Abstract
Currently, racial and ethnic differences in adverse birth outcomes and infant mortality are some of the largest and most persistent health disparities in the United States. This narrative review article synthesizes existing literature to present a conceptual model of how racism-related stress and adversity are critical determinants of such disparities. We describe how historical and ongoing racism has created conditions wherein women of color are disproportionately exposed to chronic, multilayered stress and adversity and how the biological consequences of exposure to these stressors confers risk for adverse birth outcomes. Next, we identify important priorities and considerations for future research, including the heterogeneity of racism-related stressors, biomarkers and mechanisms, chronicity and sensitive periods of exposure, developmental programming of lifespan health, resilience, and community-engaged research methodologies.
Key Words: Birth, disparities, racism, pregnancy, preterm
Highlights
-
•
Historical and ongoing racism has created conditions wherein women of color are disproportionately exposed to stress and adversity.
-
•
The consequences of exposure to racism-related stress and adversity can confer risk for health conditions implicated in adverse birth outcomes and alter maternal physiology associated with fetal development and timing of parturition.
-
•
Conjointly studying racism-related stress, biologic profiles, and birth outcomes is a priority for future research.
-
•
It is important to identify factors that mitigate the impact of racism-related stress and adversity on birth outcomes.
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-21-00142
Essential points.
-
•
Historical and ongoing racism has created conditions wherein women of color are disproportionately exposed to stress and adversity.
-
•
The consequences of exposure to racism-related stress and adversity can confer risk for health conditions implicated in adverse birth outcomes and alter maternal physiology associated with fetal development and timing of parturition.
-
•
Conjointly studying racism-related stress, biological profiles, and birth outcomes is a priority for future research.
-
•
It is important to identify factors that mitigate the impact of racism-related stress and adversity on birth outcomes.
Racial/Ethnic disparities in birth outcomes
Five decades ago, the United States had the sixth lowest infant mortality rate among industrialized countries. Today, it ranks 26th (1). During that same half century, the Black-White infant mortality ratio has increased from 1.6 to 2.2 (1). Despite significant advances in obstetric and perinatal medicine, the magnitude of this disparity is greater today than during antebellum slavery (2). Preterm birth and low birth weight comprise the leading causes of infant mortality, and unsurprisingly, these outcomes occur almost twice as often among Black women compared with White women. Although not to the same extent as Black women, Latinx/Hispanic, Asian American/Pacific Islander, Native American, and Multiethnic women also have higher rates of adverse birth outcomes compared with White women (3). The urgency of this public health crisis is compounded by the fact that in addition to being contributors to infant mortality, preterm birth and low birth weight are associated with risk for physical and mental health disorders across the lifespan and impose a significant economic burden, most recently estimated at $26.2 billion annually (4).
The imperative to study racism-related stress and adversity as drivers of disparities in birth outcomes
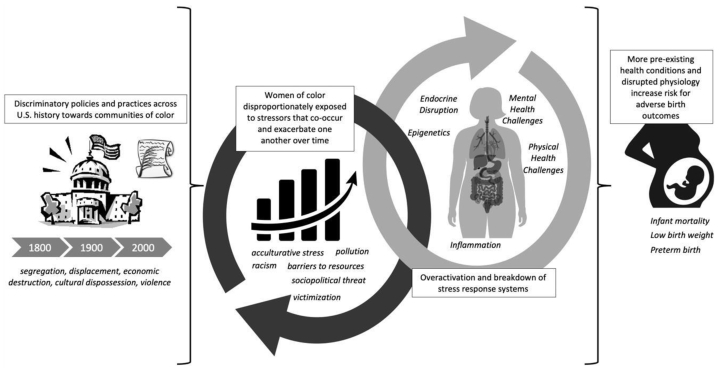
Although the existence of racial/ethnic differences in birth outcomes has been documented for more than 150 years, there has been a historical failure in the scientific field to move beyond simply naming these differences. Consequently, research has perpetuated the message that race in and of itself is a risk factor for poor health—an argument that quickly falls flat on inspection. For example, research has shown that there are more optimal birth outcomes among immigrant and refugee mothers of color residing in the United States compared with native-born mothers of color (1, 5). Similarly, racial/ethnic differences also cannot be fully explained by other sociodemographic predictors such as income or education (1)—although a Black mother with an advanced degree has lower odds of losing her child within its first year of life than a Black mother with less education, she has higher odds than a White mother with less than an 8th grade education (6). Thus, in line with growing calls in the broader field of health inequities, it is imperative that researchers recognize sociocultural context and interrogate racism, rather than race, as a critical driver of disparities in birth outcomes (1, 7). The goal of this article is to present a narrative review and conceptual model (Fig. 1) of the ways in which racism-related stress and adversity drive disparities in birth outcomes, and to outline current research gaps and recommendations.
Figure 1.
The contribution of racism-related stress and adversity to disparities in birth outcomes: a conceptual model.
Sociopolitical-historical context
US history is riddled with discriminatory policies and practices against virtually every community of color. These acts led to (and in many cases directly caused) experiences of segregation (e.g., Jim Crow Laws), displacement and separation (e.g., Indian Removal Act and Dawes Act), economic destruction (e.g., employment, mortgage, and loan discrimination), cultural dispossession (e.g., banning or discouraging certain cultural beliefs, language, practices, and expressions), and violence. This long history has embedded discriminatory beliefs and racial/ethnic inequity into the United States’ social and structural fabric, placing historically oppressed populations at greater risk of experiencing adversities that co-occur and exacerbate one another at both the interpersonal (e.g., interpersonal discrimination, violence, and incarceration) and structural (e.g., underresourced neighborhoods, unequal access to opportunity) levels (7, 8, 9, 10). Further, research on stress proliferation processes demonstrates how initial stressors beget additional new stressors (11), explaining why populations of color not only disproportionately face adversities that are linked to racism at face value such as discrimination but are also at increased risk of experiencing more “traditional” adversities such as community violence or death of a parent, compared with their White counterparts (10). For example, because of racial/ethnic disparities in life expectancy, Black youth are more likely to lose a parent at a younger age compared with White youth (10, 12). Additionally, theories of intersectionality and “double jeopardy” suggest that women of color are at even greater risk of stress and adversity because they possess multiple marginalized identities (gender and race/ethnicity), thus creating more opportunities for discrimination and stress (5, 13).
Cumulative risk, stress, and health
Cumulative risk models posit that risk for poor health increases in a graded manner with total number of adverse early life experiences (14, 15, 16). Repeated exposure to stressors can result in chronic activation of physiological systems that mobilize the response to the stressful situation and subsequently return the body to homeostasis. Over time, the overactivation and interaction of these systems (neuroendocrine, autonomic nervous, and immune) lead to their dysfunction (sometimes referred to as toxic stress, wear and tear, or weathering) and stress-related disease (12, 17, 18, 19). Regarding pregnancy and childbirth, exposure to adversity may increase risk for health conditions implicated in adverse birth outcomes, such as infection and pregnancy-induced hypertension, and/or directly alter maternal physiology associated with fetal development and timing of parturition (17, 20, 21).
Synthesizing understanding of biological consequences of chronic stress with the historical and current sociocultural context surrounding populations of color in the United States illuminates why research has found these groups to be at greater risk of worse health outcomes, including birth outcomes. The impact of these physiological health risks are compounded by structural barriers to health-promoting resources (healthcare, healthy food, exercise spaces/facilities, etc.), which occur along racial lines (22). Thus, it is likely that women of color’s disproportionate exposure and vulnerability to adversity, driven by racism, are key drivers of racial/ethnic disparities in birth outcomes (8). Following is a description of studies and evidence that support this hypothesis.
The contribution of racism-related stress and adversity to disparities in birth outcomes: empirical evidence
Existing evidence links multiple types of racism-related adversity to adverse birth outcomes. Regarding interpersonal discrimination, a recent systematic review from 2021 examined 28 studies on the relation between maternal experiences of interpersonal discrimination and pregnancy or birth related outcomes (23). Authors concluded that the majority of these studies provide evidence that maternal experiences of interpersonal discrimination predict adverse birth outcomes (23). Their findings build on those of 3 previous systematic reviews (24, 25, 26) that similarly found evidence for associations between experiences of racial discrimination, preterm birth, and low birth weight.
Qualitative reports have highlighted inadequate, unequal, and/or discriminatory treatment by healthcare providers or systems during pregnancy, at both interpersonal and structural levels; for example, a lack of culturally competent providers and staff, fragmented care, differential treatment, and provider assumptions about mothers’ lifestyle (such as being unmarried, on medical assistance, or using drugs) (9, 26, 27). Quantitative studies on healthcare utilization and provision of care complement these findings. Compared with White women, Black women are more likely to receive late or no prenatal care, partially due to structural barriers such as lack of available providers nearby, insurance type, and travel and scheduling challenges (28). There are also stark racial/ethnic differences in cesarean delivery rates. Specifically, among women with low-risk pregnancies that are most favorable for vaginal deliveries (singleton, term, cephalic, and primiparous), Black women have the highest rates of cesarean delivery, followed by Asian women (29). Conversely, Black women have the lowest rates among women with high risk pregnancies for whom cesarean delivery is usually indicated (29).
Related to healthcare more broadly, a nationally representative survey of 3,453 adults found that between a fifth and a third of Black, Native American, and Latinx respondents experienced racial discrimination in healthcare and 15%–22% avoid medical care because of concern of discrimination (30). Studies asking about specific types of racial discrimination occurring in medical settings find that experiences of discourtesy, disrespect, poor service, and condescension are most commonly endorsed (31, 32, 33). Other research has found interactions between White healthcare providers and patients of color to be briefer, less patient-centered (i.e., care that considers treatment options on the basis of a patient’s unique concerns, preferences, and values), and less positive than provider–patient interactions between White physicians and patients (34). A substantial body of literature has attributed many of these observed differences to implicit bias among medical providers or automatically activated, primarily unconscious beliefs resulting from chronic exposure to negative stereotypes (35). Recently, however, scholars have argued for new understanding of implicit bias as a cognitive reflection of systemic racism. In other words, implicit bias is “the natural outcome of a mind that generates associations based on statistical regularities, whenever that mind is immersed in an environment of systemic racism” (36, p.8). Therefore, we contend that attending to systemic racism, opposed to implicit bias, should be a priority for reducing racism-related healthcare disparities.
The impact of structural racism and birth outcomes also appears when examining neighborhood effects. Residential area characteristics have been linked to adverse birth outcomes, including racial segregation, more property damage, crime, poor air quality, and lack of green space (1). As noted in a recent review by Shonkoff et al. (22), communities of color are less likely to exist in places with access to health-promoting resources like grocery stores with healthy food options, sidewalks, or parks. Additionally, they are more likely to be targeted by tobacco and alcohol advertisements and have greater concentrations of air and water pollution, which have been linked to adverse birth outcomes (22). A review and meta-analysis of neighborhood disadvantage (comprised of poverty, deprivation, racial residential segregation or racial composition, and crime indicators) found that preterm birth and low birth weight were 27% and 11% more likely, respectively, among most disadvantaged compared with least disadvantaged neighborhoods (37). A second meta-analysis of neighborhood disadvantage studies that adjusted for race did not find these increased odds, illustrating how both race and neighborhood environment may be proxy indicators of overarching racism (37).
People of color and particularly Black men are disproportionately targeted by the justice system (38). Indicators of this issue, including rates of incarceration and police killings in this population, have been linked to poorer birth outcomes. One study’s findings suggested the Black-White gap infant mortality would be 10% lower if mass incarceration did not exist (39), while another showed greater Black-White preterm birth disparities in geographic areas with more police killings of unarmed Black people (40).
Lastly, evidence has shown that sociopolitical initiatives and policies can adversely impact health through increasing structural barriers to health and directly increasing exposure to stressors. For example, the 2020 “public charge” rule (which stated that green card applicants could be denied on the basis of past or potential use of government benefit programs) likely led many immigrant families to be fearful of seeking healthcare or enrolling in public benefit programs, including health insurance (41, 42). Research has also shown increases in adverse birth outcomes among groups disproportionately impacted by sociopolitical events, for example, among women from Muslim travel-banned countries between September 2017 and August 2019 (43), Latina women after the 2016 US presidential election (44, 45), Latina immigrant women in Arizona after Senate Bill 1070 (which targeted undocumented immigrants) (46), and among US-born and immigrant Latina women across Iowa after a major federal immigration raid in the state (47).
Recommendations for future research
Distinctive and “Traditional” Stressors
Existing evidence provides strong initial support for racism-related stress and adversity as important drivers of disparities in birth outcomes at multiple levels. At the same time, to form a comprehensive understanding of these associations and mechanisms behind them, there are several key areas where further research is needed. Novel forms of racism-related stress and adversity that are emerging in other areas of study must also be evaluated as unique contributors to disparities in birth outcomes. For example, vicarious racism (hearing or seeing racism directed toward ones’ racial group), racism-related vigilance, and appropriated racial oppression or internalized racism (internalization of negative racial stereotype against ones’ racial group) have been linked to worse physical and mental health outcomes such as depression, anxiety, cardiovascular disease, and obesity (48, 49). Notably, it is now fully acknowledged by global human rights organizations that racist police violence is widespread, systematic, and persistent in the United States (50). Accordingly, researchers have begun to illustrate how negative encounters with the police and the anticipatory stress of police brutality are both clear, distinctive social determinants of health (51). Lastly, examining differences in the pathways between well-established stressors such as socioeconomic stress and adverse childhood experiences and birth outcomes is also important, given that historical racism has created a cascading system where populations of color are disproportionately exposed to stressors such as these (10, 12). Ideally, research will take a comprehensive approach that studies how different types of racism-related stressors at both interpersonal and structural levels co-occur and interact across one’s lifespan to influence adverse birth outcomes.
Recommendations
-
•
Study forms of racism-related stress and adversity beyond interpersonal discrimination, including vicarious racism, racism-related vigilance, appropriated racial oppression/internalized racism, and police-brutality-related exposure and fear.
-
•
Study racial/ethnic differences in pathways between well-established stressors like economic hardship and adverse childhood experiences and birth outcomes.
Chronicity and Sensitive Periods
Two significant areas where further clarity is needed are related to timing of racism-related adversity exposures. A large body of research has identified pregnancy as a sensitive period where stress and adversity exposure have a clear detrimental impact on birth outcomes (52, 53, 54, 55), with a recent meta-analysis finding prenatal stressful life events to be associated with a 14%–23% higher risk of preterm birth, low birth weight, and small for gestational age outcomes (56). Several studies have also examined preconception adversity, demonstrating that it has a unique effect on adverse birth outcomes (57). One such investigation found that the magnitude of the relation between preconception stressful life events and low birth weight was magnified by 40% for those in low-income neighborhoods (58), highlighting the compounding nature of interpersonal and structural adversity.
Despite contributing important advances to understanding environmental determinants of adverse birth outcomes, research that compares the impact of exposures across different developmental periods to date has largely not focused on racism-related stressors (57). Additionally, there is variability in how researchers operationalize “preconception adversity.” In a recent review, several studies defined preconception adversity as experiencing adverse events 6 months before conception, whereas others assessed childhood stressors (57). One study by Dominguez et al. (59) highlights the importance of assessing these distinct developmental periods, finding that both childhood and lifespan exposures to racism were associated with lower birth weight. A second important timing consideration is that several racism-related stressors are not single discrete events but rather chronic and long-lasting (i.e., economic hardship, everyday discrimination). Some previous research suggests that chronic, everyday forms of discrimination (such as being followed around in stores more so than others) are more harmful to health than acute events of major discrimination (like being unfairly fired) (60, 61). Specific to birth outcomes, 1 recent study found that experiences of both major and everyday discrimination predicted low birth weight, whereas only major discrimination predicted preterm birth (62). However, much more research is needed in this area with large and diverse samples to develop a better understanding of how chronicity of mothers’ stressor exposure may influence adverse birth outcomes.
Recommendations
-
•
Separately assess maternal exposures to adversity in childhood, in adulthood before pregnancy, and during pregnancy, and examine their unique and cumulative impact on birth outcomes.
-
•
Separately assess maternal exposures to chronic and acute racism-related stressors, and examine their impact on birth outcomes. Williams' Major and Everyday Discrimination Scales (63) are examples of measures that assess both types of racism.
Biological Mechanisms
Another area that requires more research is on mechanisms through which racism-related stress and adversity impact birth outcomes. Importantly, biomechanisms linking stress to adverse birth outcomes have been identified (64, 65, 66, 67), and some of these biological profiles have also been associated with racism-related stress, including altered endocrine function (e.g., placental corticotropin-releasing hormone and cortisol), impaired immune function, and cardiovascular profiles (68, 69, 70, 71, 72, 73, 74). The prenatal maternal microbiome (including vaginal, gut, oral, and cervical) is also gaining increasing recognition for its relation to experiences of stress and adversity, birth outcomes, and fetal development (64, 75, 76). In particular, studies have found differences in the microbiome of women by race/ethnicity and between women who deliver term and those who deliver preterm (77, 78). Despite the growing body of evidence on biomechanisms related to stress and birth outcomes, few studies have tested an integrated model by examining racism-related stress, biological profiles, and birth outcomes conjointly.
Additionally, although stress impacts multiple physiological systems, most prenatal research has targeted individual biological parameters or systems. In the broader field of stress research, concerns that this approach overlooks the cumulative biological impact of stress and misses important indicators of risk have led to widespread interest in allostatic load (AL), an index measure representing cardiovascular, metabolic, immune, and endocrine system functioning (79). Allostatic load may be a key mechanism through which stress exposure affects birth outcomes (26, 80, 81), but characterizing AL in the context of pregnancy presents several challenges because of the unique physiological signature of gestation (54, 82). No consensus currently exists regarding an index of prenatal AL. Therefore, although there is strong evidence that the individual components of AL predict birth outcomes (52, 82, 83, 84, 85), the few existing studies on prenatal AL and birth outcomes present mixed findings (79).
Several converging lines of evidence also point to epigenetic processes as a promising potential link between racism-related adversity and adverse birth outcomes. First, a growing literature indicates that parental exposures to stress and adversity, including specifically environmental influences implicated in preterm birth, predict germline, placental, and fetal DNA methylation profiles (86, 87). Second, preterm birth risk persists across generations, and evidence is consistent with the premise that this observed “heritability” may be partially explained by epigenetic mechanisms (88). Lastly, the examination of DNA methylation in cord blood and placental tissue reveals differences between preterm and term births (87, 89). However, despite this promise, to date, there has been limited inclusion of racially/ethnically diverse samples and methodologically rigorous studies to push this area of research forward (89). This agenda is especially critical because of increasing awareness that epigenetic processes may not only be implicated in adverse birth outcomes but also represent a plausible pathway through which trauma exposures are transmitted intergenerationally and even transgenerationally (86).
Recommendations
-
•
Improve understanding of and ways to characterize allostatic load (AL) during gestation. One recent study used a promising approach—latent class analysis—to extract groups represented by shared profiles of relevant biomarkers (90).
-
•
Study the epigenetic processes underlying adverse birth outcomes in racially/ethnically diverse samples.
-
•
Study racism-related stress, biological profiles, and birth outcomes conjointly.
Developmental Origins of Health and Disease
Beyond birth outcomes, existing evidence supports the need to study the relation of preconception and prenatal racism-related stress and adversity on lifespan health and development. Research on the developmental origins of health and disease and specifically fetal programming has demonstrated that physical and mental health vulnerabilities can originate in the preconception or prenatal period through environmental influences on fetal development (57, 91, 92, 93). Specifically, studies have found links between prenatal stress (broadly conceptualized, including traumatic events, poor mental health, and economic hardship) and numerous physical and mental health disorders (57, 94, 95, 96). However, very little fetal programming research has been conducted with diverse populations (8, 97, 98), and few studies have examined stressors that disproportionately impact communities of color (8, 99). Examining the impact of preconception and prenatal racism-related stress and adversity beyond birth outcomes to child development, may be an important pathway toward identifying intergenerational antecedents of racial/ethnic disparities in birth outcomes and physical and mental health.
Recommendations
-
•
Incorporate the concepts of racism-related stress and adversity into developmental origins of health and disease research.
Resilience and Community Partnership
In addition to studying new racism-related predictors and pathways, there are 2 significant interrelated areas of additional consideration for this work in the realm of maternal–child health and for health disparities research more broadly. First, there has been a historical tendency to solely focus on risk pathways in this area of study. This approach can inadvertently position populations of color as only vulnerable, lacking, and/or limited and propagate the idea that disparities are intractable and endemic rather than constructed and changeable. Investigating resilience to racism-related stress and adversity is important for counteracting these narratives and critical for advancing health equity. In this vein, 1 recent study found that community cohesion and social support mitigate the relation between discrimination and low birth weight (62). Investigators interested in furthering this type of research may consider drawing on cultural assets found to mitigate other racial/ethnic health disparities, including racial socialization, racial identity, communalism, and spirituality (100). Additionally, given how little research exists in this area, qualitative methods are warranted to form a foundational understanding of resilience processes in the context of racism and birth outcomes.
It also important to note that emerging research has shown that some resilience or protective factors do not have as great a “return” on health for populations of color compared with White populations (101, 102, 103). This has primarily been shown through work demonstrating that higher socioeconomic position is not as strongly associated with improved health for Black vs. White populations (102, 104). Research has shown this effect to be present in relation to income level and birth weight as well—specifically, higher income does not appear to protect Black mothers against delivering a low-birth-weight infant to the same extent as White mothers (105). Researchers have attributed these findings to greater discrimination exposure and/or social isolation for those with higher socioeconomic status (104), but future research can confirm and expand existing understanding of these phenomena.
A second important consideration involves recognizing that traditional approaches to health disparities research have often failed to center the perspectives of those with lived experience of the domains under study, thus perpetuating their marginalization and likely hindering the utility and accuracy of the research (106). In fact, there are several examples in the US history where populations of color have been not only neglected but also abused in the name of research (i.e., Henrietta Lacks, Tuskegee Syphilis Study), including the “father of gynecology” J. Marion Sims’ experiments on enslaved Black women without anesthesia (107). In response to such historical injustice, researchers are increasingly recognizing the importance of empowerment and social justice-based research methodologies (e.g., community-based participatory research, human-centered design, and research justice frameworks) (106, 108). These community-partnered approaches are grounded in tenets that those impacted by disparities have unique expertise, wisdom, and rights to contribute to corresponding research (“nothing about us without us”) (109). In turn, these methods improve likelihood that research questions, methods, interpretations, recommendations for future research, and translation to intervention and prevention are in line with the perspectives and priorities of those communities the research is trying to serve.
Recommendations
-
•
Identify factors that mitigate the impact of racism-related stress and adversity on birth outcomes.
-
•
Utilize empowerment and social justice-based research methodologies when studying the impact of racism-related stress and adversity on birth outcomes.
Concluding thoughts and opportunities
Through presenting a conceptual model and synthesis of existing literature, this article aimed to delineate ways in which racism-related stress and adversity are some of the root determinants of the racial/ethnic disparities in birth outcomes that exist today. We also discuss priorities and considerations for future research, which span the heterogeneity of racism-related stressors, biomarkers and mechanisms, chronicity and sensitive periods of exposure, developmental programming of lifespan health, resilience, and community-based research methodologies. Importantly, doing this work will require collaboration between researchers from a broad range of disciplines, including those with expertise in community-partnered research, biological psychology, obstetrics, public health, perinatal psychology, culture, racism, and trauma.
Visibility and awareness of racism’s long reach have been growing for years in the United States but perhaps reached a tipping point in 2020–2021 with the disproportionate harm conferred on communities of color by the coronavirus disease 2019 pandemic and multiple racist hate crimes and events of police brutality, including the murder of George Floyd and the Atlanta Spa Shootings. Thus, important organizations have publicly acknowledged the long overdue need to address the health impact of racism as well as racial/ethnic disparities in maternal–child health. Thus, there may be more opportunities than ever before to conduct meaningful research in this area. The Centers for Disease Control and Prevention announced new initiatives to address the critical threat racism poses to public health (110), whereas the National Institutes of Health launched the UNITE initiative to promote racial equity, which prioritizes listening and learning from stakeholder perspectives on racism and supporting new research on health disparities, minority health, and health equity (111). Additionally, multiple National Institutes of Health institutes and centers have released a new funding opportunity for projects that study racism at a structural level, as opposed to individual-level behavior. Specific to racism and adverse birth outcomes, the White House officially instituted Black Maternal Health week in 2021 to raise awareness and action around the dire state of racial/ethnic disparities in maternal health, pregnancy, and childbirth. Further, the proclamation named systemic racism as a driving force behind such disparities (112). Although the infant mortality rates in the United States have dramatically improved over time, the Black-White infant mortality ratio has widened. With interdisciplinary collaboration, increasing funding and attention, and clear and cohesive priorities such as those outlined in this article, there is renewed promise for making progress toward racial/ethnic equity in birth outcomes.
Acknowledgments
The authors thank Curt Sandman and Elysia Poggi Davis for providing feedback on earlier drafts of this article.
Footnotes
S.R.L. has nothing to disclose. L.M.G. has nothing to disclose.
Supported by the National Institutes of Health (grant number MH096889) and the California Initiative to Advance Precision Medicine.
References
- 1.Matoba N., Collins J.W., Jr. Racial disparity in infant mortality. Semin Perinatol. 2017;41:354–359. doi: 10.1053/j.semperi.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Owens D.C., Fett S.M. Black maternal and infant health: historical legacies of slavery. Am J Public Health. 2019;109:1342–1345. doi: 10.2105/AJPH.2019.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.March of Dimes Perinatal Data Center Peristats. https://www.marchofdimes.org/peristats/Peristats.aspx Available at: Accessed July 15, 2021.
- 4.Behrman R.E., Butler A.S. National Academies Press; Washington: 2007. Preterm birth: causes, consequences, and prevention. [PubMed] [Google Scholar]
- 5.Villarosa L. New York Times; 2018. Why America’s Black mothers and babies are in a life-or-death crisis. [Google Scholar]
- 6.Reeves R.V., Matthew D.B. 6 charts showing race gaps within the American middle class. https://www.brookings.edu/blog/social-mobility-memos/2016/10/21/6-charts-showing-race-gaps-within-the-american-middle-class/ Available at: Accessed July 29, 2021.
- 7.Boyd R.W., Lindo E.G., Weeks L.D., McLemore M.R. On racism: a new standard for publishing on racial health inequities. https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/ Available at: Accessed November 16, 2021.
- 8.Conradt E., Carter S.E., Crowell S.E. Biological embedding of chronic stress across two generations within marginalized communities. Child Dev Perspect. 2020;14:208–214. [Google Scholar]
- 9.Chambers B.D., Arega H.A., Arabia S.E., Taylor B., Barron R.G., Gates B., et al. Black women’s perspectives on structural racism across the reproductive lifespan: a conceptual framework for measurement development. Matern Child Health J. 2021;25:402–413. doi: 10.1007/s10995-020-03074-3. [DOI] [PubMed] [Google Scholar]
- 10.Williams D.R. Stress and the mental health of populations of color: advancing our understanding of race-related stressors. J Health Soc Behav. 2018;59:466–485. doi: 10.1177/0022146518814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brondolo E., Byer K., Gianaros P.J., Liu C., Prather A.A., Thomas K., et al. Stress and health disparities: contexts, mechanisms, and interventions among racial/ethnic minority and low socioeconomic status populations. http://www.apa.org/pi/health-disparities/resources/stress-report.pdf Available at:
- 12.Liu S.R., Kia-Keating M., Nylund-Gibson K. Patterns of adversity and pathways to health among White, Black, and Latinx youth. Child Abuse Negl. 2018;86:89–99. doi: 10.1016/j.chiabu.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Watson L.B., DeBlaere C., Langrehr K.J., Zelaya D.G., Flores M.J. The influence of multiple oppressions on women of color’s experiences with insidious trauma. J Couns Psychol. 2016;63:656–667. doi: 10.1037/cou0000165. [DOI] [PubMed] [Google Scholar]
- 14.Evans G.W., Li D., Whipple S.S. Cumulative risk and child development. Psychol Bull. 2013;139:1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- 15.Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 16.Appleyard K., Egeland B., van Dulmen M.H., Sroufe L.A. When more is not better: the role of cumulative risk in child behavior outcomes. J Child Psychol Psychiatry. 2005;46:235–245. doi: 10.1111/j.1469-7610.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 17.Riggan K.A., Gilbert A., Allyse M.A. Acknowledging and addressing allostatic load in pregnancy care. J Racial Ethn Health Disparities. 2021;8:69–79. doi: 10.1007/s40615-020-00757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen B.S. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 19.Selye H. McGraw-Hill; New York, NY: 1956. The stress of life. [Google Scholar]
- 20.Morgan N., Christensen K., Skedros G., Kim S., Schliep K. Life stressors, hypertensive disorders of pregnancy, and preterm birth. J Psychosom Obstet Gynaecol. 2020:1–9. doi: 10.1080/0167482X.2020.1778666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian L.M., Glaser R., Porter K., Iams J.D. Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom Med. 2013;75:658–669. doi: 10.1097/PSY.0b013e31829bbc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shonkoff J.P., Slopen N., Williams D.R. Early childhood adversity, toxic stress, and the impacts of racism on the foundations of health. Annu Rev Public Health. 2021;42:115–134. doi: 10.1146/annurev-publhealth-090419-101940. [DOI] [PubMed] [Google Scholar]
- 23.Larrabee Sonderlund A., Schoenthaler A., Thilsing T. The association between maternal experiences of interpersonal discrimination and adverse birth outcomes: a systematic review of the evidence. Int J Environ Res Public Health. 2021;18:1465. doi: 10.3390/ijerph18041465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutambudzi M., Meyer J.D., Reisine S., Warren N. A review of recent literature on materialist and psychosocial models for racial and ethnic disparities in birth outcomes in the US, 2000-2014. Ethn Health. 2017;22:311–332. doi: 10.1080/13557858.2016.1247150. [DOI] [PubMed] [Google Scholar]
- 25.Giurgescu C., McFarlin B.L., Lomax J., Craddock C., Albrecht A. Racial discrimination and the Black-White gap in adverse birth outcomes: a review. J Midwifery Womens Health. 2011;56:362–370. doi: 10.1111/j.1542-2011.2011.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alhusen J.L., Bower K.M., Epstein E., Sharps P. Racial discrimination and adverse birth outcomes: an integrative review. J Midwifery Womens Health. 2016;61:707–720. doi: 10.1111/jmwh.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salm Ward T.C., Mazul M., Ngui E.M., Bridgewater F.D., Harley A.E. “You learn to go last”: perceptions of prenatal care experiences among African-American women with limited incomes. Matern Child Health J. 2013;17:1753–1759. doi: 10.1007/s10995-012-1194-5. [DOI] [PubMed] [Google Scholar]
- 28.Gadson A., Akpovi E., Mehta P.K. Exploring the social determinants of racial/ethnic disparities in prenatal care utilization and maternal outcome. Semin Perinatol. 2017;41:308–317. doi: 10.1053/j.semperi.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Valdes E.G. Examining cesarean delivery rates by race: a population-based analysis using the Robson Ten-Group Classification System. J Racial Ethn Health Disparities. 2021;8:844–851. doi: 10.1007/s40615-020-00842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Discrimination in America: final summary. https://www.rwjf.org/en/library/research/2017/10/discrimination-in-america-experiences-and-views.html Available at: Accessed July 20, 2021.
- 31.Thomas J.L., Carter S.E., Dunkel Schetter C., Sumner J.A. Racial and ethnic disparities in posttraumatic psychopathology among postpartum women. J Psychiatr Res. 2021;137:36–40. doi: 10.1016/j.jpsychires.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peek M.E., Nunez-Smith M., Drum M., Lewis T.T. Adapting the everyday discrimination scale to medical settings: reliability and validity testing in a sample of African American patients. Ethn Dis. 2011;21:502–509. [PMC free article] [PubMed] [Google Scholar]
- 33.López-Cevallos D.F., Harvey S.M. Psychometric properties of a healthcare discrimination scale among young-adult Latinos. J Racial Ethn Health Disparities. 2019;6:618–624. doi: 10.1007/s40615-018-00560-x. [DOI] [PubMed] [Google Scholar]
- 34.Major B., Mendes W.B., Dovidio J.F. Intergroup relations and health disparities: a social psychological perspective. Health Psychol. 2013;32:514–524. doi: 10.1037/a0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penner L.A., Blair I.V., Albrecht T.L., Dovidio J.F. Reducing racial health care disparities: a social psychological analysis. Policy Insights Behav Brain Sci. 2014;1:204–212. doi: 10.1177/2372732214548430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payne B.K., Hannay J.W. Implicit bias reflects systemic racism. Trends Cogn Sci. 2021;25:927–936. doi: 10.1016/j.tics.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Ncube C.N., Enquobahrie D.A., Albert S.M., Herrick A.L., Burke J.G. Association of neighborhood context with offspring risk of preterm birth and low birthweight: a systematic review and meta-analysis of population-based studies. Soc Sci Med. 2016;153:156–164. doi: 10.1016/j.socscimed.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey Z.D., Feldman J.M., Bassett M.T. How structural racism works—racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2021;384:768–773. doi: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wildeman C. Imprisonment and infant mortality. Soc Probl. 2012;59:228–257. [Google Scholar]
- 40.Yang N., Collins J.W., Jr., Burris H.H. States with more killings of unarmed Black people have larger Black-White preterm birth disparities. J Perinatol. 2021;41:358–359. doi: 10.1038/s41372-020-00914-6. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein H., Gonzalez D., Karpman M., Zuckerman S. Amid confusion over the public charge rule, immigrant families continued avoiding public benefits in 2019. https://www.urban.org/research/publication/amid-confusion-over-public-charge-rule-immigrant-families-continued-avoiding-public-benefits-2019 Available at: Accessed July 20, 2021.
- 42.Perreira K.M., Yoshikawa H., Oberlander J. A new threat to immigrants’ health—the public-charge rule. N Engl J Med. 2018;379:901–903. doi: 10.1056/NEJMp1808020. [DOI] [PubMed] [Google Scholar]
- 43.Samari G., Catalano R., Alcalá H.E., Gemmill A. The Muslim Ban and preterm birth: analysis of U.S. vital statistics data from 2009 to 2018. Soc Sci Med. 2020;265:1–7. doi: 10.1016/j.socscimed.2020.113544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gemmill A., Catalano R., Casey J.A., Karasek D., Alcalá H.E., Elser H., et al. Association of preterm births among US Latina women with the 2016 presidential election. JAMA Netw Open. 2019;2:1–10. doi: 10.1001/jamanetworkopen.2019.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gemmill A., Catalano R., Alcalá H., Karasek D., Casey J.A., Bruckner T.A. The 2016 presidential election and periviable births among Latina women. Early Hum Dev. 2020;151:1–6. doi: 10.1016/j.earlhumdev.2020.105203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torche F., Sirois C. Restrictive immigration law and birth outcomes of immigrant women. Am J Epidemiol. 2019;188:24–33. doi: 10.1093/aje/kwy218. [DOI] [PubMed] [Google Scholar]
- 47.Novak N.L., Geronimus A.T., Martinez-Cardoso A.M. Change in birth outcomes among infants born to Latina mothers after a major immigration raid. Int J Epidemiol. 2017;46:839–849. doi: 10.1093/ije/dyw346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chae D.H., Yip T., Martz C.D., Chung K., Richeson J.A., Hajat A., et al. Vicarious racism and vigilance during the COVID-19 pandemic: mental health implications among Asian and Black Americans. Public Health Rep. 2021;136:508–517. doi: 10.1177/00333549211018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.David E.J.R., Schroeder T.M., Fernandez J. Internalized racism: a systematic review of the psychological literature on racism’s most insidious consequence. J Soc Issues. 2019;75:1057–1086. [Google Scholar]
- 50.United Nations Human Rights Office of the High Commissioner UN Human Rights Chief urges immediate, transformative action to uproot systemic racism. https://www.ohchr.org/EN/NewsEvents/Pages/DisplayNews.aspx?NewsID=27218&LangID=E Available at: Accessed July 15, 2021.
- 51.Alang S., McAlpine D., McClain M. Police encounters as stressors: associations with depression and anxiety across race. Socius. 2021;7 [Google Scholar]
- 52.Dunkel Schetter C., Glynn L.M. In: The handbook of stress science: biology, psychology, and health. Contrada R.J., Baum A., editors. Springer Publishing Company; 2011. Stress in pregnancy: empirical evidence and theoretical issues to guide interdisciplinary research; pp. 321–347. [Google Scholar]
- 53.Glynn L.M., Wadhwa P.D., Dunkel-Schetter C., Chicz-DeMet A., Sandman C.A. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 54.Sandman C.A., Glynn L.M., Davis E.P. In: Fetal development: research on brain and behavior, environmental influences, and emerging technologies. Reissland N., Kisilevsky B.S., editors. Springer International Publishing; 2016. Neurobehavioral consequences of fetal exposure to gestational stress. [Google Scholar]
- 55.Davis E.P., Narayan A.J. Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Dev Psychopathol. 2020;32:1625–1639. doi: 10.1017/S0954579420001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding X., Liang M., Wu Y., Zhao T., Qu G., Zhang J., et al. The impact of prenatal stressful life events on adverse birth outcomes: a systematic review and meta-analysis. J Affect Disord. 2021;287:406–416. doi: 10.1016/j.jad.2021.03.083. [DOI] [PubMed] [Google Scholar]
- 57.Keenan K., Hipwell A.E., Class Q.A., Mbayiwa K. Extending the developmental origins of disease model: impact of preconception stress exposure on offspring neurodevelopment. Dev Psychobiol. 2018;60:753–764. doi: 10.1002/dev.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witt W.P., Cheng E.R., Wisk L.E., Litzelman K., Chatterjee D., Mandell K., et al. Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. Am J Public Health. 2014;104:81–90. doi: 10.2105/AJPH.2013.301544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dominguez T.P., Dunkel-Schetter C., Glynn L.M., Hobel C., Sandman C.A. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychol. 2008;27:194–203. doi: 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wheaton F.V., Thomas C.S., Roman C., Abdou C.M. Discrimination and depressive symptoms among African American men across the adult lifecourse. J Gerontol B Psychol Sci Soc Sci. 2018;73:208–218. doi: 10.1093/geronb/gbx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennett I.M., Culhane J.F., Webb D.A., Coyne J.C., Hogan V., Mathew L., et al. Perceived discrimination and depressive symptoms, smoking, and recent alcohol use in pregnancy. Birth. 2010;37:90–97. doi: 10.1111/j.1523-536X.2010.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu SR, Sandman CA, Davis EP, Glynn L. Sociocultural Stress, Adverse Birth Outcomes, and the Protective Roles of Social Support and Community Cohesion. In: Society for Prevention Research. 2021.
- 63.Williams D.R., Yu Y., Jackson J.S., Anderson N.B. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 64.Christian L.M. At the forefront of psychoneuroimmunology in pregnancy: implications for racial disparities in birth outcomes: PART 2: biological mechanisms. Neurosci Biobehav Rev. 2020;117:327–333. doi: 10.1016/j.neubiorev.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma D., Shastri S., Farahbakhsh N., Sharma P. Intrauterine growth restriction - part 1. J Matern Fetal Neonatal Med. 2016;29:3977–3987. doi: 10.3109/14767058.2016.1152249. [DOI] [PubMed] [Google Scholar]
- 66.Romero R., Dey S.K., Fisher S.J. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coler B.S., Shynlova O., Boros-rausch A., Lye S., Mccartney S., Leimert K.B., et al. Landscape of preterm birth therapeutics and a path forward. J Clin Med. 2021:1–34. doi: 10.3390/jcm10132912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernard D.L., Calhoun C.D., Banks D.E., Halliday C.A., Hughes-Halbert C., Danielson C.K. Making the “C-ACE” for a culturally-informed adverse childhood experiences framework to understand the pervasive mental health impact of racism on Black youth. J Child Adolesc Trauma. 2021;14:233–247. doi: 10.1007/s40653-020-00319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christian L.M., Iams J.D., Porter K., Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain Behav Immun. 2012;26:1280–1287. doi: 10.1016/j.bbi.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hilmert C.J., Dominguez T.P., Schetter C.D., Srinivas S.K., Glynn L.M., Hobel C.J., et al. Lifetime racism and blood pressure changes during pregnancy: implications for fetal growth. Health Psychol. 2014;33:43–51. doi: 10.1037/a0031160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hilmert C.J., Schetter C.D., Dominguez T.P., Abdou C., Hobel C.J., Glynn L., et al. Stress and blood pressure during pregnancy: Racial differences and associations with birthweight. Psychosom Med. 2008;70:57–64. doi: 10.1097/PSY.0b013e31815c6d96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thayer Z.M., Kuzawa C.W. Ethnic discrimination predicts poor self-rated health and cortisol in pregnancy: insights from New Zealand. Soc Sci Med. 2015;128:36–42. doi: 10.1016/j.socscimed.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Seaton E.K., Zeiders K.H. Daily racial discrimination experiences, ethnic-racial identity, and diurnal cortisol patterns among Black adults. Cultur Divers Ethnic Minor Psychol. 2021;27:145–155. doi: 10.1037/cdp0000367. [DOI] [PubMed] [Google Scholar]
- 74.Tse A.C., Rich-Edwards J.W., Koenen K., Wright R.J. Cumulative stress and maternal prenatal corticotropin-releasing hormone in an urban US cohort. Psychoneuroendocrinology. 2012;37:970–979. doi: 10.1016/j.psyneuen.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jašarević E., Bale T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front Neuroendocrinol. 2019;55:1–13. doi: 10.1016/j.yfrne.2019.100797. [DOI] [PubMed] [Google Scholar]
- 76.Vinturache A.E., Gyamfi-Bannerman C., Hwang J., Mysorekar I.U., Jacobsson B., Preterm Birth International Collaborative (PREBIC) Maternal microbiome—a pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21:94–99. doi: 10.1016/j.siny.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Hyman R.W., Fukushima M., Jiang H., Fung E., Rand L., Johnson B., et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S., Mcculle S.L., et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doan S.N. Allostatic load: developmental and conceptual considerations in a multi-system physiological indicator of chronic stress exposure. Dev Psychobiol. 2021;63:825–836. doi: 10.1002/dev.22107. [DOI] [PubMed] [Google Scholar]
- 80.Conradt E., Shakiba N., Ostlund B., Terrell S., Kaliush P., Shakib J.H., et al. Prenatal maternal hair cortisol concentrations are related to maternal prenatal emotion dysregulation but not neurodevelopmental or birth outcomes. Dev Psychobiol. 2020;62:758–767. doi: 10.1002/dev.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leimert K.B., Olson D.M. Racial disparities in pregnancy outcomes: genetics, epigenetics, and allostatic load. Curr Opin Physiol. 2020;13:155–165. [Google Scholar]
- 82.Sandman C.A., Davis E.P., Glynn L.M. In: Preterm birth - mother and child. Morrison J.C., editor. IntechOpen; 2012. Psychobiological stress and preterm birth. [Google Scholar]
- 83.Ross K.M., Carroll J.E., Dunkel Schetter C., Hobel C., Cole S.W. Pro-inflammatory immune cell gene expression during the third trimester of pregnancy is associated with shorter gestational length and lower birthweight. Am J Reprod Immunol. 2019;82 doi: 10.1111/aji.13190. [DOI] [PubMed] [Google Scholar]
- 84.Baibazarova E., van de Beek C., Cohen-Kettenis P.T., Buitelaar J., Shelton K.H., van Goozen S.H.M. Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology. 2013;38:907–915. doi: 10.1016/j.psyneuen.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 85.Gilles M., Otto H., Wolf I.A.C., Scharnholz B., Peus V., Schredl M., et al. Maternal hypothalamus-pituitary-adrenal (HPA) system activity and stress during pregnancy: effects on gestational age and infant’s anthropometric measures at birth. Psychoneuroendocrinology. 2018;94:152–161. doi: 10.1016/j.psyneuen.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 86.Yehuda R., Lehrner A. Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry. 2018;17:243–257. doi: 10.1002/wps.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park B., Khanam R., Vinayachandran V., Baqui A.H., London S.J., Biswal S. Epigenetic biomarkers and preterm birth. Environ Epigenet. 2020;6 doi: 10.1093/eep/dvaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burris H.H., Baccarelli A.A., Wright R.O., Wright R.J. Epigenetics: linking social and environmental exposures to preterm birth. Pediatr Res. 2016;79:136–140. doi: 10.1038/pr.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barcelona de Mendoza V., Wright M.L., Agaba C., Prescott L., Desir A., Crusto C.A., et al. A systematic review of DNA methylation and preterm birth in African American women. Biol Res Nurs. 2017;19:308–317. doi: 10.1177/1099800416669049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walsh K., McCormack C.A., Webster R., Pinto A., Lee S., Feng T., et al. Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc Natl Acad Sci U S A. 2019;116:23996–24005. doi: 10.1073/pnas.1905890116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barker D.J. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 92.Hentges R.F., Graham S.A., Plamondon A., Tough S., Madigan S. A developmental cascade from prenatal stress to child internalizing and externalizing problems. J Pediatr Psychol. 2019;44:1057–1067. doi: 10.1093/jpepsy/jsz044. [DOI] [PubMed] [Google Scholar]
- 93.Gluckman PD, Buklijas T, Hanson MA. The developmental origins of health and disease (DOHaD) concept: past, present, and future. The Epigenome and Developmental Origins of Health and Disease. Elsevier, 2016;1–15.
- 94.Swales D.A., Stout-Oswald S.A., Glynn L.M., Sandman C., Wing D.A., Davis E.P. Exposure to traumatic events in childhood predicts cortisol production among high risk pregnant women. Biol Psychol. 2018;139:186–192. doi: 10.1016/j.biopsycho.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davis E.P., Sandman C.A. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Irwin J.L., Davis E.P., Hobel C.J., Coussons-Read M., Dunkel Schetter C. Maternal prenatal anxiety trajectories and infant developmental outcomes in one-year-old offspring. Infant Behav Dev. 2020;60:1–9. doi: 10.1016/j.infbeh.2020.101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bush N.R., Jones-Mason K., Coccia M., Caron Z., Alkon A., Thomas M., et al. Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Dev Psychopathol. 2017;29:1553–1571. doi: 10.1017/S0954579417001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Demers C.H., Aran Ö., Glynn L.M., Davis E.P. In: Prenatal stress and child development. Wazana A., Szekely E., Oberlander T.F., editors. Springer Nature; 2020. Prenatal programming of neurodevelopment: imaging and structural changes. [Google Scholar]
- 99.D’Anna-Hernandez K.L., Aleman B., Flores A.M. Acculturative stress negatively impacts maternal depressive symptoms in Mexican-American women during pregnancy. J Affect Disord. 2015;176:35–42. doi: 10.1016/j.jad.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woods-Jaeger B., Briggs E.C., Gaylord-Harden N., Cho B., Lemon E. Translating cultural assets research into action to mitigate adverse childhood experience-related health disparities among African American youth. Am Psychol. 2021;76:326–336. doi: 10.1037/amp0000779. [DOI] [PubMed] [Google Scholar]
- 101.Liu S.R., Kia-Keating M., Nylund-Gibson K., Barnett M.L. Co-occurring youth profiles of adverse childhood experiences and protective factors: associations with health, resilience, and racial disparities. Am J Community Psychol. 2020;65:173–186. doi: 10.1002/ajcp.12387. [DOI] [PubMed] [Google Scholar]
- 102.Assari S. The benefits of higher income in protecting against chronic medical conditions are smaller for African Americans than Whites. Healthcare. 2018;6:2. doi: 10.3390/healthcare6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu S.R., Kia-Keating M., Nylund-Gibson K. Patterns of family, school, and community promotive factors and health disparities among youth: implications for prevention science. Prev Sci. 2019;20:1103–1113. doi: 10.1007/s11121-019-01021-5. [DOI] [PubMed] [Google Scholar]
- 104.Surachman A., Rice C., Bray B., Gruenewald T., Almeida D. Association between socioeconomic status mobility and inflammation markers among white and black adults in the United States: a latent class analysis. Psychosom Med. 2020;82:224–233. doi: 10.1097/PSY.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koning S.M., Ehrenthal D.B. Stressor landscapes, birth weight, and prematurity at the intersection of race and income: elucidating birth contexts through patterned life events. SSM Popul Health. 2019;8:1–10. doi: 10.1016/j.ssmph.2019.100460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kia-Keating M., Santacrose D.E., Liu S.R., Adams J. Using community-based participatory research and human-centered design to address violence-related health disparities among Latino/a youth. Fam Community Health. 2017;40:160–169. doi: 10.1097/FCH.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bailey Z.D., Krieger N., Agénor M., Graves J., Linos N., Bassett M.T. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 108.Franck L.S., McLemore M.R., Williams S., Millar K., Gordon A.Y., Williams S., et al. Research priorities of women at risk for preterm birth: findings and a call to action. BMC Pregnancy Childbirth. 2020;20:10. doi: 10.1186/s12884-019-2664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franck L.S., McLemore M., Williams S. Working in partnership with communities: listening to women’s lived experiences to help set research priorities to address the preterm birth epidemic. http://blogs.biomedcentral.com/bmcseriesblog/2020/01/13/working-in-partnership-with-communities-listening-to-womens-lived-experiences-to-help-set-research-priorities-to- address-the-preterm-birth-epidemic/ Available at: Accessed July 17, 2021.
- 110.Wamsley L. CDC Director Declares Racism A ‘Serious Public Health Threat’. https://www.npr.org/2021/04/08/985524494/cdc-director-declares-racism-a-serious-public-health-threat Available at: Accessed May 12, 2021.
- 111.Collins F.S., Adams A.B., Aklin C., Archer T.K., Bernard M.A., Boone E., et al. Affirming NIH’s commitment to addressing structural racism in the biomedical research enterprise. Cell. 2021;184:3075–3079. doi: 10.1016/j.cell.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 112.Biden J.R. A proclamation on Black maternal health week, 2021. https://www.whitehouse.g.,ov/briefing-room/presidential-actions/2021/04/13/a-proclamation-on-black-maternal-health-week-2021/ Available at: Accessed April 30, 2021.