Abstract
Introduction
Despite modern multimodal therapeutic regimens, the prognosis of esophageal adenocarcinoma (EAC) is still poor and there is a lack of biological markers estimating the patients’ prognosis. Fructose-1,6-biphosphatase (FBP1) is a key enzyme in gluconeogenesis and is associated with tumor initiation in several cancers. Therefore, this study aims to characterize its implication for EAC patients.
Methods and materials
A total of 571 EAC patients who underwent multimodal treatment between 1999 and 2017 were analyzed for FBP1 expression using immunohistochemistry.
Results
82.5% of the EACs show FBP1 expression in the tumor albeit with different intensities categorizing specimens accordingly into score 0 (no expression), score 1 (weak expression), score 2 (moderate expression) and score 3 (strong expression) (score 1 = 25.0%, score 2 = 35.9%, score 3 = 21.5%). Intratumoral FBP1 expression was significantly associated with a better prognosis (p = 0.024). This observation was particularly relevant among patients who received primary surgery without neoadjuvant treatment (p = 0.004). In multivariate analysis, elevated FBP1 expression was an independent biomarker associated with a favorable prognosis.
Discussion
Despite being associated with a favorable prognosis, the majority of patients with high FBP1 expression also require individualized therapy options to ensure long-term survival. Recently, it has been shown that the presence of the FBP1 protein increases the sensitivity of pancreatic cancer cells to the bromodomain and extraterminal domain (BET) inhibitor JQ1.
Conclusion
We described for the first time the prognostic and possibly therapeutic relevance of FBP1 in EAC. The efficiency of the BET inhibitor in EAC should be verified in clinical studies and special attention should be paid to the effects of neoadjuvant therapy on FBP1 expression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04025-x.
Keywords: Esophageal adenocarcinoma; EAC; Fructose-1,6-bisphosphatase 1 (FBP1); Biomarker; Prognosis; Biomarker; Neoadjuvant therapy; Treatment response; Neoadjuvant treatment
Introduction
The adenocarcinoma of the esophagus (EAC) has been showing an increase in incidence for years, especially in Western countries. EAC is a malignant epithelial tumor with glandular differentiation. Histologically, the tumors are mucin producing and may occasionally show foci of squamous or endocrine differentiation. Rarely, there is the formation of signet ring cells, papillary structures, or Paneth cells. Despite a slight improvement in the overall prognosis since the introduction of neoadjuvant therapy, esophageal adenocarcinoma still only shows a 5 years survival of about 25%. The main risk factor for the development of EAC is Barrett mucosa, which is associated with reflux esophagitis. Male sex and obesity have also been identified as significant risk factors. In recent years, considerable progress has been made in the genomic characterization of EAC. However, individualized therapeutic approaches except for Her2/neu blockade have not been established yet (Lambert and Halnaut 2007; Lepage et al. 2008; Xie et al. 2017; Wheeler and Reed 2012; Kim et al. 2017).
Fructose-1,6-biphosphatase (FBP1) is a key enzyme in gluconeogenesis (Yasuda et al. 2021). FBP1 inhibits katabolic metabolic pathways such as glycolysis guaranteeing tumor cells an ongoing supply of nutrients via lactate or amino acids to feed the biosynthetic pathways (Grasmann et al. 1872). Nevertheless, its role in solid tumors is still ambiguous. In colorectal cancer, inhibition of FBP1 by upregulation of its upstream target FOXC1 results in increased tumor proliferation (Li et al. 2019) caused by metabolic reprogramming of the malignant cells. Similarly, in human hepatocellular carcinoma and colon cancer promoter hypermethylation of FBP1 inhibited the tumor-suppressive effect of this enzyme (Chen et al. 2011). In esophageal cancer, only little is known about the function of FBP1 and its impact on tumor characteristics. In esophageal squamous cell cancer, it has been shown that FBP1 promotes proliferation, migration, and invasion by regulating fatty acid metabolism in in vitro experiments (He et al. 2021).
This study aims to analyze the putative prognostic impact of FBP1 in a large EAC cohort and therefore describe the possibility of this enzyme as a biomarker within this context.
Methods and materials
Study cohort and patient’s characteristics
A total of 790 patients underwent surgery (either primary or as part of a multimodality therapy concept) between 1999 and 2017 at the Department of General, Visceral, Cancer and Transplantation Surgery, University Hospital of Cologne, Germany. The standard surgical procedure was laparotomic or laparoscopic gastrolysis and right transthoracic en bloc esophagectomy including two-field lymphadenectomy of mediastinal and abdominal lymph nodes. Reconstruction was performed by high intrathoracic esophagogastrostomy as described previously (Plum et al. 2021; Hölscher et al. 2007). Patients with advanced esophageal cancer (cT1N1M0 or cT2-3N0-1M0) received preoperative chemoradiation (5-FU, cisplatin, 40 Gy as treated analog the CROSS trial) or perioperative chemotherapy following the FLOT regime (Hagen et al. 2012; Al-batran et al. 2008). All patients were followed up according to a standardized protocol. During the first 2 years, patients were followed up clinically in the hospital every 3 months. Afterward, annual examinations were carried out. These follow-up examinations included a detailed history, clinical evaluation, abdominal ultrasound, chest X-ray, and additional diagnostic procedures as required. Follow-up data were available for all patients. Patient characteristics are given in Table 1.
Table 1.
Patient’s characteristics and FBP1 protein analysis (n = 571)
| Patient’s characteristics | Total | FBP1 expression | p value | |||
|---|---|---|---|---|---|---|
| Negative | Positive | |||||
| All patients | 571 | 243 | 328 | |||
| 100.0% | 42.6% | 57.4% | ||||
| Sex | Female | No | 70 | 35 | 35 | 0.198 |
| % | 12.3% | 50.0% | 50.0% | |||
| Male | No | 501 | 208 | 293 | ||
| % | 87.7% | 41.5% | 58.5% | |||
| Age group | < 65 years | No | 301 | 138 | 163 | 0.120 |
| % | 52.7% | 45.7% | 54.3% | |||
| > 65 years | No | 270 | 105 | 165 | ||
| % | 47.3% | 39.0% | 61.0% | |||
| Tumor stage | pT1/2 | No | 155 | 69 | 86 | 0.703 |
| % | 27.4% | 44.5% | 55.5% | |||
| pT3/4 | No | 411 | 174 | 237 | ||
| % | 72.6% | 42.3% | 57.7% | |||
| Lymph node metastasis | pN0 | No | 223 | 87 | 136 | 0.192 |
| % | 39.4% | 39.0% | 61.0% | |||
| pN + | No | 343 | 154 | 189 | ||
| % | 60.6% | 44.9% | 55.1% | |||
| UICC stage | I | No | 113 | 43 | 70 | 0.201 |
| % | 20.0% | 38.1% | 61.9% | |||
| II | No | 136 | 55 | 81 | ||
| % | 24.1% | 40.4% | 59.6% | |||
| III | No | 248 | 107 | 141 | ||
| % | 44.0% | 43.1% | 56.9% | |||
| IV | No | 67 | 36 | 31 | ||
| % | 11.9% | 53.7% | 46.3% | |||
| Neoadjuvant therapy | No | No | 248 | 98 | 150 | 0.202 |
| % | 43.40% | 39.50% | 60.50% | |||
| Yes | No | 323 | 145 | 178 | ||
| % | 56.60% | 44.90% | 55.10% | |||
In this table, the patients were divided into two groups. All patients with absent FBP1 (n = 100) or weak (n = 143) FBP1 expression were combined (group: absent to low) and the patients with moderate (n = 205) or strong (n = 123) FBP1 expression in the tumor were combined (group: moderate to strong)
Protein analysis
In our cohort of 571 patients, the protein status of fructose-1,6-bisphosphatase 1 (FBP1) was determined using immunohistochemistry (IHC). We used a tissue microarray (TMA) with formalin-fixed and paraffin-embedded tumor material. For tissue microarray analysis, one tissue core from each tumor was punched out and transferred into a TMA recipient block. Tissue cylinders with a diameter of 1.2 mm each were punched from selected tumor tissue blocks using a self-constructed semi-automated precision instrument and embedded in empty recipient paraffin blocks. Four-micrometer sections of the resulting TMA blocks were transferred to an adhesive-coated slide system (Instrumedics Inc., Hackensack, NJ) for immunohistochemistry. Immunohistochemistry (IHC) was performed on TMA slides using a rabbit monoclonal antibody against FBP1 (Abcam, clone EPR4619, 1:100 with EDTA buffer) with the automated stainer from Leica Bond, Germany. FBP1 expression was detected within the cytoplasm of TMA samples deriving from the resulting surgical specimens after either primary resection or following prior neoadjuvant treatment. Preoperative tissue samples were not included in the analysis. All stains ran in one immunochemical run accompanied by a positive control. We used liver as well as kidney as positive control.
The evaluation was primarily carried out in a four-step scheme as follows: Score 0 = no expression of FBP1; Score 1 (weak expression) = up to 70% of tumor cells show a weak intensity or up to 30% of tumor cells show moderate intensity of staining; Score 2 (moderate expression) = more than 30% of tumor cells show a moderate intensity of staining or more than 70% a weak intensity of staining or up to 30% of tumor cells show a strong intensity of FBP1 expression; Score 3 = strong expression of more than 70% of tumor cells show a moderate intensity of staining or more than 30% of tumor cells show a strong intensity of staining. In a previous publication, we were able to show that this nationally commonly used scoring system correlates excellently with the internationally more common H-score (Moentenich et al. 2020). The H-score is obtained using the following formula: 3 × percentage of strongly stained cells + 2 × percentage of moderately stained cells and percentage of weakly stained tumor cells. The H-score ranges from 0 to 300. An H-score of over 200 corresponds to a score of 3.
Depending on the effect of neoadjuvant chemotherapy or chemoradiation, there is a preponderance of minor responders in the TMAs, defined as histopathological residual tumor of ≥ 10% (Schneider et al. 2008).
Statistical analysis
Clinical data were collected prospectively and analyzed according to a standardized protocol as previously described (Plum et al. 2021; Plum et al. 2020; Plum et al. 2019). SPSS Statistics for Mac (Version 21, SPSS) was used for statistical analysis. Interdependence between stainings and clinical data were calculated using the Chi-squared and Fisher’s exact tests and displayed by cross-tables. Survival curves were plotted using the Kaplan–Meier method and analyzed using the log-rank test. All tests were two sided. P values < 0.05 were considered statistically significant.
Results
Patients
All 790 patients who underwent Ivor–Lewis esophagectomy between 1999 and 2017 at our department were included on the TMA. Of these, again only 685 (86.7%) patients were immunohistochemically “interpretable” for FBP1. Reasons for the non-informative cases were missing tissue samples or the absence of distinct cancer tissue in the corresponding TMA spot. Furthermore, there were postoperative follow-up data from 571 of these evaluable patients on the TMA, so ultimately a total of 571 patients who underwent surgery during the specified period qualified for the present study. Overall, 571 patients were included in our analysis, 87.7% were male. More than half of all patients (56.9%) had an advanced tumor stage (UICC stage 3 and stage 4). 56.6% of the analyzed tumor samples were from patients who had received neoadjuvant therapy with either chemotherapy alone (according to FLOT regimen) or combined chemoradiation (according to CROSS protocol). There was no significant difference in UICC stages, sex, lymph node positivity rate, and condition after neoadjuvant therapy in patients with no or weak FBP1 expression and patients with a moderate or strong expression (p = 0.201) within the total cohort (see Table 1).
Protein analysis of fructose-1,6-bisphosphatase 1 (FBP1) and overall survival
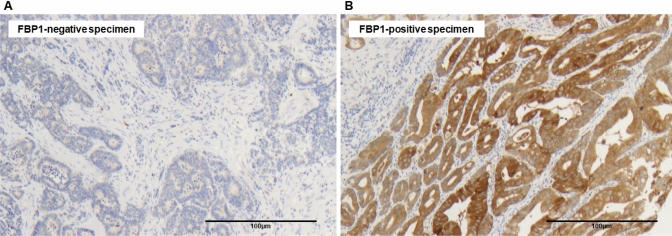
571 patients were examined via immunochemistry for FBP1 expression. 82.5% of them showed FBP1 expression in the tumor albeit with different intensities (score 1 = 25.0%, score 2 = 35.9%, score 3 = 21.5%). Representative images of the immunohistochemical staining are illustrated in Fig. 1.
Fig. 1.
Representative images of immunohistochemistry (IHC) from A FBP1-negative (score 0) and B FBP1-positive (score 3) specimens of esophageal adenocarcinoma from the tissue microarray. Magnification 100×. Example of a strong FBP1-positive adenocarcinoma (score 3). Homogeneous vigorous expression of FBP1 in the cytoplasm of tumor cells. The surrounding stroma is negative as well as an example of an FBP1 negative EACs
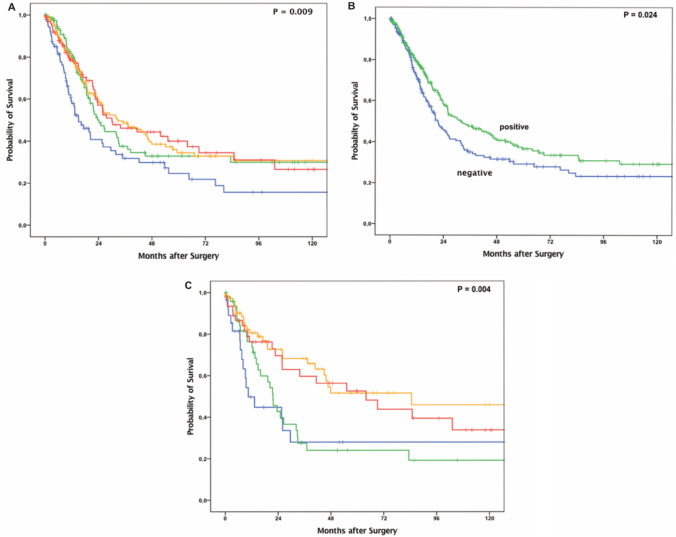
The patient characteristics are summarized in Table 1. In the entire patient cohort, any FBP1 expression showed a better overall survival than negative FBP1 (p = 0.24, Fig. 2B). We performeded survival analysis of the patients with respect to FBP1 expression (Fig. 2A) revealing median survival times for score 0 = 15.1 months (95% CI 8.26–21.84), score 1 = 24.3 months (95% CI 19.19–29.31), score 2 = 32.7 months (95% CI 18.47–46.85) and score 3 = 30.1 months (95% CI 14.1–46.03), respectively. In the group that underwent primary surgery, patients whose tumors had absent (score 0) and low (score 1) FBP1 expression showed a significantly lower median survival [score 0 = 10.5 months (95% CI 4.45–16.51) and score 1 = 21.6 months (95% CI 16.9–26.40)] than those with medium (score 2) and high FBP1 expression (score 2 = 84.6 (95% CI 9.22–15.98), score 3 = 63.9 months (95% CI 24.62–103.25, p = 0.004) (Fig. 2C; Figure S1). This difference was not observed in the group that had received neoadjuvant therapy (Figure S2).
Fig. 2.
Survival analysis of A the entire patient cohort (n = 571) according to the different scores of protein expression of FBP1 within the tumor, B the entire patient cohort stratified by negative or positive FBP1 expression within the tumor specimens, and C FBP1 protein expression in patients with primary surgery (n = 248). Patients who had tumors that showed any expression of FBP1 had a better median overall survival. Among patients without neoadjuvant therapy, moderate (red) and strong (orange) FBP1-positive EAC showed a statistically significant better prognosis in comparison to weak positive or negative tumors. Blue = score 0, green = score 1, red = score 2, orange = score 3
In a multivariate analysis of the entire cohort (taking into account the tumor stages and the nodal stage), FBP1 turned out to be an independent biomarker associated with a favorable prognosis (HR 0.762, 95% CI 0.589, 0.986; p value 0.039, Table 2).
Table 2.
Multivariate analysis revealing positive FBP1 expression associated with statistically significantly lowered HR
| Hazard ratio | 95% confidence interval | |||
|---|---|---|---|---|
| Lower | Upper | p value | ||
| Sex | ||||
| Male vs. female | 1.117 | 0.724 | 1.723 | 0.618 |
| Age group | ||||
| < 65 vs. > 65 years | 1.329 | 1.027 | 1.72 | 0.031 |
| Tumor stage | ||||
| pT1/2 vs. pT3/4 | 1.37 | 0.973 | 1.928 | 0.071 |
| Lymph node metastasis | ||||
| pN0 vs. pN + | 3.048 | 2.239 | 4.148 | < 0.001 |
| FBP1 expression | ||||
| Negative vs. positive | 0.762 | 0.589 | 0.986 | 0.039 |
FBP1 and other molecular markers
We also correlated the immunohistochemical expression of FBP1 in EAC specimens with other prognostic markers such as HER2, IDO, VISTA, GATA6, and CD3 that we have previously published (Plum et al. 2020; Plum et al. 2019; Schoemmel et al. 2021; Loeser et al. 2019; Loeser et al. 2020). High FBP1 expression correlated with increased levels of HER2 (p = 0.007) as well as IDO (p = 0.004) and higher numbers of infiltrating CD3 lymphocytes (p = 0.039), while there was no correlation between FBP1 expression and GATA6 (p = 0.540) or VISTA levels (p = 0.126) (data not shown).
Discussion
The role of FBP1 in various cancer entities is undergoing scrutiny in the last decade and it is found to be up-regulated in different tumor types (Li et al. 2016a). According to the RNA expression data of TCGA, this is also true for adenocarcinoma of the esophagus (Gao et al. 2013). While FBP1 is ascribed a tumor-inhibiting function in various tumor entities, nothing is known about the role of FBP1 in esophageal adenocarcinoma (Grasmann et al. 1872).
We evaluated the significance of FBP1 expression at the protein level in 571 patients with EAC. We could show for the first time and in a large dataset that FBP1 protein can be identified through immunohistochemistry in the majority of tumors (82% of tumors are FBP1 positive) with different intensities. Our results at the protein level match the RNA expression results of the TCGA data (Figure S3). FBP1 was associated with a favorable prognosis in EAC in our collective of patients. In particular, strongly positive tumors show a favorable disease progression compared to the FBP1 weakly positive or negative tumors. The value of FBP1 as a prognostic marker was underlined in the multivariate analysis of our cohort, where FBP1 was an independent prognostic marker (Table 2). This is in line with the fact that FBP1 is also associated with a better prognosis in other tumor entities supporting the relevance of this cross-entity biological effect. Also, in vitro data in other tumor entities such as prostate, gastric and hepatocellular cancer found FBP1 gene silencing, leading to promotion of epithelial to mesenchymal transition, invasion, and metastasis (He et al. 2021; Li et al. 2016; Li et al. 2020). Data on FBP1’s role in EAC are still scarce, but a recent study in esophageal squamous cell carcinoma also found the loss of FBP1 being associated with increased proliferation, migration, and invasion (He et al. 2021). Interestingly, we identified a positive correlation between higher expression of HER2 (a tyrosine kinase regulating intracellular signal pathways resulting in upregulation of proliferation) and elevated FBP1 levels within our EAC specimens. This is concordant to previous findings describing HER2 amplification to be associated with improved survival in EAC (Plum et al. 2019; Yoon et al. 2012). Similarly, higher FBP1 levels within our EAC cohort were connected to high amounts of CD3-positive T cell infiltration within the tumor a phenomenon for which survival benefit has already been described in this tumor entity (Schoemmel et al. 2021). After all, this data emphasizes the putative prognostic relevance of FBP1 demonstrating its potential as a biomarker (alone or in combination with other molecular markers) for prognostic estimations among EAC patients.
Regardless of its positive prognostic value on overall survival, the majority of patients with high FBP1 still died because of the disease in our cohort. Those patients still need individualized therapy options, as adjuvant therapy regimens available to date seem to be most effective in the short term. Recently, it was shown that the presence of the FBP1 protein increases the sensitivity of pancreatic cancer cells to the bromodomain and extraterminal domain (BET) inhibitor JQ1, highlighting FBP1’s possible role in individualized cancer therapy (Wang et al. 2018). In ovarian cancer, FBP1 was found to regulate proliferation, metastasis, and chemoresistance. Ovarian cancer cell lines with increased FBP1 expression were also sensitized to cisplatin-induced apoptosis (Li et al. 2019). Therefore, adjuvant treatment with a platin-containing regimen could theoretically explain the survival benefit of increased FBP1 expression in our cohort, especially in patients that underwent primary resection without pre-treatment. If proven in further studies, the increased chemosensitivity to platin-based drugs in EAC could help in tailoring individual patient therapies. It is also interesting that FBP1 was found to enhance radiosensitivity in nasopharyngeal carcinoma cells (Zhang et al. 2021). We found the most pronounced survival difference in patients that had not received neoadjuvant treatment. Therefore, according to our data, the role of FBP1’s radiosensitizing effect needs to undergo further study in EAC.
The prognostic relevance of FBP1 was highest in our cohort of patients that underwent surgery without any pre-treatment. Neoadjuvant therapy may influence the FBP1 protein expression measured by immunohistochemistry, an observation that needs to be considered when interpreting the results of FBP1 expression. Especially, if future studies use FBP1 expression to investigate individualized therapies.
Conclusion
We describe, for the first time, the prognostic and possibly therapeutic relevance of FBP1 in a large cohort of patients with esophageal adenocarcinoma. Treatment options should be further evaluated and possible changes of FBP1 expression through neoadjuvant therapy further investigated.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
AD, PSP, FG, and AQ conceived and designed the study; AD, PSP, and FG enrolled the patients and collected the clinical data, while AQ performed the pathological analysis; AD and FG carried out the statistical analyses; AD, PSP, FG, WS, TZ and AQ contributed to the interpretation of data. AD, PSP, FG, and AQ drafted the manuscript. All authors were involved in critically revising the manuscript for important intellectual content and approving the submitted version.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets generated and/or analyzed during this current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
Ethics approval This retrospective study was performed according to the criteria of the ethics committee of the University Hospital of Cologne (No. 13–091 and 10–242) and in accordance with the relevant version of the Helsinki Declaration.
Consent to participate/publication
All patients declared their participation and written consent was obtained before participation in the study (No. 13-091 and 10-242). The objective of the project was primarily in the field of diagnostics and quality assurance.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19:1882–1887. doi: 10.1093/annonc/mdn403. [DOI] [PubMed] [Google Scholar]
- Chen M, Zhang J, Li N, et al. Promoter hypermethylation mediated downregulation of FBP1 in human hepatocellular carcinoma and colon cancer. PLoS One. 2011 doi: 10.1371/journal.pone.0025564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013 doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasmann G, Smolle E, Olschewski H, Leithner K. Gluconeogenesis in cancer cells—repurposing of a starvation-induced metabolic pathway? Biochim Biophys Acta Rev Cancer. 1872;24–36:2019. doi: 10.1016/j.bbcan.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hua R, Li B, Gu H, Sun Y, Li Z. Loss of FBP1 promotes proliferation, migration, and invasion by regulating fatty acid metabolism in esophageal squamous cell carcinoma. Aging (Albany NY) 2021;13:4986–4988. doi: 10.18632/aging.103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher AH, Schneider PM, Gutschow C, Schröder W. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg. 2007;245:241–246. doi: 10.1097/01.sla.0000245847.40779.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Bowlby R, Mungall AJ, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–174. doi: 10.1038/541464d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R, Halnaut P. Esophageal cancer: cases and causes (part I) Endoscopy. 2007;39:550–555. doi: 10.1055/s-2007-966530. [DOI] [PubMed] [Google Scholar]
- Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103:2694–2699. doi: 10.1111/j.1572-0241.2008.02191.x. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Li QG, et al. Downregulation of FBP1 promotes tumor metastasis and indicates poor prognosis in gastric cancer via regulating epithelial-mesenchymal transition. PLoS One. 2016 doi: 10.1371/journal.pone.0167857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Ying M, Feng D, et al. Fructose-1,6-bisphosphatase is a novel regulator of Wnt/β-Catenin pathway in breast cancer. Biomed Pharmacother. 2016;84:1144–1149. doi: 10.1016/j.biopha.2016.10.050. [DOI] [PubMed] [Google Scholar]
- Li Q, Wei P, Wu J, et al. The FOXC1/FBP1 signaling axis promotes colorectal cancer proliferation by enhancing the Warburg effect. Oncogene. 2019;38:483–496. doi: 10.1038/s41388-018-0469-8. [DOI] [PubMed] [Google Scholar]
- Li F, Huangyang P, Burrows M, et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat Cell Biol. 2020;22:728–739. doi: 10.1038/s41556-020-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser H, Kraemer M, Gebauer F, et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology. 2019;8:e1581546. doi: 10.1080/2162402X.2019.1581546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser H, Kraemer M, Gebauer F, et al. Indoleamine 2,3-dioxygenase (IDO) expression is an independent prognostic marker in esophageal adenocarcinoma. J Immunol Res. 2020;2020:2862647. doi: 10.1155/2020/2862647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moentenich V, Comut E, Gebauer F, et al. Mesothelin expression in esophageal adenocarcinoma and squamous cell carcinoma and its possible impact on future treatment strategies. Ther Adv Med Oncol. 2020;12:1758835920917571. doi: 10.1177/1758835920917571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum PS, Gebauer F, Krämer M, et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer. 2019;19:38. doi: 10.1186/s12885-018-5242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum PS, Löser H, Zander T, et al. GATA binding protein 6 (GATA6) is co-amplified with PIK3CA in patients with esophageal adenocarcinoma and is linked to neoadjuvant therapy. J Cancer Res Clin Oncol. 2020 doi: 10.1007/s00432-020-03486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum PS, Damanakis A, Buschmann L, et al. Short-term outcome of Ivor Lewis esophagectomy following neoadjuvant chemoradiation versus perioperative chemotherapy in patients with locally advanced adenocarcinoma of the esophagus and gastroesophageal junction: a propensity score-matched analysis. J Cancer Res Clin Oncol. 2021 doi: 10.1007/s00432-021-03720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider PM, Metzger R, Schaefer H, et al. Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann Surg. 2008;248:902–908. doi: 10.1097/SLA.0b013e31818f3afb. [DOI] [PubMed] [Google Scholar]
- Schoemmel M, Loeser H, Kraemer M, et al. Distribution of tumor-infiltrating-T-lymphocytes and possible tumor-escape mechanisms avoiding immune cell attack in locally advanced adenocarcinomas of the esophagus. Clin Transl Oncol. 2021;23:1601–1610. doi: 10.1007/s12094-021-02556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- Wang B, Fan P, Zhao J, Wu H, Jin X, Wu H. FBP1 loss contributes to BET inhibitors resistance by undermining c-Myc expression in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2018 doi: 10.1186/s13046-018-0888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JB, Reed CE. Epidemiology of esophageal cancer. Surg Clin North Am. 2012;92:1077–1087. doi: 10.1016/j.suc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Xie SH, Mattsson F, Lagergren J. Incidence trends in oesophageal cancer by histological type: an updated analysis in Sweden. Cancer Epidemiol. 2017;47:114–117. doi: 10.1016/j.canep.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Ishimoto T, Baba H. Conflicting metabolic alterations in cancer stem cells and regulation by the stromal niche. Regen Ther. 2021;17:8–12. doi: 10.1016/j.reth.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;18:546–554. doi: 10.1158/1078-0432.CCR-11-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Shao Y, Quan F, Liu L, Yang J. FBP1 enhances the radiosensitivity by suppressing glycolysis via the FBXW7/mTOR axis in nasopharyngeal carcinoma cells. Life Sci. 2021;283:119840. doi: 10.1016/j.lfs.2021.119840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during this current study are available from the corresponding author on reasonable request.