strongly correlated with age [20], and this is mainly due to the age-related increase in the prevalence of comorbidities. Male sex has also been shown to be a risk factor for severe disease and death [21]. Patients in our cohort followed this pattern. The mean age was significantly higher in the “ex. letalis” group (72.5 ± 8.9 years) versus only 45.2 ± 19.4 years in the “mild” group (and approximately 62 years old in the “moderate” and “severe” groups). And except in the “moderate” group (0.86), the proportion of men was always higher, with a male/female (m/f) ratio of 1.55, 1.94 and 1.89 in “mild”, “severe” and “ex. letalis” groups, respectively.
4.2. Trace element status
As highlighted above, many trace elements are essential for maintaining the integrity of human immune system functions and, consequently, for an adequate host response to fight against infections, particularly viral ones. Thus, its deficiency will increase the individual’s susceptibility to the disease [15], [22]. On the other hand, several trace elements also play a key role in the infection process on the virus side, either as components of metalloproteins responsible for the virus attachment to host cells or participating in various genomic processes, from reverse transcription and initial integration, to the protection of newly synthesized DNA and genome maturation [22]. Furthermore, through different mechanisms, viral infection can induce a dysregulation of trace element homeostasis [23] in the patient's body, which makes the interpretation of plasma concentration data somewhat complicated.
Magnesium is the fourth most abundant cation in the human body (after Na, K and Ca), and the second most abundant cation within cells (after K) [24]. It has many biological functions, from structural roles and modulation of cell proliferation and differentiation to activation of numerous enzymes (it is required for the functioning of more than 300 enzymes) [24], [25]. The general evidence that Mg deficiency may confer a greater predisposition to infectious diseases is mainly derived from animal models, where Mg has been shown to be involved in the host’s immune response in several ways, including immune cell adhesion, IgM lymphocyte binding, antibody-dependent cytolysis, and as a cofactor for immunoglobulin synthesis [25]. Thus, a depressed immune response may be a clinical sign of an eventual Mg deficiency [24]. In humans, the key role of Mg (as a free intracellular cation) in the immune system response was highlighted by the discovery of XMEN (X-linked immunodeficiency with magnesium defect, Epstein–Barr virus infection, and neoplasia) disease, which is characterized by chronic Epstein-Barr virus infection [26].
In blood plasma, Mg is present mainly as free ions, while approximately 30% is bound to albumin [25]. The reference range (i.e., the range of expected values in healthy individuals) for total Mg in adult serum is 17–23 mg/L, but deficiency symptoms usually do not appear until values below 10 mg/L [27].
In our study, serum Mg concentrations were well within this reference range in all groups, with values slightly higher in the “mild” and “moderate” groups than in the “severe” and “ex. letalis” groups (mean values of ca. 20 mg/L vs. 19 mg/L), but without reaching statistical significance. This is in close agreement with a recent study by Skalny et al., where no significant difference was observed between patients (“mild”, “moderate” and “severe” groups) and controls, and also no correlation between serum Mg levels and disease severity [28]. However, although serum Mg is the standard test for assessing Mg status, it should be noted that serum Mg represents only approximately 0.3% of total body content and serum concentrations are poor predictors of both total body content and intracellular levels [29]. Low serum concentrations reliably indicate deficiency, but normal values do not exclude significant Mg tissue depletion.
Existing evidence from experimental and clinical studies on the relevance of Mg homeostasis in COVID‑19 has been recently reviewed. Most evidence supports the importance of Mg levels, but the literature is somewhat contradictory. Specifically, COVID-19 patients have shown lower levels than controls and severe disease cases and mortality have been associated with low Mg levels [30]. On the other hand, although almost half of hospitalized patients had low Mg levels, hypermagnesemia was found more prevalent in patients who required ICU admission, and pregnant women with COVID-19 showed higher Mg levels than controls in the first and third trimesters. In another study, hypermagnesemia was found to be correlated with acute phase reactants [31], which led the authors to hypothesize an eventual detrimental impact of increased Mg levels on the course of the disease.
Overall, existing evidence indicates that, especially during the current pandemic, it is important to monitor Mg status in the general population (and correct it when necessary), as this can help prevent SARS-CoV-2 infection, reduce the severity of COVID-19 symptoms and improve recovery [30].
Iron, a transition metal, is essential for many cellular processes, including ATP generation, nucleic acid synthesis, and various enzymatic and non-enzymatic processes such as oxygen-binding and electron-transfer reactions [32]. It plays a key role in immunity and Fe dyshomeostasis influences the functioning of both the innate and adaptive arms of the immune system [33]. Iron also plays a significant role in viral infections. During virus replication, ATP is needed, and Fe is required for ATP synthesis. In addition, expression of the Fe regulatory hormone hepcidin is increased by several cytokines (e.g., IL-6, IL-1), and increased hepcidin levels are known to be related to low plasma Fe levels (a non-specific host defense mechanism based on Fe deprivation to invading pathogens) [34]. Hepcidin exerts its effects by binding and mediating the degradation of ferroportin, the only known cellular iron exporter, present in the cell membrane of enterocytes and macrophages, thus preventing the efflux of Fe into the plasma from those cells [33]. Recent research found a potential sequence similarity between the cysteine-rich cytoplasmic tail of the coronavirus spike protein and hepcidin [35]. As SARS-CoV-2 infection causes overproduction of cytokines, it will cause overexpression of hepcidin and consequent accumulation of Fe within enterocytes and macrophages and high Fe content in cells may benefit virus replication [36].
The reference range for Fe in serum is sex-dependent: 500–1500 µg/L in males and 350–1450 µg/L in females [37]. The results obtained in our study were well within this interval, but a slight tendency towards a decrease in the serum Fe concentration according to the disease severity was observed. The difference reached statistical significance for “mild” vs. “ex. letalis” groups (1233 ± 531 vs. 943 ± 530 mg/L).
Serum Fe deficiency has been found in COVID-19 patients, and disease severity and mortality have been shown to be closely correlated with serum Fe levels (low serum Fe was shown to be an independent risk factor for death in COVID-19 patients). In particular, Fe deficiency showed to be valuable in predicting the transition from mild to severe illness, with low serum Fe levels being an independent risk factor for death in COVID-19 patients [38], [39]. Furthermore, disturbances in Fe homeostasis can persist for a few months after the onset of the disease and are closely associated with non-resolving lung pathologies and impaired physical performance [40]. In the recent study by Skalny et al., COVID-19 patients had significantly lower serum Fe (1.33 ± 0.7 mg/L in severe disease) compared with controls (1.87 ± 0.66 mg/L), and serum Fe (as well as Se and Zn to a lesser extent) was directly correlated with SpO2 [28]. In another recent retrospective cohort study [41], low Fe status was associated with an increased risk of severe disease and adverse outcomes. In particular, decreased serum Fe was found to be associated with acute respiratory distress syndrome (ARDS) and acute organ injuries. Accordingly, the authors suggested that Fe status may be indicative of a poor prognosis for COVID-19. As emphasized above, upregulation of hepcidin by inflammatory stress response pathways induces a decrease in serum Fe. In our study, serum Fe was inversely correlated with the nonspecific inflammatory marker C-reactive protein (CRP), but only in the “mild” and “moderate” disease severity groups (Supplementary Materials, Tables S5 and S6).
Copper is an essential transition metal with a remarkable ability to be involved in redox reactions. In particular, it plays a role as a cofactor for specific enzymes involved in antioxidant metabolism, namely superoxide dismutase (Cu,Zn-SOD), a key player in the defense against oxidative stress, promoting the superoxide radical neutralization [42]. Copper also appears to be essential for the maintenance of immune function, and increased susceptibility to infection, as well as an inadequate immune response, with changes in several immunological markers, have been associated with Cu deficiency [43]. In particular, Cu exhibits strong virucidal effects, acting on the virus itself, and its role in inhibiting SARS-CoV-2 cell entry and replication has been highlighted [44], and it has even proposed as a potential adjunct therapy for critically ill COVID-19 patients [45].
In blood plasma, Cu mainly binds to ceruloplasmin, which serves as a Cu transport protein and is an acute-phase reactive protein [46]. Women tend to have higher serum Cu levels [47]. Commonly accepted reference intervals are 700–1400 µg/L for men and 800–1550 µg/L for women [48].
Our results showed serum Cu concentrations within the normal range only in the “mild” disease severity group (1186 ± 283 µg/L). The other groups (“moderate”, “severe” and “ex. letalis”) had Cu levels around 20% higher, already above the reference range, without significant differences between them. The slightly higher mean value in the “moderate” group was related to the higher proportion of women in this group. These findings confirm the close positive relationship of serum concentrations of Cu (and ceruloplasmin) with infection and inflammation, as part of the acute-phase response [49]. However, a significant positive correlation between serum Cu and CRP was obtained only for the “mild” and “moderate” severity groups, not existing in the higher severity groups (Supplementary Materials, Tables S5-S8). This may be related to the fact that during the inflammatory process serum CRP levels seem to rise much earlier than ceruloplasmin and last for a much shorter period of time [50]. Skalny et al. also reported increased serum Cu levels in COVID-19 patients compared to controls, but no significant differences between “mild”, “moderate” and “severe” groups [28]. In a recent small study by Hackler et al., conflicting results were obtained, with surviving patients showing higher mean serum Cu compared to non-survivors (mean ± SEM: 1475.9 ± 22.7 vs. 1317.9 ± 43.9 µg/L) [46]. Furthermore, the authors found relatively stable levels of Cu over the course of hospital stay and a complex disease-dependent relationship between Cu and Se, a different finding than what would be expected from severe inflammation alone, with a positive linear correlation of serum Cu and Se, instead of an increase in Cu and a decrease in Se. The relationship between Cu dyshomeostasis in COVID-19 patients and clinical symptoms was studied by Skalny et al., who found serum Cu (and especially the Cu/Zn ratio) closely associated with lung damage, inflammation and fever, and inversely associated with blood oxygen saturation (SpO2) [28].
Zinc is one of the most important essential trace elements, a structural component of numerous biologically relevant molecules and a cofactor of hundreds of enzymes [51]. In particular, it is a key factor in maintaining adequate innate and adaptive immune responses, and Zn deficiency affects inflammatory processes, exacerbating the inflammatory response and host tissue damage [52]. In vitro studies suggest that free Zn may have potent antiviral effects, and dietary Zn supplementation has been shown to improve the immune response in Zn-deficient patients [53] and specifically inhibit viral replication and infection-related symptoms [54]. Thus, Zn has been proposed as one of the most important components of strategies aimed at boosting the immune system and treating COVID-19 patients [55], [56], [57].
Systemic and intracellular Zn is tightly regulated. In plasma, Zn circulates mainly bound to albumin [54], a well-established negative acute-phase reactant [49]. The decrease in serum Zn concentration is mediated by interleukins (IL-1 and IL-6), which promote the uptake of Zn by the liver, and as for Fe, this is considered a host defense mechanism, since it deprives microorganisms of an essential trace element for their growth and replication [49].
In our study, low serum Zn concentrations were obtained, on the lower side of the commonly accepted reference interval (600–1200 µg/L), or even clearly below [58]. There was also a steady and significant trend of decreasing levels according to the disease severity (from 814 ± 164 in the “mild” group to 574 ± 218 µg/L in the “ex. letalis” group), with a significant negative correlation with CRP levels in all groups, except “ex. letalis” group (Supplementary Materials, Tables S5-S8). This Zn behavior is a typical finding in infectious and inflammatory processes. Decreasing serum Zn levels in association with COVID-19 severity, with levels lower than in healthy controls, has also been recently reported [28].
Copper / Zinc ratio – The normal Cu/Zn ratio is close to 1:1 [59]. Due to the well-known antagonistic effect between these metals, the Cu/Zn ratio is considered clinically more relevant than just the individual concentration of each element [60]. As discussed above, Cu and Zn levels followed opposite trends. Thus, the Cu/Zn ratio gradually increased in association with COVID-19 severity, from 1.55 ± 0.56 in the “mild” to 3.09 ± 2.85 in the “ex. letalis” group. That is, patients who eventually died from the disease had a mean Cu/Zn ratio that was almost double that observed in patients with mild symptoms. The Cu/Zn ratio thus proved to be a very sensitive predictor of fatality. Interestingly, much greater inter-individual variability was observed in this group, higher in men, which may be due to the presence of important co-morbidities. In the study by Skalny et al., a similar trend was observed and the Cu/Zn ratio was shown to be inversely associated with blood oxygen saturation [28] .
Selenium is an essential non-metallic trace element. Most of its biochemical actions are exerted through a group of essential proteins (selenoproteins), where it is incorporated in the form of selenocysteine (the 21st proteinogenic amino acid). Selenoproteins include glutathione peroxidase (GPX) and thioredoxin reductases, essential components of the human antioxidant defense [61]. Insufficient levels of Se in the human body impair cellular immunity, and individuals with low plasma concentrations of Se show increased oxidative stress, with increased production of reactive oxygen species (ROS). In particular, this can result in viral RNA mutations, which can result in more virulent and pathogenic variants [62], [63], [64]. There is ample evidence of the antiviral role of Se and specifically in relation to SARS-CoV-2. The association between soil Se levels and the incidence of COVID-19 has been reported [64], and a recent ecological study found an association between Se deficiency (assessed from the Se content in crops and topsoil) and increased COVID-19 case fatality rate [65]. On the other hand, in a recent population-based retrospective analysis of patient data from China, an association was also shown between reported COVID-19 cure rates and Se status (assessed by hair Se levels) [63].
In blood plasma, selenoprotein P is the main Se-containing protein and is a negative acute-phase reactant [66]. A widely accepted reference range for serum Se is 70–150 µg/L [67]. In our study, the mean serum Se concentration significantly decreased from “mild (85.9 ± 18.0 µg/L) to ”ex. letalis” (64.7 ± 19.8 µg/L) groups, with the “moderate” and “severe” groups showing intermediate and similar Se levels. An inverse correlation of Se levels with CRP in the four patient’s groups was also observed (Supplementary Materials, Tables S5-S8). In the recent study by Skalny et al., decreased serum Se levels were also found (patients vs. controls), with a decrease related to the severity of the disease [28]. In addition, Se levels were shown to be an independent predictor of lung damage. These are expected findings as oxidative stress and hyperinflammation are hallmarks of COVID-19 and Se deficiency has been reported in COVID-19 patients [68], in association with disease severity [69]. Dietary supplementation with sodium selenite (which can oxidize thiol groups in the virus protein disulfide isomerase, rendering it unable to penetrate the healthy cell membrane) [70] has been suggested as a strategy to combat SARS-CoV-2 infection, especially in the context of low dietary intake [71].
Iodine, an essential halogen, is crucial for the proper functioning of virtually every system in the human body. Its effects are primarily mediated by thyroid function, as iodine is a key component necessary for the synthesis of thyroid hormones (T3 and T4) [72]. Thyroid hormones can directly affect several aspects of innate and adaptive immune responses, namely B cell differentiation, phagocytosis, cytolytic activity of natural killer cells against virus-infected targets and cytokine synthesis [73], [74]. In contrast, the direct effects of inorganic iodine (I2) or iodide anion (I-) on immune system activity have not yet been fully elucidated. However, in vitro studies showed increased cytokine synthesis in leukocytes treated with NaI or Lugol solution (iodine plus KI), and an epidemiological study showed a decreased immune response in iodine-deficient schoolchildren, despite normal thyroid hormone levels [74]. Interestingly, in Japan, a country known for its high iodine intake by the general population, relatively low mortality from COVID-19 has been observed despite having the eldest population in the world and having adopted relatively moderate lockdown measures [75]. However, both iodine deficiency and excess iodine are associated with an increased risk of thyroid disorders, and excessive iodine intake can precipitate hyper- or hypothyroidism and thyroid autoimmunity in some individuals [76].
Dietary iodine intake is usually assessed by measuring the urinary iodine concentration (UIC). However, the large day-to-day variation of UIC makes it difficult to quantify individual iodine intake [77]. Serum iodine concentrations, the determination performed in the present study, showed a strong nonlinear correlation with UIC and thyroid function [78], [79], and may be a better index of dietary iodine intake and iodine overload. A serum iodine level higher than 100 μg/L has been considered a risk factor for thyroid diseases [78]. The results obtained in the present study were close to this risk threshold for thyroid diseases, with no significant differences between groups (93.9 ± 18.9 µg/L in the “mild” group; 104.0 ± 35.6 µg/L in the “ex. letalis” group). There is a strong rationale for suspecting that patients with hypo- or hyperthyroidism may have an increased susceptibility to infection and a worse prognosis. However, a population-based case-control study using data from the Danish COVID-19 cohort suggests that patients treated for hypothyroidism or hyperthyroidism are not at increased risk of SARS-CoV-2 infection and therapy does not influence the prognosis of COVID-19 [80]. Another large retrospective cohort study conducted in the US also showed that hypothyroidism is not associated with an increased risk of COVID-19 related hospitalization or a worse outcome, including death [81].
5. Conclusions
Significant imbalances in the serum levels of important trace elements related to the immune response were observed in this cohort of COVID-19 patients. With the exception of Mg, which presented serum levels well within the reference (normal) range and without significant differences depending on the disease severity (“mild”, “moderate”, “severe” and “exitus letalis” groups), it was possible to observe: i) for iodine – relatively high levels (in all disease severity categories), with no differences between groups; ii) for Cu – increased levels in all disease severity categories (except in the “mild” group, which had normal levels), without differences between them; iii) for Se – significantly decreasing serum levels according to the disease severity, reaching sub-normal values in the groups of higher severity; iv) for Fe – serum levels within the reference range, decreasing slightly with disease severity (not reaching statistical significance.); v) for Zn – significantly decreasing serum levels according to the disease severity, reaching sub-normal values in the groups of higher severity. The Cu/Zn ratio proved to be a very sensitive predictor of fatality. It gradually increased in association with disease severity, from 1.55 ± 0.56 in the “mild” group to 3.09 ± 2.85 in the “ex. letalis” group. In the separate analysis after sex partitioning, the main differences between sexes were observed in “ex. letalis” group, with women having significantly lower Se and Zn levels (and significantly higher Cu/Zn ratios).
Overall, our findings are in agreement with those of similar studies and reinforce the importance of monitoring trace element levels in COVID-19 patients and conducting relevant interventions, namely Zn and Se supplementation. Further studies are needed to discriminate to what extent the observed imbalances in trace element levels were already present at the time of infection (and were responsible for an increased susceptibility) or were triggered by the infection itself. Furthermore, it would be important to study how much of the differences observed between the four groups could simply be due to the significantly different mean age of the individuals in each group using strictly age-matched control groups (healthy individuals).
Funding
This research was funded by Ministry of Science, Higher Education and Youth of Canton Sarajevo, grant number 27-02-11-4375-10/21. This work was also supported by UIDB/50006/2020 with funding from FCT/MCTES (Portugal) through national funds.
CRediT authorship contribution statement
Tamer Bego: Conceptualization, Software, Data curation, Writing – original draft, Funding acquisition. Neven Meseldžić: Data curation, Writing – original draft. Besim Prnjavorac: Investigation. Lejla Prnjavorac: Project administration. Damir Marjanović: Writing — review & editing, Supervision. Rui Azevedo: Validation. Edgar Pinto: Validation. Mary Duro: Writing – review & editing. Cristina Couto: Writing – review & editing. Agostinho Almeida: Methodology, Software, Validation, Data curation, Writing – original draft, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
We thank Anel Mahmutović MD, Elvira Čorhodžić MD, for the tremendous support in patient recruitment, as well as Emir Hondo MD, medical biochemistry specialist, Head of Department of Laboratory Diagnostics, General Hospital Tešanj. Also, we thank Nermin Kotorić and Ehlimana Pobrić for their technical and administrative support.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of GENERAL HOSPITAL TEŠANJ (01-4-18/21, 11th January, 2021).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jtemb.2022.127055.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Government of Canada. COVID-19 signs, symptoms and severity of disease: A clinician guide. Available online: 〈https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/signs-symptoms-severity.html〉 (Accessed on 16/11/2021).
- 2.Mutiawati E., Fahriani M., Mamada S.S., Fajar J.K., Frediansyah A., Maliga H.A., Ilmawan M., Emran T.B., Ophinni Y., Ichsan I., Musadir N., Rabaan A.A., Dhama K., Syahrul S., Nainu F., Harapan H. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms - a systematic review and meta-analysis. F1000Research. 2021;10:40. doi: 10.12688/f1000research.28393.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farshbafnadi M., Kamali Zonouzi S., Sabahi M., Dolatshahi M., Aarabi M.H. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors. Exp. Gerontol. 2021;154 doi: 10.1016/j.exger.2021.111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Coronavirus (COVID-19) Dashboard. Available online: 〈https://covid19.who.int/〉 (accessed on 6/12/2021).
- 5.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paces J., Strizova Z., Smrz D., Cerny J. COVID-19 and the immune system. Physiol. Res. 2020;69(3):379–388. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olea B., Albert E., Torres I., Amat P., Remigia M.J., Gozalbo-Rovira R., Rodriguez-Diaz J., Buesa J., Blasco M.L., Redon J., Signes-Costa J., Navarro D. Adaptive immune responses to SARS-CoV-2 in recovered severe COVID-19 patients. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2021;142 doi: 10.1016/j.jcv.2021.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carsetti R., Zaffina S., Piano Mortari E., Terreri S., Corrente F., Capponi C., Palomba P., Mirabella M., Cascioli S., Palange P., Cuccaro I., Milito C., Zumla A., Maeurer M., Camisa V., Vinci M.R., Santoro A., Cimini E., Marchioni L., Nicastri E., Palmieri F., Agrati C., Ippolito G., Porzio O., Concato C., Onetti Muda A., Raponi M., Quintarelli C., Quinti I., Locatelli F. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra R.K. Nutrition and the immune system: an introduction. Am. J. Clin. Nutr. 1997;66(2):460S–463S. doi: 10.1093/ajcn/66.2.460S. [DOI] [PubMed] [Google Scholar]
- 10.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S., Corsico A.G., Di Sabatino A., Belliato M., Calvi M., Iacona I., Grugnetti G., Bonadeo E., Muzzi A., Cereda E. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo J.L., Marques M. The effects of some essential and toxic metals/metalloids in COVID-19: a review. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021;152 doi: 10.1016/j.fct.2021.112161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler M.J., Barrientos R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav., Immun. 2020;87:53–54. doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaqoob P. Ageing alters the impact of nutrition on immune function. Proc. Nutr. Soc. 2017;76(3):347–351. doi: 10.1017/S0029665116000781. [DOI] [PubMed] [Google Scholar]
- 15.Taheri S., Asadi S., Nilashi M., Ali Abumalloh R., Ghabban N.M.A., Mohd Yusuf S.Y., Supriyanto E., Samad S. A literature review on beneficial role of vitamins and trace elements: evidence from published clinical studies. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2021;67 doi: 10.1016/j.jtemb.2021.126789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calder P.C. Nutrition and immunity: lessons for COVID-19. Eur. J. Clin. Nutr. 2021;75(9):1309–1318. doi: 10.1038/s41430-021-00949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baj J., Karakula-Juchnowicz H., Teresinski G., Buszewicz G., Ciesielka M., Sitarz E., Forma A., Karakula K., Flieger W., Portincasa P., Maciejewski R. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J. Clin. Med. 2020;9(6) doi: 10.3390/jcm9061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulle J.P., Mahieu L., Castermant J., Neveu N., Bonneau L., Laine G., Bouige D., Lacroix C. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Ref. Values Forensic Sci. Int. 2005;153(1):39–44. doi: 10.1016/j.forsciint.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Urine Iodine ICPMS. Centers for Disease Control and Prevention (CDC) Laboratory Procedure Manual, 2001. Available online: 〈https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06uio_c_met_urine_iodine_icpms.pdf〉 (Accessed on 3/12/2021).
- 20.Risk for COVID-19 infection, hospitalization, and death by age group. Available online: 〈https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html〉 (Accessed on 18/11/2021).
- 21.Reilev M., Kristensen K.B., Pottegard A., Lund L.C., Hallas J., Ernst M.T., Christiansen C.F., Sorensen H.T., Johansen N.B., Brun N.C., Voldstedlund M., Stovring H., Thomsen M.K., Christensen S., Gubbels S., Krause T.G., Molbak K., Thomsen R.W. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int. J. Epidemiol. 2020;49(5):1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jesus J.R., Andrade T.D. Understanding the relationship between viral infections and trace elements from a metallomics perspective: implications for COVID-19. Metallomics. 2020;12(12):1912–1930. doi: 10.1039/d0mt00220h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharmalingam K., Birdi A., Tomo S., Sreenivasulu K., Charan J., Yadav D., Purohit P., Sharma P. Trace elements as immunoregulators in SARS-CoV-2 and other viral infections. Indian J. Clin. Biochem.: IJCB. 2021:1–11. doi: 10.1007/s12291-021-00961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiNicolantonio J.J., O'Keefe J.H., Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Heart. 2018;5(1) doi: 10.1136/openhrt-2017-000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam M., Gomez S., Gonzalez-Gross M., Marcos A. Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 2003;57(10):1193–1197. doi: 10.1038/sj.ejcn.1601689. [DOI] [PubMed] [Google Scholar]
- 26.Ravell J., Chaigne-Delalande B., Lenardo M. X-linked immunodeficiency with magnesium defect, Epstein-Barr virus infection, and neoplasia disease: a combined immune deficiency with magnesium defect. Curr. Opin. Pediatr. 2014;26(6):713–719. doi: 10.1097/MOP.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo Clinic Laboratories – Magnesium. Available onliine: 〈https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/8448〉 (accessed on 21/11/2021).
- 28.Skalny A.V., Timashev P.S., Aschner M., Aaseth J., Chernova L.N., Belyaev V.E., Grabeklis A.R., Notova S.V., Lobinski R., Tsatsakis A., Svistunov A.A., Fomin V.V., Tinkov A.A., Glybochko P.V. Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites. 2021;11(4) doi: 10.3390/metabo11040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahnen-Dechent W., Ketteler M. Magnesium basics. Clin. Kidney J. 2012;5(Suppl 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapani V., Rosanoff A., Baniasadi S., Barbagallo M., Castiglioni S., Guerrero-Romero F., Iotti S., Mazur A., Micke O., Pourdowlat G., Scarpati G., Wolf F.I., Maier J.A. The relevance of magnesium homeostasis in COVID-19. Eur. J. Nutr. 2021 doi: 10.1007/s00394-021-02704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anuk A.T., Polat N., Akdas S., Erol S.A., Tanacan A., Biriken D., Keskin H.L., Moraloglu Tekin O., Yazihan N., Sahin D. The relation between trace element status (Zinc, Copper, Magnesium) and clinical outcomes in COVID-19 infection during pregnancy. Biol. Trace Elem. Res. 2021;199(10):3608–3617. doi: 10.1007/s12011-020-02496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galaris D., Barbouti A., Pantopoulos K. Iron homeostasis and oxidative stress: an intimate relationship, Biochimica et biophysica acta. Mol. Cell Res. 2019;1866(12) doi: 10.1016/j.bbamcr.2019.118535. [DOI] [PubMed] [Google Scholar]
- 33.Cherayil B.J. Iron and immunity: immunological consequences of iron deficiency and overload. Arch. Immunol. Et. Ther. Exp. 2010;58(6):407–415. doi: 10.1007/s00005-010-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arezes J., Nemeth E. Hepcidin and iron disorders: new biology and clinical approaches. Int. J. Lab. Hematol. 2015;37(Suppl 1):92–98. doi: 10.1111/ijlh.12358. [DOI] [PubMed] [Google Scholar]
- 35.Ehsani S. COVID-19 and iron dysregulation: distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol. Direct. 2020;15(1):19. doi: 10.1186/s13062-020-00275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taheri M., Bahrami A., Habibi P., Nouri F. A review on the serum electrolytes and trace elements role in the pathophysiology of COVID-19. Biol. Trace Elem. Res. 2021;199(7):2475–2481. doi: 10.1007/s12011-020-02377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo Clinic Laboratories. Iron and Total Iron-Binding Capacity, Serum. Available online: 〈https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/34624〉 (Accessed on 6/12/2021).
- 38.Zhou C., Chen Y., Ji Y., He X., Xue D. Increased serum levels of hepcidin and ferritin are associated with severity of COVID-19. Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.926178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect. Dis. 2020;7(7) doi: 10.1093/ofid/ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., Kurz K., Koppelstatter S., Haschka D., Petzer V., Hilbe R., Theurl M., Lehner D., Nairz M., Puchner B., Luger A., Schwabl C., Bellmann-Weiler R., Woll E., Widmann G., Tancevski I., Judith Loffler R., Weiss G. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir. Res. 2020;21(1):276. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv Y., Chen L., Liang X., Liu X., Gao M., Wang Q., Wei Q., Liu L. Association between iron status and the risk of adverse outcomes in COVID-19. Clin. Nutr. 2021;40(5):3462–3469. doi: 10.1016/j.clnu.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uauy R., Olivares M., Gonzalez M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998;67(5 Suppl):952S–959S. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 43.Bonham M., O'Connor J.M., Hannigan B.M., Strain J.J. The immune system as a physiological indicator of marginal copper status? Br. J. Nutr. 2002;87(5):393–403. doi: 10.1079/BJNBJN2002558. [DOI] [PubMed] [Google Scholar]
- 44.Andreou A., Trantza S., Filippou D., Sipsas N., Tsiodras S. COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. vivo. 2020;34(3 Suppl):1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fooladi S., Matin S., Mahmoodpoor A. Copper as a potential adjunct therapy for critically ill COVID-19 patients. Clin. Nutr. Espen. 2020;40:90–91. doi: 10.1016/j.clnesp.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hackler J., Heller R.A., Sun Q., Schwarzer M., Diegmann J., Bachmann M., Moghaddam A., Schomburg L. Relation of serum copper status to survival in COVID-19. Nutrients. 2021;13(6) doi: 10.3390/nu13061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buxaderas S.C., Farre-Rovira R. Whole blood and serum copper levels in relation to sex and age. Rev. Esp. De. Fisiol. 1986;42(2):213–217. [PubMed] [Google Scholar]
- 48.ARUP Laboratories. Copper, Serum or Plasma. Available online: 〈https://ltd.aruplab.com/Tests/Pub/0020096〉 (Accessed on 06/12/2021).
- 49.Galloway P., McMillan D.C., Sattar N. Effect of the inflammatory response on trace element and vitamin status. Ann. Clin. Biochem. 2000;37(Pt 3):289–297. doi: 10.1258/0004563001899429. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed M.S., Jadhav A.B., Hassan A., Meng Q.H. Acute phase reactants as novel predictors of cardiovascular disease. ISRN Inflamm. 2012;2012 doi: 10.5402/2012/953461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv. Nutr. 2013;4(1):82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gammoh N.Z., Rink L. Zinc in infection and inflammation. Nutrients. 2017;9(6) doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnett J.B., Dao M.C., Hamer D.H., Kandel R., Brandeis G., Wu D., Dallal G.E., Jacques P.F., Schreiber R., Kong E., Meydani S.N. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2016;103(3):942–951. doi: 10.3945/ajcn.115.115188. [DOI] [PubMed] [Google Scholar]
- 54.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma P., Reddy P.K., Kumar B. Trace element zinc, a nature's gift to fight unprecedented global pandemic COVID-19. Biol. Trace Elem. Res. 2021;199(9):3213–3221. doi: 10.1007/s12011-020-02462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asl S.H., Nikfarjam S., Majidi Zolbanin N., Nassiri R., Jafari R. Immunopharmacological perspective on zinc in SARS-CoV-2 infection. Int. Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joachimiak M.P. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl. Trop. Dis. 2021;15(1) doi: 10.1016/j.intimp.2021.107630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ARUP Laboratories. Zinc, Serum or Plasma. Available online: 〈https://ltd.aruplab.com/Tests/Pub/0020097〉 (Accessed on 25/11/2021).
- 59.Bockerman P., Bryson A., Viinikainen J., Viikari J., Lehtimaki T., Vuori E., Keltikangas-Jarvinen L., Raitakari O., Pehkonen J. The serum copper/zinc ratio in childhood and educational attainment: a population-based study. J. Public Health. 2016;38(4):696–703. doi: 10.1093/pubmed/fdv187. [DOI] [PubMed] [Google Scholar]
- 60.Osredkar J. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clin. Toxicol. 2011 [Google Scholar]
- 61.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9) doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harthill M. Review: micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011;143(3):1325–1336. doi: 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q.Y., Zhao X.L., Ma J., Mu Y.S., Wang Y., Yang S.H., Wu Y.H., Wu F.C., Zhou Y.Z. Selenium (Se) plays a key role in the biological effects of some viruses: Implications for COVID-19. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H.Y., Zhang A.R., Lu Q.B., Zhang X.A., Zhang Z.J., Guan X.G., Che T.L., Yang Y., Li H., Liu W., Fang L.Q. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021;21(1) doi: 10.1186/s12879-021-06167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forceville X., Mostert V., Pierantoni A., Vitoux D., Le Toumelin P., Plouvier E., Dehoux M., Thuillier F., Combes A., Selenoprotein P. Rather than Glutathione Peroxidase, as a Potential Marker of Septic Shock and Related Syndromes. Eur. Surg. Res. 2009;43(4):338–347. doi: 10.1159/000239763. [DOI] [PubMed] [Google Scholar]
- 67.Mayo Clinic Laboratories. Selenium, Serum. Available online: 〈https://www.mayocliniclabs.com/test-catalog/overview/9765#Clinical-and-Interpretive〉 (accessed on 6/12/2021).
- 68.Majeed M., Nagabhushanam K., Gowda S., Mundkur L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition. 2021;82 doi: 10.1016/j.nut.2020.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khatiwada S., Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19. Curr. Nutr. Rep. 2021;10(2):125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8) doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmermann M.B., Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endo. 2015;3(4):286–295. doi: 10.1016/S2213-8587(14)70225-6. [DOI] [PubMed] [Google Scholar]
- 73.Montesinos M.D., Pellizas C. Thyroid hormone action on Innate Immunity. Front. Endocrinol. 2019;10 doi: 10.3389/fendo.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bilal M.Y., Dambaeva S., Kwak-Kim J., Gilman-Sachs A., Beaman K.D. A role for iodide and thyroglobulin in modulating the function of human immune cells. Front. Immunol. 2017;8:1573. doi: 10.3389/fimmu.2017.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verheesen R.H., Traksel R.A.M. Iodine, a preventive and curative agent in the COVID-19 pandemic? Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farebrother J., Zimmermann M.B., Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci. 2019;1446(1):44–65. doi: 10.1111/nyas.14041. [DOI] [PubMed] [Google Scholar]
- 77.Chung H.R. Iodine and thyroid function. Ann. Pediatr. Endocrinol. Metab. 2014;19(1):8–12. doi: 10.6065/apem.2014.19.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin X., Jiang P., Liu L., Jia Q., Liu P., Meng F., Zhang X., Guan Y., Pang Y., Lu Z., Shen H. The application of serum iodine in assessing individual iodine status. Clin. Endocrinol. 2017;87(6):807–814. doi: 10.1111/cen.13421. [DOI] [PubMed] [Google Scholar]
- 79.Pan Z., Cui T., Chen W., Gao S., Pearce E.N., Wang W., Chen Y., Guo W., Tan L., Shen J., Zhang W. Serum iodine concentration in pregnant women and its association with urinary iodine concentration and thyroid function. Clin. Endocrinol. 2019;90(5):711–718. doi: 10.1111/cen.13945. [DOI] [PubMed] [Google Scholar]
- 80.Brix T.H., Hegedus L., Hallas J., Lund L.C. Risk and course of SARS-CoV-2 infection in patients treated for hypothyroidism and hyperthyroidism. Lancet Diabetes Endocrinol. 2021;9(4):197–199. doi: 10.1016/S2213-8587(21)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Gerwen M., Alsen M., Little C., Barlow J., Naymagon L., Tremblay D., Sinclair C.F., Genden E. Outcomes of patients with hypothyroidism and COVID-19: a retrospective Cohort study. Front. Endocrinol. 2020;11:565. doi: 10.3389/fendo.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material