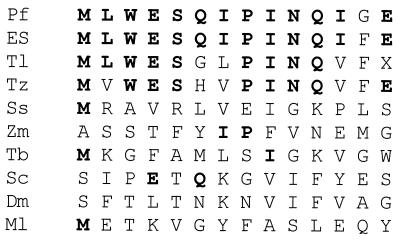Abstract
Pyrococcus furiosus is a hyperthermophilic archaeon that grows optimally at 100°C by the fermentation of peptides and carbohydrates to produce acetate, CO2, and H2, together with minor amounts of ethanol. The organism also generates H2S in the presence of elemental sulfur (S0). Cell extracts contained NADP-dependent alcohol dehydrogenase activity (0.2 to 0.5 U/mg) with ethanol as the substrate, the specific activity of which was comparable in cells grown with and without S0. The enzyme was purified by multistep column chromatography. It has a subunit molecular weight of 48,000 ± 1,000, appears to be a homohexamer, and contains iron (∼1.0 g-atom/subunit) and zinc (∼1.0 g-atom/subunit) as determined by chemical analysis and plasma emission spectroscopy. Neither other metals nor acid-labile sulfur was detected. Analysis using electron paramagnetic resonance spectroscopy indicated that the iron was present as low-spin Fe(II). The enzyme is oxygen sensitive and has a half-life in air of about 1 h at 23°C. It is stable under anaerobic conditions even at high temperature, with half-lives at 85 and 95°C of 160 and 7 h, respectively. The optimum pH for ethanol oxidation was between 9.4 and 10.2 (at 80°C), and the apparent Kms (at 80°C) for ethanol, acetaldehyde, NADP, and NAD were 29.4, 0.17, 0.071, and 20 mM, respectively. P. furiosus alcohol dehydrogenase utilizes a range of alcohols and aldehydes, including ethanol, 2-phenylethanol, tryptophol, 1,3-propanediol, acetaldehyde, phenylacetaldehyde, and methyl glyoxal. Kinetic analyses indicated a marked preference for catalyzing aldehyde reduction with NADPH as the electron donor. Accordingly, the proposed physiological role of this unusual alcohol dehydrogenase is in the production of alcohols. This reaction simultaneously disposes of excess reducing equivalents and removes toxic aldehydes, both of which are products of fermentation.
Hyperthermophiles are a group of microorganisms that grow at temperatures of 90°C and above (1, 61–63). They are found in geothermally heated environments (62), and all but two of them are classified as members of the domain Archaea (71). The majority are anaerobic organisms, and for many of them significant growth is dependent on the reduction of elemental sulfur (S0) to H2S. Various organic compounds or molecular H2 serve as electron donors (1, 9, 56, 58). The organisms differ, however, in their dependence on S0. For example, the growth of Thermococcus strain ES-1 is obligately dependent on S0, and the amount of S0 added to the growth medium has a major effect on the activities of enzymes such as alcohol dehydrogenase (ADH), hydrogenase, and formate ferredoxin oxidoreductase (FMOR) (37). Thus, the specific activities of these enzymes are dramatically higher in cells grown under S0 limitation than in S0-sufficient cells (37), while the activities of various other catabolic enzymes are similar in the two cell types. It was therefore postulated that ADH, together with hydrogenase and FMOR, served to dispose of excess reducing equivalents when Thermococcus strain ES-1 is grown under S0 limitation and that S0, presumably via the cellular redox potential, regulated the expression of these enzymes (37).
In contrast to Thermococcus strain ES-1, several hyperthermophilic species that are able to reduce S0 also grow well in its absence by a fermentative-type metabolism. One of the best studied of this class is Pyrococcus furiosus, which grows optimally at 100°C. In this case, the addition of S0 stimulates growth by an as yet unknown mechanism (20, 59). If S0 reduction affects the internal redox balance of P. furiosus, and thus the bioenergetics of fermentation, then one might expect that enzymes involved in the disposal of the reducing equivalents generated during fermentation to be affected by the presence of S0. The initial objective of the present study was, therefore, to determine to what extent, if any, the presence of S0 affects the activities of key metabolic enzymes, in particular, ADH, hydrogenase, and FMOR, in P. furiosus. Our results show that there is a fundamental difference in the response to S0 between fermentative-type hyperthermophiles, like P. furiosus, and those that appear to respire S0, such as Thermococcus strain ES-1, with regard to key metabolic enzymes such as ADH, hydrogenase, and FMOR. It was therefore of interest to determine if differences existed in the nature of these enzymes in the two types of organism. Since ADH, but not hydrogenase or FMOR, had been previously purified from Thermococcus strain ES-1 (37), we focused on characterizing the ADH from P. furiosus. We show here the P. furiosus enzyme is part of the same ADH family as that from Thermococcus strain ES-1 but differs in that it contains zinc as well as iron. The proposed physiological role of ADH in P. furiosus is to both dispose of excess reducing equivalents and to detoxify the aldehydes produced by the fermentative pathways.
MATERIALS AND METHODS
Growth of organism.
P. furiosus (DSM 3638) was routinely grown at 85°C in a 600-liter fermentor as described previously (12).
Enzyme assays.
All assays were carried out under anaerobic conditions at 80°C. The activities of ADH and glutamate dehydrogenase (GDH) were measured by the ethanol-dependent and glutamate-dependent reduction of NADP, respectively, as described previously (34, 37, 53). The substrate specificity and kinetic parameters of purified ADH were determined by the same assay procedures except that the substrates and electron carrier were varied. The activities of pyruvate ferredoxin oxidoreductase (POR), 2-ketoglutarate ferredoxin oxidoreductase (KGOR), indolepyruvate ferredoxin oxidoreductase (IOR), and 2-ketoisovalerate ferredoxin oxidoreductase (VOR) were determined by measuring the substrate-dependent reduction of methyl viologen in the presence of coenzyme A (CoA) with pyruvate, 2-ketoglutarate, indolepyruvate, and 2-ketoisovalerate, respectively, as the substrates (final concentration, 5 mM) (7, 8, 40, 41). Crotonaldehyde (43), formaldehyde (44), glyceraldehyde-3-phosphate, formate (37), and NADH were used as substrates for aldehyde ferredoxin oxidoreductase (AOR), formaldehyde ferredoxin oxidoreductase (FOR), glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR), FMOR, and NAD(P)H benzyl viologen oxidoreductase (BVOR), respectively (36); the assays were carried out as described in the references. For all of these enzymes, 1 U of activity represents 1 μmol of substrate oxidized per min. Hydrogenase activity was determined by the production of H2 from dithionite-reduced methyl viologen, and 1 U equals 1 μmol of H2 produced per min. The pH dependence of ADH activity was measured by using 100 mM EPPS [N-(2-hydroxyethyl)piperazine-N′-3-propanesulfonic acid; pH 7.2 to 8.8] and 50 mM CAPS [3-(cyclohexylamino)-1-propanesulfonic acid; pH 10.2 to 10.8]. All pH values were measured at 23°C. The effect of temperature on ADH activity was measured by using 50 mM CHES (2-[N-cyclohexylamino]ethanesulfonic acid; pH 9.6).
Enzyme purification.
ADH was purified from 150 g (wet weight) of P. furiosus cells under anaerobic conditions (34, 37) at 23°C. Frozen cells were thawed anaerobically in approximately 9 volumes of 50 mM Tris-HCl buffer (pH 7.8) containing DNase I (10 μg/ml), 2 mM sodium dithionite, and 2 mM dithiothreitol and were incubated at 23°C for 3 h with constant stirring. Cell lysis by this procedure was confirmed by microscopic examination. A cell extract was obtained by centrifugation at 50,000 × g for 120 min at 4°C and used directly for determining enzymatic activities. For purification, the extract was loaded onto a column (5 by 10 cm) of DEAE-Sepharose Fast Flow (Pharmacia, Piscataway, N.J.) equilibrated with buffer A (50 mM Tris-HCl [pH 7.8] containing 5% [vol/vol] glycerol, 2 mM dithionite, and 2 mM dithiothreitol). The column was eluted with a 2.5-liter linear gradient (0 to 0.6 M NaCl) in buffer A. The flow rate was 8 ml/min, and 90-ml fractions were collected. ADH activity started to elute from the column as 0.22 M NaCl was applied. Fractions containing ADH activity above 1.0 U/mg were combined (300 ml) and loaded onto a column (5 by 10 cm) of hydroxyapatite (Bio-Rad) equilibrated with buffer A. The flow rate was 3 ml/min, and 60-ml fractions were collected. The column was eluted with 1.0-liter linear gradient (0 to 0.5 M potassium phosphate) in buffer A. The ADH activity started to elute as 0.25 M potassium phosphate was applied to the column. Fractions containing ADH activity above 1.2 U/mg were combined (200 ml), and 1.0 M (NH4)2SO4 was added to give a final concentration of 0.6 M. This loaded onto a column of Phenyl-Sepharose (5 by 10 cm) equilibrated with buffer A containing 10 μM Fe(NH4)2(SO4)2 and 0.6 M (NH4)2SO4. The column was eluted with a 600-ml linear gradient from 0.6 to 0 M (NH4)2SO4 in buffer A containing 10 μM Fe(NH4)2(SO4)2. The flow rate was 4 ml/min, and 50-ml fractions were collected. ADH activity started slowly to elute from the column as 0 M of (NH4)2SO4 was applied. Fractions containing ADH activity above 10 U/mg were combined and concentrated by ultrafiltration (Amicon type ultrafilter using a PM30 membrane). The concentrated fractions (20 ml) were applied to a column of Superdex 200 (6 by 60 cm; Pharmacia LKB) equilibrated with buffer A containing 100 mM NaCl. The flow rate was 4 ml/min, and 30-ml fractions were collected. Fractions containing ADH activity above 40 U/mg were combined (120 ml) and loaded onto a Q-Sepharose High Performance column (2.5 by 10 cm) equilibrated with buffer A. The flow rate was 6 ml/min, and 40-ml fractions were collected. The column was eluted with a 480-ml linear gradient (0 to 0.4 M NaCl) in buffer A. The ADH activity started to elute as 0.2 M NaCl was applied to the column. Those fractions containing pure ADH as judged by electrophoretic analysis were combined (50 ml), concentrated by ultrafiltration to 4 ml, and stored as pellets in liquid N2.
Metal analyses.
A complete metal analysis (32 elements, including zinc and iron) was performed by plasma emission spectroscopy using a Jarrel Ash Plasma Comp 750 instrument at the Riverbend Research Laboratories, University of Georgia. The iron contents of pure ADH were measured by using o-phenanthroline (33). ADH as isolated (0.4 ml) was dialyzed overnight in 3.5 liter 50 mM Tris-HCl buffer (pH 7.8) containing 5% glycerol, 2 mM dithiothreitol, and 2 mM sodium dithionite at 4°C. ADH as isolated (0.4 ml) was also treated with 5 mM EDTA for 1 h at room temperature and then dialyzed overnight in 3.5 liters of 50 mM Tris-HCl buffer (pH 7.8) containing 5% glycerol, 2 mM dithiothreitol, 2 mM sodium dithionite, and 0.5 mM EDTA at 4°C. For dialysis experiments, all samples were unavoidably exposed shortly to air when the samples were transferred into the dialysis tubing.
Other methods.
The molecular weight of ADH was estimated by gel filtration on a column of Superdex 200 (1.6 by 60 cm; Pharmacia LKB) with ferritin (molecular weight, 450,000), catalase (240,000), lactate dehydrogenase (140,000), yeast alcohol dehydrogenase (150,000), bovine serum albumin (67,000), and egg albumin (45,000) as the standard proteins. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed by the method of Laemmli (28). SDS molecular weight markers were purchased from Sigma Chemical Co. (St. Louis, Mo.). Protein concentrations were routinely estimated by the method of Bradford (11), with bovine serum albumin as the standard. The protein content of samples of pure ADH was also determined by quantitative recovery of amino acids from compositional analyses (17). The acid-labile sulfide contents of pure ADH were measured by methylene blue formation (13). The N-terminal sequence was determined with an Applied Biosystems model 477 sequencer (17). Amino acid analyses were performed with an Applied Biosystems model 4240A analyzer after hydrolysis of the protein under Ar at 165°C for 1 h in the presence of 6 M HCl, 1% (wt/vol) phenol, and 8% (wt/vol) thioglycolic acid (17). Serine and threonine amounts were corrected for destruction. The melting temperature of pure ADH was measured with a differential scanning calorimeter (Hart Scientific, Pleasant Grove, Utah). Mass spectrometry was carried out by using a Bruker Reflex time-of-flight mass spectrometer located in Department of Chemistry, University of Georgia. Electron paramagnetic resonance (EPR) spectra were recorded on an IBM-Bruker ER 300D spectrometer interfaced to an ESP 3220 data system and equipped with an Oxford Instrument ITC-4 flow cryostat.
RESULTS
ADH activity.
In cell extracts of cells grown either with S0 (sublimed powder, 1 g/liter) or without it, there was no significant difference in the activities of GDH, hydrogenase, BVOR, POR, KGOR, IOR, and VOR (Table 1). Sulfhydrogenase, α-glucosidase, and protease were previously reported to be unaffected by the presence of S0 in the medium (59). On the other hand, the activities of the three tungsten-containing enzymes, AOR, FOR, and GAPOR, were significantly (≥2-fold) higher in cells grown in the presence of S0 (Table 1). These increased activities may be due to the activation of these enzymes by sulfide, a reaction that has been demonstrated with purified enzyme in vitro (55), although it is not known if the intracellular sulfide concentration increases when S0 is added to the growth medium. In contrast to what was reported for Thermococcus strain ES-1 (37), FMOR activity could not be detected in extracts of P. furiosus, independent of S0 in the growth medium. Similarly, while the ADH activity of Thermococcus strain ES-1 increased dramatically when S0 was limiting (37), extracts of P. furiosus cells grown in the presence or absence of S0 had comparable specific activities of ADH (0.35 ± 0.15 U/mg). Clearly, obligate S0 reducers such as Thermococcus strain ES-1 have a very different response to S0 than does P. furiosus, in particular, with regard to key metabolic enzymes such as ADH, hydrogenase, and FMOR.
TABLE 1.
Activities of oxidoreductase-type enzymes from P. furiosus cells grown with or without S0
| Enzyme | Sp act (U/mg)a
|
+S0/−S0 | |
|---|---|---|---|
| −S0 | +S0 | ||
| ADH | 0.18 ± 0.03 | 0.24 ± 0.04 | 1.3 |
| GDH | 2.3 ± 0.2 | 1.3 ± 0.1 | 0.6 |
| Hydrogenase | 5.6 ± 0.3 | 6.6 ± 0.4 | 1.2 |
| FMOR | ND | ND | |
| BVOR | 1.8 ± 0.1 | 1.4 ± 0.1 | 0.8 |
| POR | 1.9 ± 0.1 | 1.4 ± 0.1 | 0.7 |
| KGOR | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.7 |
| IOR | 0.25 ± 0.02 | 0.13 ± 0.02 | 1.9 |
| VOR | 0.45 ± 0.03 | 0.66 ± 0.03 | 0.5 |
| AOR | 2.4 ± 0.2 | 7.1 ± 0.5 | 3.0 |
| FOR | 3.2 ± 0.2 | 14.3 ± 0.6 | 4.5 |
| GAPOR | 1.6 ± 0.2 | 3.5 ± 0.4 | 2.2 |
Measured at 80°C as described in Materials and Methods; average ± standard deviation; ND, not detected.
Purification and physical properties of ADH.
More than 90% of the ADH activity was found in the supernatant after centrifugation of the cell extract of P. furiosus, indicating that the enzyme is a cytoplasmic protein. The results of a typical purification are shown in Table 2. ADH was purified about 131-fold, with a yield of 34%. The enzyme was very oxygen sensitive (see below), and anaerobic and reducing conditions were required to prevent significant losses of activity during purification. The purified enzyme gave rise to a single protein band after SDS-gel electrophoresis (Fig. 1), and this band corresponded to an Mr of 48,000. Mass spectrometric analysis of the purified enzyme gave a similar value (Mr of 44,840). By gel filtration, the apparent Mr of the holoenzyme was estimated to be 270,000 ± 20,000 (data not shown), suggesting that the enzyme may have a hexameric structure. Amino-terminal sequence analysis of a solution of ADH gave rise to a single sequence (Fig. 2), consistent with the presence of a single type of subunit. A comparison of the N-terminal amino acid sequence of P. furiosus ADH with those of the ADHs from methanogenic, aerobic, and hyperthermophilic archaea, from thermophilic and mesophilic bacteria, and from eukaryotes shows that it has significant sequence similarity only to the enzymes from the hyperthermophiles Thermococcus litoralis, Thermococcus strain ES-1, and T. zilligii (formerly Thermococcus strain AN1) (31, 54). However, the amino acid compositions of these hyperthermophilic ADHs were quite distinct, with large differences in potentially key amino acid residues such as His and Met (Table 3). For example, the P. furiosus enzyme contains eight His residues, some of which are presumably involved in metal binding (see below), yet T. litoralis ADH contains only one such residue (24).
TABLE 2.
Purification of ADH from P. furiosus
| Purification step | Total protein (mg) | Activity (U) | Sp act (U/mg) | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Cell extract | 7,280 | 4,030 | 0.55 | 1 | 100 |
| DEAE-Sepharose Fast Flow | 1,836 | 2,851 | 1.55 | 2.8 | 71 |
| Hydroxyapatite | 632 | 1,491 | 2.36 | 4.3 | 37 |
| Phenyl-Sepharose | 71 | 2,103 | 29.6 | 53.9 | 52 |
| Superdex 200 | 29 | 1,973 | 68.0 | 124.0 | 49 |
| Q-Sepharose High Performance | 19.2 | 1,388 | 72.3 | 131.0 | 34 |
FIG. 1.
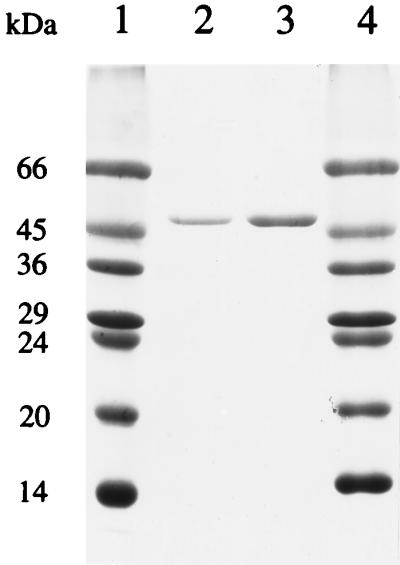
SDS-polyacrylamide electrophoresis gel (12.5%) of ADH purified from P. furiosus. Lanes 1 and 4, molecular mass markers; lanes 2 and 3, 1 and 2 μg of ADH, respectively.
FIG. 2.
Amino-terminal amino acid sequences of ADHs from various sources. Abbreviations and references: Pf, P. furiosus (this work); ES, Thermococcus strain ES-1 (37); Tl, T. litoralis (34); Tz, T. zilligii (31); Ss, Sulfolobus solfataricus (2); Zm, Z. mobilis (adh2 [46]); Tb, Thermoanaerobium brockii (47); Sc, Saccharomyces cerevisiae (5); Dm, Drosophila melanogaster (68); Ml, Methanogenium liminatans (8). Residues identical to those in the P. furiosus enzyme are in boldface. X, unidentified residue.
TABLE 3.
Amino acid compositions of ADHs from P. furiosus, Thermococcus strain ES-1, T. litoralis, and T. zilligii
| Amino acid(s) | No. of residues in ADH from:
|
|||
|---|---|---|---|---|
| P. furiosusa | ES-1b | T. litoralisc | T. zilligiid | |
| Asn + Asp | 38 | 29 | 28 | 32 |
| Gln + Glu | 46 | 59 | 57 | 35 |
| Ser | 18 | 13 | 13 | 19 |
| Gly | 29 | 46 | 53 | 26 |
| Arg | 16 | 14 | 9 | 19 |
| Thr | 22 | 20 | 19 | 23 |
| Ala | 44 | 43 | 61 | 46 |
| Pro | 28 | 16 | 30 | 26 |
| Tyr | 21 | 5 | 17 | 19 |
| Val | 30 | 34 | 36 | 31 |
| Met | 8 | 1 | 6 | 6 |
| Trp | 4 | 8 | 11 | 3 |
| Cys | 3 | 2 | 3 | 4 |
| Ile | 35 | 14 | 39 | 28 |
| Leu | 46 | 58 | 52 | 42 |
| Phe | 14 | 17 | 4 | 14 |
| Lys | 25 | 39 | 15 | 22 |
| His | 8 | 8 | 1 | 11 |
| Total | 435 | 426 | 454 | 406 |
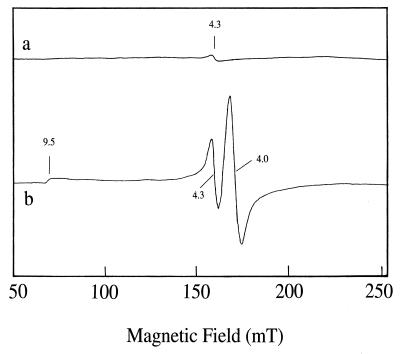
P. furiosus ADH as purified contained both Fe and Zn at concentrations of approximately 1 g-atom/subunit as determined by chemical analysis and plasma emission spectroscopy (Table 4). The Fe content decreased to less than 0.5 g-atom/subunit after dialysis of the enzyme against buffer containing EDTA, but there was also a corresponding loss of activity (Table 4). The zinc content of the enzyme decreased only slightly after dialysis and remained at approximately stoichiometric amounts (Table 4). No other metals were present in significant amounts (>0.05 g-atom/subunit), and no acid-labile sulfide was detected by colorimetric analysis. By EPR analyses, the enzyme as purified gave rise to only a very minor resonance near g = 4.3, indicative of high-spin ferric iron (Fig. 3a), suggesting that bulk of the iron within the enzyme was predominantly EPR silent, i.e., low-spin ferrous (S = 0). Attempts to oxidize this iron by treating the enzyme with air at 25 or 80°C (for 5 min) were unsuccessful, as additional EPR absorption was not evident. The presence of ferrous iron in the enzyme was confirmed by treating it with NO, which led to new EPR resonances at low magnetic field (Fig. 3b). These were in addition to an intense signal centered near g = 1.95 (data not shown), which arises from the NO radical (the same EPR absorption was seen in the absence of enzyme). The NO-induced resonances seen at low field could be resolved into two distinct species (g = 4.3 and 9.5 and g = 4.0) according to their power and temperature dependence (data not shown). The g = 4.0 resonance is characteristic of species with an S = 3/2 ground state and can be reasonably assigned to a ferrous-NO complex (3, 31, 72), while the additional resonances (g = 4.3 and 9.5) were assumed to arise from the ferric site (S = 5/2).
TABLE 4.
Metal content of P. furiosus ADH
| Treatment | Avg metal content ± SD (g-atoms/subunit)a
|
Avg sp act (U/mg) ± SD | Activity (%) | |
|---|---|---|---|---|
| Fe | Zn | |||
| None | 1.1 ± 0.2 | 1.4 ± 0.3 | 29 ± 2 | 100 |
| Dialysis | ||||
| −EDTA | 0.9 ± 0.1 | 0.8 ± 0.2 | 24 ± 2 | 83 |
| +EDTA | 0.4 ± 0.1 | 0.8 ± 0.2 | 14.5 ± 2 | 50 |
Of the enzyme as purified and after dialysis in the presence and absence of EDTA as described in Materials and Methods.
FIG. 3.
EPR spectra of P. furiosus ADH. ADH was used at a concentration of 3.6 mg/ml. a, ADH as isolated in 50 mM Tris-HCl (pH 7.8) containing 5% (vol/vol) glycerol and 2 mM dithiothreitol. Spectrum was recorded at 4 K with 5 mW of microwave power. b, ADH treated with NO. The enzyme (3.6 mg/ml in 50 mM Tris-HCl [pH 7.8]) was gently bubbled with NO for 3 min at 25°C prior to being frozen in liquid N2. The spectrum was recorded with 40 mW of microwave power at 4 K. The spectrometer settings were as follows: microwave frequency, 9.597 GHz; modulation frequency, 100 kHz; modulation amplitude, 5 G; time constant, 163.84 ms; gain, 2 × 105; and scale, 16.
Oxygen sensitivity and thermal stability.
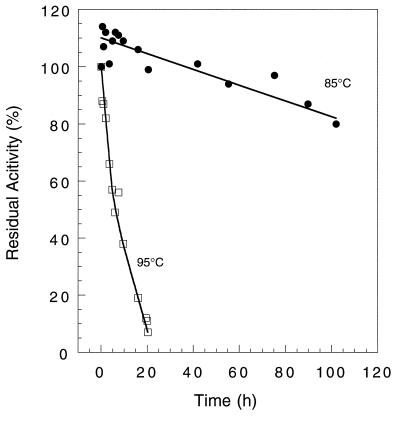
Purified ADH was irreversibly inactivated by oxygen. The time required for a 50% loss in catalytic activity in the presence of air was 1.1 h at 23°C, and this decreased to 15 min if air was replaced by oxygen (100% in the gas phase). In both cases, there was no significant increase in activity when the sample was degassed and flushed with Ar, followed by the addition of sodium dithionite (3 mM). Incubation of an oxygen-inactivated sample (≤10% of the original activity) at 80°C for 1 min either in the presence or in the absence of dithionite did not restore any activity. In contrast, under anaerobic conditions the enzyme as purified lost no activity even after 24 h (at 23°C). As anticipated, purified ADH was very thermostable. The times required for a 50% loss in catalytic activity (using a protein concentration of 3.6 mg/ml in 50 mM EPPS (pH 8.0) at 85 and 95°C were about 160 and 7 h, respectively (Fig. 4).
FIG. 4.
Effects of temperature on the stability of P. furiosus ADH. The enzyme (3.6 mg/ml in 50 mM Tris-HCl [pH 7.8] containing 2.0 mM dithiothreitol) was incubated in stoppered glass vials at 85°C (●) or 95°C (□). Samples were removed at intervals and assayed by the NADP-dependent oxidation of ethanol at 80°C.
Catalytic properties.
P. furiosus ADH catalyzed the oxidation of a range of aliphatic (C2 to C8) and aromatic primary alcohols at 80°C with NADP as the electron acceptor, but it did not oxidize methanol and showed little if any activity with polyols (Table 5). NAD also functioned as an electron acceptor for ethanol oxidation, but the enzyme had an extremely low affinity for this cofactor (apparent Km of 20 mM) compared to that for NADP (apparent Km of 71 μM [Table 6]). For ethanol oxidation at 80°C, the optimal pH was between 9.4 and 10.2, with approximately 50% of the maximal activity at pH 8.5 and at pH 10.8. At pH 9.6, the activity increased with increasing temperature from 30°C (1.5 U/mg) to 90°C (33 U/mg), with an optimum above 90°C. The corresponding Arrhenius plot showed no obvious transition point over this temperature range. ADH also catalyzed the reduction of acetaldehyde and phenylacetaldehyde with NADPH as the electron donor. The enzyme had no detectable activity as an acetyl-CoA reductase, as was found with the ADH from Escherichia coli (14, 26, 27). As shown in Table 6, although the maximal specific activities for aldehyde reduction and alcohol oxidation were comparable, the enzyme was much more efficient in catalyzing aldehyde reduction. That is, the apparent Kms were less than 170 μM for both the aldehyde substrate and the cofactor (NADPH), compared with a value of 29 mM for ethanol. Clearly, the physiological role of ADH is more likely to be aldehyde reduction than alcohol oxidation, and NADP(H) rather than NAD(H) is the preferred cofactor.
TABLE 5.
Substrate specificity of P. furiosus ADH
| Substrate | Activity (U/mg) | % |
|---|---|---|
| Alcohols (60 mM) | ||
| Methanol | 0 | 0 |
| Ethanol | 18.2 | 100a |
| 1-Propanol | 28.5 | 156 |
| 2-Propanol | 0 | 0 |
| 1-butanol | 30.8 | 169 |
| Isobutanol | 22.2 | 122 |
| 1-Pentanol | 31.4 | 172 |
| 1-Hexanol | 17.4 | 95 |
| 1-Heptanol | 15.7 | 86 |
| 1-Octanol | 10.5 | 57 |
| 1,3-Propanediol | 1.1 | 6 |
| 2-Phenylethanol | 13.8 | 76 |
| Tryptophol (10 mM) | 2.1 | 11 |
| Glycerol | 0 | 0 |
| d-Sorbitol | 0 | 0 |
| Aldehydes (2 mM) | ||
| Acetaldehyde | 8.4 | 46 |
| Phenylacetaldehyde | 13.8 | 76 |
| Methylglyoxal | 4.9 | 27 |
Equals 18.2 U/mg at 80°C.
TABLE 6.
Kinetic parameters of ADH from P. furiosus
| Substrate (mMa) | Cosubstrate (mM) | Avg apparent Km (mM) ± SDb | Apparent Kcat (min−1) | Apparent Kcat/apparent Km (min−1 mM−1) |
|---|---|---|---|---|
| Ethanol (13–130) | NADP (0.4) | 29.4 ± 2.5 | 1,150 | 39 |
| Acetaldehyde (0.05–2) | NADPH (0.25) | 0.17 ± 0.02 | 329 | 1,940 |
| NADP (0.03–0.4) | Ethanol (91) | 0.071 ± 0.003 | 945 | 13,300 |
| NAD (0.1–0.6) | Ethanol (91) | 20.0 ± 2.0 | 144 | 7 |
| NADPH (0.03–0.25) | Acetaldehyde (2.0) | 0.071 ± 0.003 | 378 | 5,320 |
| 2-Phenylethanol (2–40) | NADP (0.4) | 8.0 ± 0.2 | 540 | 69 |
| Tryptophol (1–10) | NADP (0.4) | 6.3 ± 0.1 | 99 | 16 |
| Phenylacetaldehyde (0.05–2) | NADPH (0.3) | 0.091 ± 0.003 | 621 | 6,820 |
| Methylglyoxal (0.07–2) | NADPH (0.3) | 0.2 ± 0.02 | 221 | 1,100 |
Concentration range used to determine the kinetic constants.
For the corresponding substrate, not the cosubstrate.
DISCUSSION
ADHs are widely distributed in all three domains of life, and they can be classified into three different groups based on their molecular properties (51). Group I contains long-chain ADHs represented by the well-studied horse liver ADH, which contains Zn at its active site (19, 64). Some of these ADHs contain additional Zn, which has a structural function, as in the horse liver enzyme (64), while some contain only catalytic Zn, as in ADH from Thermoanaerobacter brockii (10). Group II contains short-chain ADHs, and these enzymes, such as the ADH from Drosophila melanogaster (48, 68), lack metals. Group III includes only a small number of Fe-dependent ADHs, represented by ADH2 from Zymomonas mobilis (60).
Recently, two types of ADH were isolated from hyperthermophilic organisms and characterized. One was a group I, Zn-containing ADH from Sulfolobus solfataricus (50, 52), while the other type has been obtained from three Thermococcus species (Table 7). The complete sequence of one of the Thermococcus enzymes, i.e., that from T. zilligii (formerly Thermococcus strain AN1 [31, 54]) is available and shows similarities with sequences of the group III Fe-dependent ADHs (31). Mesophilic ADHs of group III do not contain Fe after purification; rather, Fe must be present in the assay mixture to obtain ADH activity (60, 67). These enzymes are therefore termed Fe-activated ADHs (4, 60). In contrast, the hyperthermophilic ADHs from Thermococcus strain ES-1 and T. litoralis do contain Fe after purification (34, 37), and we will refer to these as Fe-containing (rather than Fe-activated) ADHs.
TABLE 7.
Comparison of P. furiosus ADH with other archaeal ADHs
| Property | P. furiosus | ES-1 | T. litoralis | T. zilligii | S. solfataricus |
|---|---|---|---|---|---|
| Mol wt (structure) | 48,000 (α6) | 46,000 (α4) | 48,000 (α4) | 46,700 (α4) | 37,580 (α2) |
| Metal (g-atoms/mol) | Fe (0.9) | Fe (0.95) | Fe (0.45) | NDa | Zn (2) |
| Zn (0.8) | |||||
| Electron carrier | NADPH | NADPH | NADPH | NADPH | NADH |
| Apparent Km, alcohol (mM) | 29.4 | 8.0 | 11.0 | 10.0 | 0.019 |
| Apparent Km, aldehyde (mM) | 0.17 | 0.25 | 0.4 | 0.13 | 0.003 |
| Apparent Vmax (U/mg) | 72 | 62 | 32 | 3.2 | 3.9 |
| Optimal pH | 9.4–10.2 | 8.8–10.4 | 8.8 | 6.8–7.0 | 7.5–8.5 |
| Optimal temp (°C) | >95 | >95 | 85 | 85 | >95 |
| Stability (t1/2, h) | 160 (85°C), 7 (95°C) | 35 (85°C), 4 (95°C) | 5 (85°C), 0.3 (96°C) | 0.3 (80°C) | 5 (70°C), 3 (85°C) |
| Reference(s) | This work | 37 | 34 | 31 | 2, 50 |
ND, not determined.
As indicated in Fig. 2, there is high similarity between the N-terminal amino acid sequences of the ADHs from Thermococcus species and that purified from P. furiosus, but these show no similarity with the sequences of the Fe-activated mesophilic enzymes; this suggests that the P. furiosus enzyme belongs to the Fe-containing type ADH. On the other hand, P. furiosus ADH as isolated contains Zn in addition to Fe, which is in contrast to the zinc-free ADHs from the Thermococcus species. Since the P. furiosus enzyme contains eight histidine residues, it has at least two potential sites to bind metal ions, possibly one for Fe and other for Zn. The latter would be expected to have a structural rather than catalytic role, since it is not present in the ADHs from the Thermococcus species. Interestingly, the P. furiosus enzyme represents the most thermostable ADH known (Table 7), and so perhaps Zn does play a structural role to enhance thermostability. Obviously, the catalytic and/or structural functions of the two metals in this enzyme need to be established, and such studies using structural and spectroscopic analyses are under way.
Among the mesophilic group III ADHs, that of Z. mobilis is regulated by oxygen (65), while the concentration of the enzyme in E. coli responds to the redox status of the cell (29, 30) and is regulated by the catabolite repressor activator protein, Cra (43). It was suggested that repression by Cra results in the stringent control of adhE transcription under aerobic conditions. The expression of Fe-containing ADH of Thermococcus strain ES-1 also appears to be regulated by the cellular redox balance, since its activity increases dramatically under S0 limitation (37). Thus, all three of these ADHs seem to be regulated by the cellular redox potential, as determined by the presence or absence of oxidants such as O2 or S0. In contrast, this does not appear to be the case with the ADH from P. furiosus, as its activity was similar in cells grown in the presence and absence of S0. Moreover, in contrast to the other ADHs, P. furiosus ADH was very oxygen sensitive and had to be purified under strictly anaerobic, reducing conditions. The mechanism of inactivation is not clear at present since treatment of the as-purified enzyme with oxygen did not lead to the oxidation of its ferrous iron site, as monitored by EPR spectroscopy.
It was reported that P. furiosus contains two ADH-encoding genes (adhA and adhB) which are coregulated with celB, a gene that is expressed only in the presence of cellobiose, a growth substrate (23, 69). The complete sequences of adhA and adhB were obtained from the genome sequence of P. furiosus, which is currently being completed (70). However, based on its amino-terminal sequence, the ADH reported herein is not encoded by either of these genes. Such a conclusion is supported by the fact that the P. furiosus cells used to purify ADH in the present study were grown in the absence of cellobiose. The genome sequence of the related organism P. horikoshii (21) has been published (45), but a search of the database using the N-terminal amino acid sequence of P. furiosus ADH gave no match. The growth of P. horikoshii is greatly stimulated by the presence of S0 (21) but an ADH from this organism has not yet been characterized.
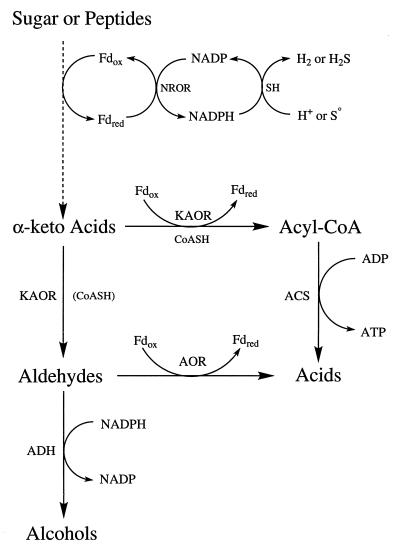
The kinetic data obtained with P. furiosus ADH clearly show that the enzyme preferentially catalyzes aldehyde reduction using NADPH as the electron donor (Table 6). NADPH is also the physiological electron donor to the hydrogenase of P. furiosus (39). This enzyme can also reduce S0 to H2S and is termed sulfhydrogenase (38). S0 reduction by P. furiosus was thought to be a mechanism to remove inhibitory H2 (20), but H2S production appears to have a bioenergetic role, as the equivalent of approximately 0.5 mol of ATP is generated per mol of sulfide produced (59, 66). The other S0-reducing enzyme in P. furiosus is an iron-sulfur flavoprotein termed sulfide dehydrogenase (36), but like sulfhydrogenase, it is a cytoplasmic enzyme. It is not clear, therefore, how S0 reduction can lead to energy conservation in this organism. One potential mechanism stems from the discovery that a key enzyme in the fermentation pathway, POR, which produces acetyl-CoA from pyruvate, can also function as a pyruvate decarboxylase to generate acetaldehyde (35) (Fig. 5). Thus, while acetate production from acetyl-CoA is coupled to ATP synthesis, this is not the case with acetaldehyde-to-acetate conversion, which is catalyzed by AOR (43). The production of acetyl-CoA and acetaldehyde from pyruvate catalyzed by POR appears to be regulated by the cellular redox potential (35). Presumably, this is affected by whether reductant is disposed of as H2 or as H2S. Hence, if S0 reduction is a more efficient means of removing excess reductant than is H2 production, this process would promote the oxidative rather than the nonoxidative decarboxylation of pyruvate by POR, and more ATP would be generated via acetyl-CoA (56) (Fig. 5). This indirect mechanism might explain how S0 reduction by hyperthermophiles that do not grow by S0 respiration can still result in the overall conservation of energy (35).
FIG. 5.
Proposed metabolic role of P. furiosus ADH. Abbreviations: Fdox, oxidized ferredoxin; Fdred, reduced ferredoxin; KAOR, α-keto acid ferredoxin oxidoreductases; CoASH, coenzyme A; AOR, aldehyde ferredoxin oxidoreductase; ACS, acetyl-CoA synthetases; FNOR, ferredoxin NADP oxidoreductase; SH, sulfhydrogenase (or hydrogenase).
We therefore propose that the aldehydes which are the substrates for P. furiosus ADH arise from the nonoxidative decarboxylation of 2-keto acids, produced from glycolysis and amino acid transamination and catalyzed by POR and the related enzymes IOR, KGOR, and VOR. Such aldehydes are very reactive compounds and ultimately toxic, especially at the growth temperature of P. furiosus (16, 25, 37, 49). Hence, ADH is proposed to function in P. furiosus as an aldehyde-scavenging enzyme (Fig. 5). Another aldehyde-oxidizing enzyme, AOR (43), is thought to have a similar role in P. furiosus and related species (25, 37). Note that aldehyde oxidation by AOR generates reduced ferredoxin, which would favor the decarboxylation of 2-keto acids to produce more aldehydes by POR and related enzymes rather their oxidation (35). In contrast, ADH reduces aldehydes and produces an oxidized electron carrier (NADP), which is a substrate for ferredoxin:NADP oxidoreductase (FNOR) to produce oxidized ferredoxin (36), and this in turn serves to decrease the amount of aldehyde produced by the 2-keto acid oxidoreductases (35).
The ADH enzymes that have purified from facultative S0 reducers such as P. furiosus and obligate S0 reducers such as Thermococcus strain ES-1 are, therefore, quite similar in their molecular and biochemical properties (Table 7). However, they differ in that the former type contains a zinc atom, possibly for structural stability, and the cellular concentration of the enzyme appears to be independent of the S0 content of the growth medium. In spite of this, the two types of ADH are assigned the same physiological role, namely, the reduction of toxic aldehydes. In addition to ADH, the obligate S0 reducers also regulate expression of hydrogenase and FMOR in response to S0, whereas the hydrogenase of P. furiosus appeared not to be affected by S0, and FMOR activity could not be detected, regardless of whether cells were grown with S0. Curiously, the genome of P. furiosus contains a gene encoding a putative formate dehydrogenase (70), but the growth condition under which it is expressed has not been determined. In any event, these two types of hyperthermophilic archaea clearly differ in how they regulate enzymes thought to be involved in reductant disposal, and the mechanism by which the facultative S0 reducers such as P. furiosus appear to gain a bioenergetic benefit from S0 reduction has yet to be proven. The properties of its ADH do not argue for or against the mechanism proposed herein, whereby S0 reduction effectively decreases the amount of aldehydes produced by the 2-keto acid oxidoreductases. Direct measurements of aldehyde production might prove or disprove such a mechanism, and such a study is under way.
ACKNOWLEDGMENT
This research was supported by grant BCS-9632657 from the National Science Foundation.
REFERENCES
- 1.Adams M W W. Biochemical diversity among sulfur-dependent hyperthermophilic microorganisms. FEMS Microbiol Rev. 1994;15:267–277. doi: 10.1111/j.1574-6976.1994.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 2.Ammendola S, Raia C A, Caruso C, Camardella L, D’Auria S, De Rosa M, Rossi M. Thermostable NAD-dependent alcohol dehydrogenase from Sulfolobus solfataricus: gene and protein sequence determination and relationship to other alcohol dehydrogenases. Biochemistry. 1992;31:12514–12523. doi: 10.1021/bi00164a031. [DOI] [PubMed] [Google Scholar]
- 3.Arciero D M, Orville A M, Lipscomb J D. 17O-water and nitric oxide binding by protocatechuate 4,5 dioxygenase and catechol 2,3 dioxygenase. J Biol Chem. 1985;260:14035–14044. [PubMed] [Google Scholar]
- 4.Bakshi E N, Tse P, Murray K S, Hanson G R, Scopes R K, Wedd A G. Iron-activated alcohol dehydrogenase from Zymomonas mobilis: spectroscopic and magnetic properties. J Am Chem Soc. 1989;189:8707–8713. [Google Scholar]
- 5.Bennetzen J L, Hall B D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase I. J Biol Chem. 1982;257:3018–3025. [PubMed] [Google Scholar]
- 6.Blamey J M, Adams M W W. Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus. Biochim Biophys Acta. 1993;1161:19–27. doi: 10.1016/0167-4838(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 7.Blamey J M, Adams M W W. Characterization of an ancestral-type of pyruvate ferredoxin oxidoreductase from the hyperthermophlic bacterium, Thermotoga maritima. Biochemistry. 1994;33:1000–1007. doi: 10.1021/bi00170a019. [DOI] [PubMed] [Google Scholar]
- 8.Bleicher K, Winter J. Purification and properties of F420- and NADP-dependent alcohol dehydrogenases of Methanogenium liminatans and Methanobacterium palustre, specific for secondary alcohols. Eur J Biochem. 1991;200:43–51. doi: 10.1111/j.1432-1033.1991.tb21046.x. [DOI] [PubMed] [Google Scholar]
- 9.Blumentals I I, Itoh M, Olson G J, Kelly R M. Role of polysulfides in reduction of elemental sulfur by the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990;56:1255–1262. doi: 10.1128/aem.56.5.1255-1262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogin O, Peretz M, Burstein Y. Thermoanaerobacter brockii alcohol dehydrogenase: characterization of the active site metal and its ligand amino acids. Protein Sci. 1997;6:450–458. doi: 10.1002/pro.5560060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 12.Bryant F O, Adams M W W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989;264:5070–5079. [PubMed] [Google Scholar]
- 13.Chen J-S, Mortenson L E. Inhibition of methylene blue formation during determination of acid-labile sulfide of iron-sulfur protein samples containing dithionite. Anal Biochem. 1977;79:157–165. doi: 10.1016/0003-2697(77)90390-6. [DOI] [PubMed] [Google Scholar]
- 14.Clarke D P. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;63:223–234. doi: 10.1016/0168-6445(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 15.Conway T, Sewell G W, Osman Y A, Ingram L O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987;169:2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng W, Hamilton-Kemp T R, Nielsen M T, Andersen R A, Collins G B, Hildebrand D F. Effects of six-carbon aldehydes and alcohols on bacterial proliferation. J Agric Food Chem. 1993;41:506–510. [Google Scholar]
- 17.Deutscher M P. Guide to protein purification. Methods Enzymol. 1990;182:588–604. [PubMed] [Google Scholar]
- 18.Drewke C, Ciriacy M. Overexpression, purification and properties of alcohol dehydrogenase IV from Saccharomyces. Biochim Biophys Acta. 1988;950:54–60. doi: 10.1016/0167-4781(88)90072-3. [DOI] [PubMed] [Google Scholar]
- 19.Eklund H, Nordstrom B, Zeppezauer E, Soderlund G, Ohlsson I, Boiwe T, Soderberg B O, Tapia O, Branden C I, Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2.4 Å resolution. J Mol Biol. 1976;102:27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- 20.Fiala G, Stetter K O. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch Microbiol. 1986;145:56–61. [Google Scholar]
- 21.González J M, Masuchi Y, Robb F T, Ammerman J W, Maeder D L, Yanagibayashi M, Tamaoka J, Kato C. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles. 1998;2:123–130. doi: 10.1007/s007920050051. [DOI] [PubMed] [Google Scholar]
- 22.Goodlove P E, Bury S M, Sawyer L. Cloning and sequence analysis of the fermentative alcohol-dehydrogenase-encoding gene of Escherichia coli. Gene. 1989;85:209–214. doi: 10.1016/0378-1119(89)90483-6. [DOI] [PubMed] [Google Scholar]
- 23.Gueguen Y, Voorhorst W G B, van der Oost J, de Vos W M. Molecular and biochemical characterization of an endo-β-1,3-glucanase of the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1997;272:31258–31264. doi: 10.1074/jbc.272.50.31258. [DOI] [PubMed] [Google Scholar]
- 24.Hegg E L, Que L., Jr The 2-His-1-carboxylate facial triad: an emerging structural motif in mononuclear non-heme iron(II) enzymes. Eur J Biochem. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- 25.Heider J, Ma K, Adams M W W. Purification, characterization, and metabolic function of tungsten-containing aldehyde ferredoxin oxidoreductase from the hyperthermophilic and proteolytic archeon Thermococcus strain ES-1. J Bacteriol. 1995;177:4757–4764. doi: 10.1128/jb.177.16.4757-4764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler D, Leibrecht I, Knappe J. Pyruvate-formate-lyase-deactivase and acetyl-CoA reductase activities of Escherichia coli reside on a polymeric operon particle encoded by adhE. FEBS Lett. 1991;281:59–63. doi: 10.1016/0014-5793(91)80358-a. [DOI] [PubMed] [Google Scholar]
- 27.Kessler D, Herth W, Knappe J. Ultrastructure and pyruvate-quenching property of the multienzyme AdhE protein of Escherichia coli. J Biol Chem. 1992;267:18073–18079. [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Leonardo M R, Cunningham P R, Clark D P. Anaerobic regulation of the adhE gene, encoding the fermentative alcohol dehydrogenase of Escherichia coli. J Bacteriol. 1993;175:870–878. doi: 10.1128/jb.175.3.870-878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonardo M R, Dailly Y, Clark D P. Role of NAD in regulating the adhE gene of Escherichia coli. J Bacteriol. 1996;178:6013–6018. doi: 10.1128/jb.178.20.6013-6018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Stevenson K J. Purification and sequence analysis of a novel NADP(H) dependent type III alcohol dehydrogenase from Thermococcus strain AN1. J Bacteriol. 1997;179:4433–4437. doi: 10.1128/jb.179.13.4433-4437.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipscomb J D, Orville A M. Mechanistic aspects of dihydroxybenzoate dioxygenases. In: Sigel H, Sigel A, editors. Metal ions in biological systems. Vol. 28. New York, N.Y: Marcel Dekker; 1992. pp. 243–298. [Google Scholar]
- 33.Lovenberg W, Buchanan B B, Rabinowitz J C. Studies on the chemical nature of ferredoxin. J Biol Chem. 1963;238:3899–3913. [PubMed] [Google Scholar]
- 34.Ma K, Robb F T, Adams M W W. Purification and characterization of NADP-specific alcohol dehydrogenase and NADP-specific glutamate dehydrogenase from the hyperthermophilic archaeon Thermococcus litoralis. Appl Environ Microbiol. 1994;60:562–568. doi: 10.1128/aem.60.2.562-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma K, Hutchins A, Sung S-J S, Adams M W W. Pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus, functions as a CoA-dependent pyruvate decarboxylase. Proc Natl Acad Sci USA. 1997;94:9608–9613. doi: 10.1073/pnas.94.18.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma K, Adams M W W. Sulfide dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus: a new multifunctional enzyme involved in the reduction of elemental sulfur. J Bacteriol. 1994;176:6509–6517. doi: 10.1128/jb.176.21.6509-6517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma K, Loessner H, Heider J, Johnson M K, Adams M W W. Effects of elemental sulfur on the metabolism of the deep-sea hyperthermophilic archaeon Thermococcus strain ES-1: characterization of a sulfur-regulated, non-heme iron alcohol dehydrogenase. J Bacteriol. 1995;177:4748–4756. doi: 10.1128/jb.177.16.4748-4756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma K, Schicho R N, Kelly R M, Adams M W W. Hydrogenase of the hyperthermophile Pyrococcus furiosus is an elemental sulfur reductase or sulfhydrogenase: evidence for a sulfur-reducing hydrogenase ancestor. Proc Natl Acad Sci USA. 1993;90:5341–5344. doi: 10.1073/pnas.90.11.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma K, Zhou Z H, Adams M W W. Hydrogen production from pyruvate by enzymes purified from the hyperthermophilic archaeon, Pyrococcus furiosus: a key role for NADPH. FEMS Microbiol Lett. 1994;122:263–266. [Google Scholar]
- 40.Mai X, Adams M W W. Characterization of aromatic and aliphatic 2-ketoacid oxidoreductases from hyperthermophilic archaea. J Inorg Chem. 1993;51:459. [Google Scholar]
- 41.Mai X, Adams M W W. Indolepyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus: a new enzyme involved in peptide fermentation. J Biol Chem. 1994;269:16726–16732. [PubMed] [Google Scholar]
- 42.Mikulskis A, Aristarkhov A, Lin E C. Regulation of expression of the ethanol dehydrogenase gene (adhE) in Escherichia coli by catabolite repressor activator protein Cra. J Bacteriol. 1997;179:7129–7134. doi: 10.1128/jb.179.22.7129-7134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukund S, Adams M W W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase: evidence for its participation in a unique glycolytic pathway. J Biol Chem. 1991;266:14208–14216. [PubMed] [Google Scholar]
- 44.Mukund S, Adams M W W. Characterization of a novel tungsten-containing formaldehyde ferredoxin oxidoreductase from the extremely thermophilic archaeon, Thermococcus litoralis. A role for tungsten in peptide catabolism. J Biol Chem. 1993;268:13592–13600. [PubMed] [Google Scholar]
- 45.National Institute of Technology and Evaluation.http://www.bio.nite.go.jp/E-home/biomenu-e2.html. National Institute of Technology and Evaluation, Tokyo, Japan.
- 46.Neale A D, Scope R K, Kelly J M, Wettenhall R E H. The two alcohol dehydrogenases of Zymomonas mobilis—purification by different dye ligand chromatography, molecular characterization and physiological roles. Eur J Biochem. 1986;154:119–124. doi: 10.1111/j.1432-1033.1986.tb09366.x. [DOI] [PubMed] [Google Scholar]
- 47.Peretz M, Burstein Y. Amino acid sequence of alcohol dehydrogenase from the thermophilic bacterium Thermoanaerobium brockii. Biochem J. 1989;28:6549–6555. doi: 10.1021/bi00442a004. [DOI] [PubMed] [Google Scholar]
- 48.Persson B, Krook M, Jörnvall Characterization of short-chain alcohol dehydrogenases and related enzymes. Eur J Biochem. 1991;200:537–543. doi: 10.1111/j.1432-1033.1991.tb16215.x. [DOI] [PubMed] [Google Scholar]
- 49.Pospísil S, Sedmera P, Havlícek V, Prikrylová V. Excretion of butyraldehyde, isobutyraldehyde and valeraldehyde by Streptomyces cinnamonensis. FEMS Microbiol Lett. 1994;119:95–98. [Google Scholar]
- 50.Raia C A, Caruso C, Marino M, Vespa N, Rossi M. Activation of Sulfolobus solfataricus alcohol dehydrogenase by modification of cysteine residue 38 with iodoacetic acid. Biochemistry. 1996;35:638–647. doi: 10.1021/bi9502093. [DOI] [PubMed] [Google Scholar]
- 51.Reid M F, Fewson C A. Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol. 1994;20:13–56. doi: 10.3109/10408419409113545. [DOI] [PubMed] [Google Scholar]
- 52.Rella R, Raia C A, Pensa M, Pisani F M, Gambacorta A, De Rosa M, Rossi M. A novel archaebacterial NAD-dependent alcohol dehydrogenase: purification and properties. J Biochem. 1987;167:475–479. doi: 10.1111/j.1432-1033.1987.tb13361.x. [DOI] [PubMed] [Google Scholar]
- 53.Robb F T, Park J-B, Adams M W W. Characterization of an extremely thermostable glutamate dehydrogenase: a key enzyme in the primary metabolism of the hyperthermophilic archaebacterium, Pyrococcus furiosus. Biochim Biophys Acta. 1992;1120:267–272. doi: 10.1016/0167-4838(92)90247-b. [DOI] [PubMed] [Google Scholar]
- 54.Ronimus R S, Reysenbach A-L, Musgrave D R, Morgan H W. The phylogenetic position of the Thermococcus isolate AN1 based on 16S rRNA gene sequence analysis: a proposal that AN1 represents a new species, Thermococcus zilligii sp. nov. Arch Microbiol. 1997;168:245–248. doi: 10.1007/s002030050495. [DOI] [PubMed] [Google Scholar]
- 55.Roy, R., and M. W. W. Adams. 1998. Unpublished results.
- 56.Schäfer T, Selig M, Schönheit P. Acetyl CoA synthetase (ADP-forming) in archaea, a novel enzyme involved in acetate formation and ATP synthesis. Arch Microbiol. 1993;159:72–83. [Google Scholar]
- 57.Schauder R, Kröger A. Bacterial sulfur respiration. Arch Microbiol. 1993;159:491–497. [Google Scholar]
- 58.Schauder R, Müller E. Polysulfide as a possible substrate for sulfur-reducing bacteria. Arch Microbiol. 1993;160:377–382. [Google Scholar]
- 59.Schico R N, Ma K, Adams M W W, Kelly R M. Bioenergetics of sulfur reduction in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 1993;175:1823–1830. doi: 10.1128/jb.175.6.1823-1830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scopes R K. An iron-activated alcohol dehydrogenase. FEBS Lett. 1983;156:303–306. doi: 10.1016/0014-5793(83)80517-1. [DOI] [PubMed] [Google Scholar]
- 61.Stetter K O. Diversity of extremely thermophilic archaebacteria. In: Brock T D, editor. The thermophiles: general, molecular, and applied microbiology. New York, N.Y: John Wiley; 1986. pp. 39–74. [Google Scholar]
- 62.Stetter K O. Hyperthermophilic procaryotes. FEMS Microbiol Rev. 1996;18:149–158. [Google Scholar]
- 63.Stetter K O, Fiala G, Huber G, Huber R, Segerer G. Hyperthermophilic microorganisms. FEMS Microbiol Rev. 1990;75:117–124. [Google Scholar]
- 64.Sytkowski A J, Vallee B L. Chemical reactivities of catalytic and noncatalytic zinc or cobalt atoms of horse liver alcohol dehydrogenase: differentiation by their thermodynamic and kinetic properties. Proc Natl Acad Sci USA. 1976;73:344–348. doi: 10.1073/pnas.73.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamarit J, Cabiscol E, Aguilar J, Ros J. Differential inactivation of alcohol dehydrogenase isoenzymes in Zymomonas mobilis by oxygen. J Bacteriol. 1997;179:1102–1104. doi: 10.1128/jb.179.4.1102-1104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tse P, Scopes R K, Wedd A G. An iron-activated alcohol dehydrogenase: metal dissociation constants and magnetic and spectroscopic properties. J Am Chem Soc. 1988;110:1295–1297. [Google Scholar]
- 68.Villarroya A, Juan E, Egestad B, Joenvall H. The primary structure of alcohol dehydrogenase from Drosophila lebanonensis: intensive variation within insect ’short-chain’ alcohol dehydrogenase lacking zinc. Eur J Biochem. 1989;180:191–197. doi: 10.1111/j.1432-1033.1989.tb14632.x. [DOI] [PubMed] [Google Scholar]
- 69.Voorhorst W G B, Eggen R I L, Luesink E J, de Vos W M. Characterization of the celB gene coding for β-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus and its expression and site-directed mutation in Escherichia coli. J Bacteriol. 1995;177:7105–7111. doi: 10.1128/jb.177.24.7105-7111.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss, R. B. Unpublished data.
- 71.Woese C R, Kandler O, Wheelis M L. Towards a natural system of organisms: proposal for the domains of Archaea, Bacteria and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolgel S A, Dege J E, Perkins-Olson P E, Juarez-Garcia C H, Crawford R L, Münck E, Lipscomb J D. Purification and characterization of protocatechuate 2,3-dioxygenase from Bacillus macerans: a new extradiol catecholic dioxygenase. J Bacteriol. 1993;175:4414–4426. doi: 10.1128/jb.175.14.4414-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]