Abstract
Malignant pleural mesothelioma (MPM) is a rare and deadly malignancy with an extremely poor prognosis. The median overall survival (OS) of this disease is 12–18 months. However, the oncogenic driver mutations of MPM are rarely understood, and the targeted therapy for it is still under investigation. In this report, we describe a case of MPM with CD74-ROS1 fusion who obtains complete and durable response after receiving crizotinib. By the time of submission, the progression-free survival (PFS) with crizotinib has been 6.0 years, and the patient has survived for 7.6 years. Currently, he is still in complete remission (CR). To the best of our knowledge, this case represents the first report of CD74-ROS1 fusion identified in MPM. Meanwhile, it is also the first report of complete and long-term response to crizotinib in a patient with MPM positive for CD74-ROS1 fusion. This case report might contribute to the tumorigenesis and targeted therapy of this deadly disease.
Keywords: Malignant pleural mesothelioma, CD74-ROS1 fusion, Crizotinib, Targeted therapy
Malignant pleural mesothelioma (MPM) is a rare and highly deadly cancer. Its prognosis is extremely poor, with a reported median overall survival (OS) of 12–18 months, and no definitive therapy is available for this lethal disease. Moreover, MPM is refractory to the trimodal therapy consisting of chemotherapy, radiotherapy and surgery (Scherpereel et al. 2018). To date, there has not been approved targeted therapy for it. Over the recent years, advances in the fields of genomics and functional genomics have achieved a breakthrough in the complex genetic landscape of MPM. However, limited information is available regarding gene fusions in this fatal disease. ROS proto-oncogene 1 (ROS1) fusion is one of oncogenic driver mutations in 1–2% of patients with non-small-cell lung cancer (NSCLC), with CD74-ROS1 fusion being the most common one in light or non-smokers (Bergethon et al. 2012). In 2016, the Food and Drug Administration (FDA) of the United States approved crizotinib as first-line therapy for ROS1-positive advanced NSCLC. However, to date, CD74-ROS1 fusion and the corresponding targeted therapy have not been reported in patients with MPM. Herein, we describe the first case of MPM with CD74-ROS1 fusion who achieves complete and long-term response after receiving treatment with crizotinib.
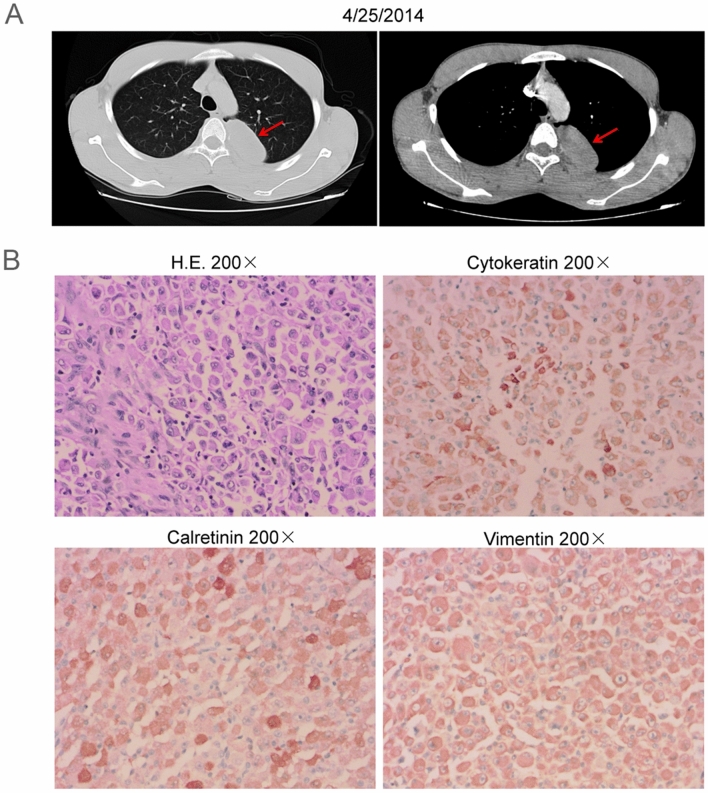
In 2014, a 19-year-old-male patient was admitted to a local hospital due to chest pain without obvious inducement. Thoracic computed tomography (CT) showed a space-occupying lesion under the left upper pleura (Fig. 1A). Preliminary diagnosis was left posterior mediastinal tumor, more likely to be malignant.
Fig. 1.
Radiographic imaging at diagnosis and pathology. A Computed tomography (CT) showed a space-occupying lesion under the left upper pleura. B Hematoxylin and eosin (H.E.) staining revealed the space-occupying lesion was malignant pleural mesothelioma (MPM). Immunohistochemistry (IHC) showed that tumor cells were positive for cytokeratin, calretinin and vimentin
On April 29, 2014, the patient underwent video-assisted thoracoscopic surgery (VATS) and thoracoscopic wedge resection (TWR) of the left upper lobe. Postoperative pathology showed the space-occupying lesion was epithelioid malignant pleural mesothelioma invading pulmonary parenchyma. Meanwhile, tumor thrombus in vessels was observed. Immunohistochemistry (IHC) showed that tumor cells were positive for cytokeratin, vimentin, calretinin, and negative for alpha smooth muscle actin (α-SMA), desmin, MyoD1, myogenin, p63, CD45, CD38, CD138, S-100, C5/6, mesothelial cells (Fig. 1B). The final diagnosis was stage II MPM (pT2NxM0). Subsequently, he received six cycles of postoperative adjuvant chemotherapy combined with pemetrexed and cisplatin, and no adverse events were reported from him.
In July 2015, hoarseness was developed in this patient. Meanwhile, thoracic CT showed enlargement of mediastinal lymph node indicating relapse. On September 10, 2015, he received six cycles of first-line chemotherapy combined with pemetrexed and carboplatin. After two cycles, thoracic CT showed the mediastinal lymph node reduced from 3.94 cm × 4.16 cm in size to 1.0 cm in diameter. Therefore, the clinical response was evaluated as partial remission (PR). However, after six cycles, thoracic CT showed that the mediastinal lymph node was enlarged indicating progressive disease (PD).
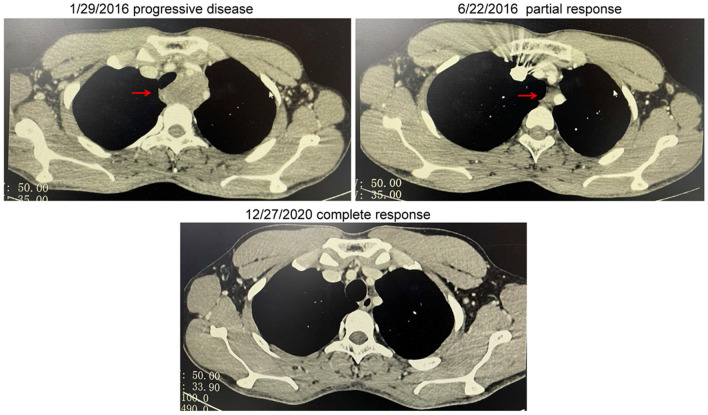
To seek personalized therapy strategies, paraffin-embedded sections of tumor tissues resected from the patient were subjected to real-time PCR (RT-PCR), and the results showed there were no EGFR mutations, MET amplifications or ALK fusions, but ROS1 fusion identified in the patient. However, the partner type of ROS1 could not be determined through this method. From February 2016, he began to receive second-line therapy with oral crizotinib. On June 22, 2016, thoracic CT revealed that the mediastinal lymph node shrank remarkably indicating PR. Furthermore, on December 27, 2020, chest CT scan showed that the mediastinal lymph node disappeared suggesting complete remission (CR). By the time of submitting this manuscript, the progression-free survival (PFS) of second-line therapy with oral crizotinib has been 6.0 years, and he has survived for 7.6 years. Therefore, the patient achieved complete and durable remission (Fig. 2). At present, the patient is still in CR.
Fig. 2.
Dynamic imaging of mediastinal lymph node at different stages of the treatment. The mediastinal lymph node markedly shrank and finally disappeared after treatment with crizotinib
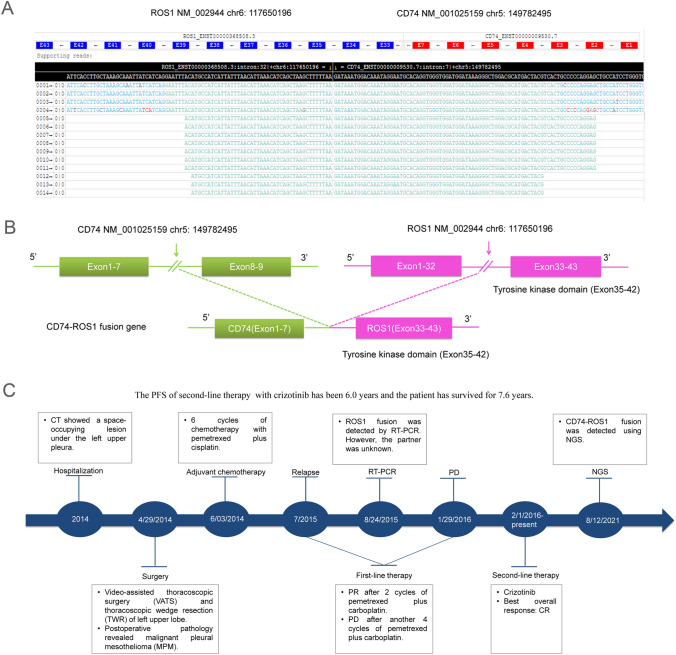
To find out the partner type of ROS1 in the patient, paraffin-embedded sections of tumor tissue resected from him were subjected to next-generation sequencing (NGS) through a 599-gene panel (ChosenMed Technology [Beijing] Co. Ltd, Beijing, China) on August 12, 2021. The results revealed he harbored CD74-ROS1 fusion (Fig. 3A). This fusion included exons 1–7 of CD74 and exons 33–43 of ROS1, which retained the complete tyrosine kinase domain of ROS1 (Fig. 3B). The somatic and germline mutations in the patient were showed in Tables 1 and 2, respectively. The timeline of the patient was demonstrated as Fig. 3C.
Fig. 3.
Next-generation sequencing findings of the primary malignant pleural mesothelioma tissue samples and case timeline. A Visualization of the CD74-ROS1 fusion using the Integrative Genomics Viewer browser (IGV). B An intergenic region between CD74 exon 1–7 and ROS proto-oncogene 1 (ROS1) exon 33–43 fusion variant was identified. C The timeline of the patient
Table 1.
Somatic mutations in the patient
| Gene | Transcript | Exon | Nucleotide change | Alteration | Mutant allele frequency | Variation type |
|---|---|---|---|---|---|---|
| CD74-ROS1 fusion | NM_001025159 | 1–7 | CD74 (exon 1–7)—ROS1(exon 33 to 43) | 1.40% | II | |
| NM_002944 | 33–43 | |||||
| CDKN2A | NM_000077 | 1 | c.35C > T | p.S12L | 15.25% | II |
| CHEK2 | NM_007194 | 11 | c.1116_1117inv | p.K373E | 8.36% | II |
| FLCN | NM_144997 | 11 | c.1285dup | p.H429fs | 2.82% | II |
| PMS2 | NM_000535 | 11 | c.1239dup | p.D414fs | 2.96% | II |
| ACVR1 | NM_001105 | 4 | c.111_112dup | p.E38fs | 2.08% | III |
| AKT3 | NM_005465 | 8 | c.739C > T | p.R247C | 2.24% | III |
| APC | NM_000038 | 16 | c.6386C > T | p.S2129L | 2.63% | III |
| ASXL2 | NM_018263 | 10 | c.1037-1G > A | – | 10.00% | III |
| EPHB4 | NM_004444 | 7 | c.1339C > T | p.P447S | 9.10% | III |
| GNAQ | NM_002072 | 2 | c.303C > A | p.Y101a | 10.05% | III |
| HNF1A | NM_000545 | 4 | c.865dup | p.G292fs | 8.59% | III |
| KEL | NM_000420 | 9 | c.1006G > A | p.V336M | 2.04% | III |
| RTEL1 | NM_032957 | 3 | c.287_289del | p.A96del | 2.96% | III |
| TCF7L2 | NM_030756 | 14 | c.1385dup | p.C463fs | 5.25% | III |
| TET1 | NM_030625 | 12 | c.5531C > T | p.A1844V | 2.15% | III |
| ZFHX3 | NM_006885 | 10 | c.10164_10166del | p.Q3389del | 2.51% | III |
aA premature stop codon due to a nonsense mutation
Table 2.
Germline mutations in the patient
| Gene | Transcript | Chromosome | Exon | Nucleotide change | Alteration | Homozygous/heterozygous | Clinical significance |
|---|---|---|---|---|---|---|---|
| ATRX | NM_000489 | chrX | 9 | c.2806G > C | p.V936L | Homozygous | VUS |
| BCOR | NM_017745 | chrX | 4 | c.935_937del | p.Q312del | Homozygous | Possibly benign |
| E2F3 | NM_001243076 | chr6 | 6 | c.637G > A | p.G213R | Heterozygous | VUS |
| ETV1 | NM_001163147 | chr7 | 3 | c.55G > A | p.G19R | Heterozygous | VUS |
| FLCN | NM_144997 | chr17 | 14 | c.1580G > A | p.R527Q | Heterozygous | VUS |
| FOXL2 | NM_023067 | chr3 | 1 | c.118G > C | p.G40R | Heterozygous | VUS |
| KMT2A | NM_001197104 | chr11 | 3 | c.1512C > A | p.S504R | Heterozygous | VUS |
| LRP1B | NM_018557 | chr2 | 34 | c.5531G > A | p.G1844E | Heterozygous | VUS |
| PMS1 | NM_000534 | chr2 | 11 | c.2440A > G | p.T814A | Heterozygous | VUS |
| PRDM1 | NM_001198 | chr6 | 2 | c.170A > G | p.K57R | Heterozygous | VUS |
| RECQL4 | NM_004260 | chr8 | 5 | c.520C > A | p.H174N | Heterozygous | VUS |
VUS variant of uncertain significance
Besides in NSCLC, ROS1 fusions have been identified in non-NSCLC solid tumors, such as brain tumors and gastrointestinal tumors. According to the study of Huang et al. (Huang et al. 2021), it seems CD74 is a common partner of ROS1 fusion in patients with NSCLC (49.8%), while a rare one in patients with non-NSCLC cases (4.9%). However, it has not been reported in patients with MPM. Crizotinib is a small-molecule inhibitor targeting ALK, MET, and ROS1 tyrosine kinases. To the best of our knowledge, the clinical efficacy of crizotinib in ROS1-positive MPM has not been reported.
In this case report, positive ROS1 fusion was initially detected through RT-PCR in the patient. Subsequently, the partner CD74 was determined using NGS. Of note, the patient responded very well to crizotinib for a durable time.
Crizotinib was the first oral targeted treatment approved for ROS1-positive advanced NSCLC. A long-term clinical benefit has been observed for the patients with ROS1-rearranged metastatic NSCLC since the application of crizotinib. However, the PFS of the patient in this case report (6.0 years) is far superior to the median PFS in Study OO12-01 (15.9 months), PROFILE 1001 (19.2 months), EUROS1 (9.1 months), EUCROSS (20.0 months), and METROS (22.8 months) (Landi et al. 2019; Mazieres et al. 2015; Michels et al. 2019; Shaw et al. 2019; Wu et al. 2018). Furthermore, the patient in this case report has survived for 7.6 years, which is longer than the median OS in Study OO12-01 (32.5 months) and PROFILE 1001 (51.4 months) (Shaw et al. 2019; Wu et al. 2018).
In this case report, the patient is an atypical case being only 19 years old. According to the patient’s dictation, his family has no history of malignant tumors, and he had no history of exposure to asbestos, therefore, this report might highlight the role of genomic testing particularly in those younger patients without an inherited/familial syndrome or no history of previous exposure to asbestos.
To the best of our knowledge, this case represents the first report of CD74-ROS1 fusion identified in MPM. Meanwhile, it is also the first report of remarkable efficacy of crizotinib in a patient with MPM harboring CD74-ROS1 fusion. Moreover, the patient obtained long-term clinical benefit from crizotinib. Therefore, this case illustrates the potential role of genomic testing and targeted therapy in selected cases of MPM.
Acknowledgements
The authors wish to thank the patient for participating in this study.
Author contributions
Conceptualization: WH and BN. Attending physicians for the patient: XX and MY. Writing-original draft: EM. Collecting data: SW.
Funding
This case report was supported by a grant awarded to Mengxing You by Scientific Research Project of Putian University (#2021060).
Data availability
All of the data supporting the findings in this study are available upon reasonable request from the corresponding author (Weiming Huang).
Code availability
Not applicable.
Declarations
Conflict of interest
Erhong Meng, Shunyou Wang and Beifang Niu are employees at ChosenMed Technology. The remaining authors declare that there is no conflict of interest.
Ethics approval
This report was approved by the Ethics Committee of the First Hospital of Putian City. The study has been performed in accordance with the Declaration of Helsinki.
Consent to participate
Written consent was obtained from the patient for the participation.
Consent to publish
Written informed consent was obtained from the patient for the publication of clinical details and images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuehua Xie, and Mengxing You have contributed equally to this work.
References
- Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Iafrate AJ. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RSP, Haberberger J, Sokol E, Schrock AB, Danziger N, Madison R, Ross JS. Clinicopathologic, genomic and protein expression characterization of 356 ROS1 fusion driven solid tumors cases. Int J Cancer. 2021;148(7):1778–1788. doi: 10.1002/ijc.33447. [DOI] [PubMed] [Google Scholar]
- Landi L, Chiari R, Tiseo M, D'Inca F, Dazzi C, Chella A, Cappuzzo F. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non-small cell lung cancer (METROS): a phase II, prospective, multicenter two-arms trial. Clin Cancer Res. 2019;25(24):7312–7319. doi: 10.1158/1078-0432.CCR-19-0994. [DOI] [PubMed] [Google Scholar]
- Mazieres J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T, Gautschi O. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- Michels S, Massuti B, Schildhaus HU, Franklin J, Sebastian M, Felip E, Wolf J. Safety and efficacy of crizotinib in patients with advanced or metastatic ROS1-rearranged lung cancer (EUCROSS): a European phase II clinical trial. J Thorac Oncol. 2019;14(7):1266–1276. doi: 10.1016/j.jtho.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Scherpereel A, Wallyn F, Albelda SM, Munck C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19(3):e161–e172. doi: 10.1016/S1470-2045(18)30100-1. [DOI] [PubMed] [Google Scholar]
- Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, Ou SI. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, Goto K. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–1411. doi: 10.1200/JCO.2017.75.5587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data supporting the findings in this study are available upon reasonable request from the corresponding author (Weiming Huang).
Not applicable.