Abstract
Breast milk is a pivotal source to provide passive immunity in newborns over the first few months of life. Very little is known about the antibody transfer levels over the period of breastfeeding. We conducted a prospective study in which we evaluated concentrations of anti-SARS-CoV-2 Spike IgA and RBD IgG/M/A antibodies in maternal serum and breast milk over a duration of up to 6 months after delivery. We compared antibody levels in women with confirmed COVID-19 infection during pregnancy (n = 16) to women with prenatal SARS-CoV-2 vaccination (n = 5). Among the recovered women, n = 7 (44%) had been vaccinated during the lactation period as well. We observed intraindividual moderate positive correlations between antibody levels in maternal serum and breast milk (r = 0.73, p-value<0.0001), whereupon the median levels were generally higher in serum. Anti-RBD IgA/M/G transfer into breast milk was significantly higher in women recovered from COVID-19 and vaccinated during lactation (35.15 AU/ml; IQR 21.96–66.89 AU/ml) compared to the nonvaccinated recovered group (1.26 AU/ml; IQR 0.49–3.81 AU/ml), as well as in the vaccinated only group (4.52 AU/ml; IQR 3.19–6.23 AU/ml). Notably, the antibody level in breast milk post SARS-CoV-2 infection sharply increased following a single dose of vaccine. Breast milk antibodies in all groups showed neutralization capacities against an early pandemic SARS-CoV-2 isolate (HH-1) and moreover, also against the Omicron variant, although with lower antibody titer. Our findings highlight the importance of booster vaccinations especially after SARS-CoV-2 infection in pregnancy in order to optimize protection in mother and newborn.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-19, coronavirus disease 2019; VOC, variants of concern; Ig, immunoglobulins; RBD, receptor binding domain; mRNA, messenger ribonucleid acid; NC, nucleocapsid; S1, Spike protein subunit; BAU, Binding Antibody Units; OD, optical density; ELISA, enzyme-linked immunosorbent assay; BMT162b2, Pfizer-BioNTech COVID-19 Vaccine; “R” group, recovered group; “R & V” group, recovered and vaccinated group; “V” group, vaccinated group
Keywords: SARS-CoV-2, Pregnancy, Passive immunity, Breast milk, Antibodies, Humoral immune response
1. Introduction
The SARS-CoV-2 pandemic has been going on for more than two years and currently the Omicron variant (linage B.1.1.529) is dominating in many countries of the world leading to high infection numbers and health burden (Sharma et al., 2022, Vaughan, 2021). Although presumably millions of children are infected with SARS-CoV-2 worldwide, there is paucity of data available on the pediatric burden suffering from underreporting of the cases and limitation on SARS-CoV-2 testing (Chow and Englund, 2022). As of January 2022, UNICEF reported over 12 ´ 300 deaths in children younger than 20 years and of them 42% were among children aged 0–9 (“Child mortality and COVID-19 - UNICEF DATA", 2022). Severe short-term outcomes of neonatal SARS-CoV-2 infection are rare but long-term effects on the neurological and physical development are still unclear (Ryan et al., 2022). Currently prevention including active immunization via SARS-CoV-2 mRNA vaccine is the main protective measure against COVID-19 disease in children (Chow and Englund, 2022). So far, young children aged 0–4 years are excluded form the vaccination against SARS-CoV-2.
It is well known that newborns are relatively well protected from infections due to the vertical transfer of pathogen-specific IgG antibodies from mother to the fetus via the placenta, followed by the transfer of IgA antibodies through breast milk (Albrecht and Arck, 2020, Flannery et al., 2021, Song et al., 2021). Thus, this phenomenon – referred to as passive immunity - reverts the window of vulnerability for early life infections during the first few months of life (Kollmann et al., 2020). To take advantage of this natural ways of protection from infection, vaccination recommendations during pregnancy have been re-evaluated, e.g. against pertussis and tetanus (Amirthalingam et al., 2014, Blencowe et al., 2010). Just recently it could be shown that maternal mRNA Covid-19 vaccination during pregnancy is associated with a reduced risk for COVID-19 hospitalization among infants aged < 6 months (Halasa et al., 2022).
In this context, the transfer of passive immunity via breast milk is less well studied compared to the transfer of antibodies via the placenta. Breast milk is composed of a wealth of specific immunoprotective factors. Among these, human milk antibodies comprise largely of secretory immunoglobulins A (sIgA) (> 90%), as well as secretory immunoglobulins M (sIgM, 8%) and immunoglobulins G (IgG, 2%) (Hurley and Theil, 2011). With respect to SARS-CoV-2 both, BNT162b2 messenger RNA Covid-19 vaccination and natural infection with SARS-CoV-2 virus lead to the formation of anti-SARS-CoV-2 antibodies in the mother which exert their neutralizing capacity by binding different regions of the virus spike glycoprotein. These neutralizing antibodies are also secreted into breast milk (Collier et al., 2021, Fow et al., 2020, Pace et al., 2021, Perl et al., 2021, Romero Ramírez et al., 2021). With ongoing SARS-CoV-2 pandemic the number of people with the binomial situation of “recovered or vaccinated” are diminishing, while more complex situations like convalescent and vaccinated with one or more vaccine booster are taking place. However, real life data are still scant. Here, we performed a cohort study to assess the transfer of passive immunity via breast milk in newborns up to 6 months after delivery against SARS-CoV-2 an early pandemic SARS-CoV-2 isolate (HH-1) and Omicron variant.
2. Methods
2.1. Study design and population
We conducted an exploratory, descriptive, prospective longitudinal study from February through December 2021.
In total, 21 pregnant women giving birth at our hospital were recruited around the time of delivery. Of these, 16 women had recovered from COVID-19 during pregnancy, which was diagnosed by a positive SARS-CoV-2-PCR in a nasopharyngeal swab. Among these women, 7 had been vaccinated with one dose (30 µg) of BNT162b2 messenger RNA Covid 19 Vaccine in the lactation period.These groups are referred to as R for recovered from COVID-19 only and RV for recovered and vaccinated. Furthermore, we included 5 pregnant women who have not been infected with SARS-CoV-2, but vaccinated twice with 30 µg BNT162b2 messenger RNA Covid 19 Vaccine during pregnancy within an interval of 6 weeks, referred to as V. All participants signed informed consent forms and the study protocol was approved by the ethics committee of the Hamburg Chamber of Physicians under the license number PV 7312, in accordance with the Declaration of Helsinki for Medical Research involving Human Subjects.
For those patients with a previous SARS-CoV-2 infection, the first date with positive RT-PCR or the onset of previously existing symptoms were used to calculate the time post infection.
Paired samples of breast milk and blood were taken monthly during the lactation period for a maximum of 6-months after delivery. At least one paired sample of breast milk and blood was required for inclusion. The venous blood samples were collected by peripheral venopuncture, while breast milk was collected after hand washing either by milk pumps or manual expression. The milk was sampled into sterile plastic containers and stored at 5–7 °C for a maximum duration of 6 h until cryopreservation. Blood samples were centrifugated at 2000 g for 15 min and then the isolated serum stored at − 80 °C. Breast milk samples were centrifugated at 750 g for 20 min with acceleration on 9 and break on 0. The lipid- and milk-layers were separated from the cell pellet and then centrifugated at 750 g for 10 min. Then the milk-layers were transferred into a separate 1.5 ml reaction tube by pipetting through the lipid-layer, then stored at − 80 °C.
Table 1 displays the vaccination and sampling schedule for each study participant.
Table 1.
Vaccination/Infection status and sampling schedule of the study participants.
| Study participant | Months before delivery | Months after delivery (x = Serum & Breast milk samples) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 3 | 2 | 1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Recovered & Vaccinated | |||||||||||
| RV 1 |  |
x
|
x | x | x | x | x | ||||
| RV 2 |  |
x | x | x
|
x | x | x | ||||
| RV 3 |  |
x | x
|
x | x | x | x | ||||
| RV 4 |  |
x | x | x
|
x | x | x | ||||
| RV 5 |  |
x | x | x 
|
x | ||||||
| RV 6 |  |
x | x | x | x 
|
x | x | ||||
| RV 7 |  |
x | x 
|
x | x | x | |||||
| Recovered | |||||||||||
| R1 |  |
x | x | x | x | x | x | ||||
| R2 |  |
x | x | x | x | x | x | ||||
| R3 |  |
x | x | x | x | x | |||||
| R4 |  |
x | x | x | x | x | x | ||||
| R5 |  |
x | |||||||||
| R6 |  |
x | |||||||||
| R7 |  |
x | |||||||||
| R8 |  |
x | x | x | |||||||
| R9 |  |
x | x | x | x | ||||||
| Vaccinated | |||||||||||
| V1 |  |
 |
x | x | |||||||
| V2 |  |
 |
x | x | |||||||
| V3 |  |
 |
x | x | |||||||
| V4 |  |
 |
x | x | |||||||
| V5 |  |
 |
x | x | |||||||
2.2. Serological assays
The serological confirmation or exclusion of a previous SARS-CoV-2 infection was assessed by the qualitative anti–NC–SARS-CoV-2 Ig assay (Elecsys Anti-SARS-CoV-2, Roche; cut off ≥1 COI/ml). To evaluate the humoral immune response to SARS-CoV-2 infection and/or vaccination, three assays were performed: the quantitative anti-S1-RBD-SARS-CoV-2 assay (Elecsys Anti-SARS-CoV-2 Spike, Roche; Mannheim, Germany; cut off 0.8 U/ml), the qualitative anti-SARS-CoV-2 IgA enzyme-linked immunosorbent assay (ELISA, Euroimmun Medizinische Labordiagnostika, Lübeck, Germany;) and anti- SARS-CoV-2 TrimericS IgG assay ( LIAISON; DiaSorin, cut off ≥ 33.8 BAU/ml). For the anti-SARS-CoV-2 IgA ELISA, the results were reported by calculating the ratio of the extinction of the control or patient sample over the extinction of the calibrator, S/P ratio. Samples with titers in the Elecsys Anti-SARS-CoV-2 Spike above 250 U/ml were manually diluted 1:100 in dilution buffer according to the manufacturer's recommendations to increase the linear range to 25000 U/ml.
Although sensitivity and specificity of the manufacturer for the total test anti-S1-RBD-SARS-CoV-2 assay and the SARS-CoV-2 TrimericS IgG assay were similar (sensitivity of 99.5% (95%CI 97.0 – 100%) and specificity of 99.8% (95%CI 99.7 – 99.9%) for the first (“Elecsys anti-SARS-CoV-2. Roche,” n.d.) and of 98.7% (95%CI 94.5%−99.6%) and 99.5% (95%CI: 99.0–99.7%) respectively for the latter(“Anti- SARS-CoV-2 TrimericS IgG assay. Liaison DiaSorin,” n.d.), the reported sensitivity for Euroimmune Anti-SARS-CoV-2 IgA ELISA in serum beyond 60 days was reported 84.6%. Furthermore, specificity in serum among pregnant women (n = 99) was reported to 97% (“Anti-SARS-CoV-2 IgA. Euroimmun,” n.d.). None of these assays were validated for breast milk by the manufacturer.
2.3. Neutralisation assay
HH-1 isolate (Pfefferle et al., 2020) and Omicron variant isolates were used for neutralization tests. Briefly, after incubation at 37 °C for one hour, the serum/virus mixtures were transferred to 96-well plates containing 5.0 × 106 cells/plate of Vero cells (ATCC CRL-1008) seeded the previous day. Following incubation for 96 h at 37 °C, supernatants were discarded. The plates were fixed in 4% formaldehyde and stained with crystal violet. The highest dilution protecting 2 of 3 wells from cytopathic effect was taken as the neutralizing antibody titer.
2.4. Statistical analyses
Data were presented as median with interquartile range (IQR). A two-tailed Mann–Whitney U test was used to analyze the median antibody titers between subgroups, while Spearman correlation was performed between antibodies titers in serum and in breast milk.
P-values lower than 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism, version 9 for macOS (GraphPad Software, La Jolla, California, USA).
3. Results
3.1. Clinical characteristics
Study population characteristics including severity of the SARS-CoV-2 infection, vaccination status, pregnancy course and breastfeeding status are shown in Table 2.
Table 2.
Maternal and infant characteristics of the study population.
| Characteristics | Recovered (n = 16) | Vaccinated (n = 5) |
|---|---|---|
| Maternal age, median (IQR) | 35 (31–38) | 37 (30–41) |
| Ethnicity, n (%) | ||
| European | 12 (75%) | 5 (100%) |
| Eastern Asia | 1 (6%) | none |
| Middle East | 3 (19%) | none |
| Body-Mass-Index, mean (SD) [range] | 24,2 (2,8) [19,6–31] | 25,7 (4,1) [20–31,2] |
| Parity, mean (SD) [range] | 1,3 (0,5) [1,2] | 1,4 (0,5) [1,2] |
| Severity of SARS-CoV-infection, n (%) | NA | |
| mild/moderate¹ | 14 (88%) | |
| severe² | 2 (12%) | |
| Interval infection – delivery (d), median (IQR) | 90 (17–133) | NA |
| Vaccination | ||
| 2 doses Bnt162b2 (Pfizer BioNTech) | NA | 5 (100%) |
| 1 booster dose Bnt162b2 (after infection) | 7 (44%) | NA |
| Vaccine associated side effects, n (%) | ||
| Local3 | none | 2 (40%) |
| Systemic⁴ | 5 (71%) | 3 (6%) |
| None | 2 (29%) | none |
| Preterm delivery due to, n (%) | none | |
| preterm labour | 1 (6%) | |
| pregnancy complications (pathological CTG, preeclampsia) |
2 (13%) | |
| severe maternal COVID-19 | 1 (6%) | |
| Delivery mode, n (%) | ||
| vaginal | 11 (69%) | 3 (60%) |
| cesarean | 5 (31%) | 2 (40%) |
| Gestational age at delivery (in weeks), median (IQR) | 39,4 (37,5–41) | 39,9 (37,8–40,5) |
| Birth weight (in g), median (IQR) | 3390 (3042–3588) | 3350 (2735–3518) |
| Infant sex, n (%) | ||
| female | 9 (56%) | 2 (40%) |
| male | 7 (44%) | 3 (60%) |
| Breastfeeding status, n (%) | ||
| exclusive | 10 (63%) | 4 (80%) |
| mixed | 6 (37%) | 1 (20%) |
¹ NIH‐criteria: ¹ NIH-criteria: COVID-19 symptoms, oxygen saturation ≥ 94% on room air at sea level
² NIH-criteria: COVID-19 symptoms, SpO2 ≤ 94% on room air at sea level, ICU and oxygen supply
3pain at the injection site, redness, swelling
4 tiredness, headache, muscle patin, fever, nausea
NA = not applicable
The median age of all participants (n = 21) was 36 years (IQR 31–41 years). 16/21 participants were SARS-CoV-2 infected, of those 2/16 had severe COVID-19 (defined by NHI criteria, COVID-19 Treatment Guidelines, 2021) with intensive care management. 7 recovered participants from SARS-CoV-2 received one dose Pfizer BTN162b2 vaccine, while 5 of the vaccinated group received two doses. No serious side effects were reported. Median interval between infection/or vaccination and delivery for all 3 groups are shown in supplementary table 1.
3.2. Humoral immune response
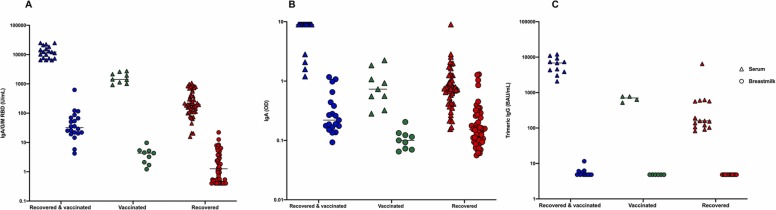
Serological response against the viral N protein which is not encoded in the mRNA vaccine confirmed infection in the R and RV groups (16/16 participants anti SARS-CoV-2 N Ig assay positive) and excluded previous occult SARS-CoV-2 infections in the V group (0/5 positive). Neutralizing antibodies against SARS-CoV-2 predominately target the viral spike protein with the S1 and S2 domains. Median total Ig S1-RBD antibody levels in serum were 12223 AU/ml (IQR 7491–18627 AU/ml), 1427 AU/ml (IQR 1137–2143 AU/ml) and 198 AU/ml (IQR 109.6–435.6 AU/ml) for the groups RV, V and R respectively. In breast milk median total Ig S1-RBD antibody levels were lower: 35.15 AU/ml (IQR 21.96–66.89 AU/ml), 4.52 AU/ml (IQR 3.19–6.23 AU/ml) and 1.26 AU/ml (IQR 0.49–3.81 AU/ml) for the groups RV, V and R respectively ( Fig. 1A), but a significant positive correlation between serum and breast milk was observed (Sperman r = 0.73, p-value< 0.0001, supplementary figure 1). A similar pattern with higher median levels in serum (9 OD, 0.73 OD, 0.74 OD) compared to breast milk (0.22 OD, 0.12 OD, 0.15 OD) for the groups RV, V and R was observed using the qualitative anti-SARS-CoV-2 IgA assay (Fig. 1 B). To selectively analyze the IgG response we performed the anti-SARS-CoV-2 TrimericS IgG assay. Median antibody levels in serum were 6860 BAU/ml (IQR 3860–9240 BAU/ml), 704 BAU/ml (IQR 576–775 BAU/ml) and 159 BAU/ml (IQR 106–569 BAU/ml) for the groups RV,V and R respectively while antibody levels in breast milk were below the threshold for detection (Fig. 1C) indicating anti-SARS-CoV-2 S specific antibodies in breast milk predominately comprise the IgA class.
Fig. 1.
Comparison between median antibody levels in serum (triangle) and breast milk (circle) according to the three different groups RV (blue), V (green) and R (red) assessed by anti-S1-RBD-SARS-CoV-2 assay (A), anti-SARS-CoV-2 IgA ELISA (B) and anti- SARS-CoV-2 TrimericS IgG (C). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
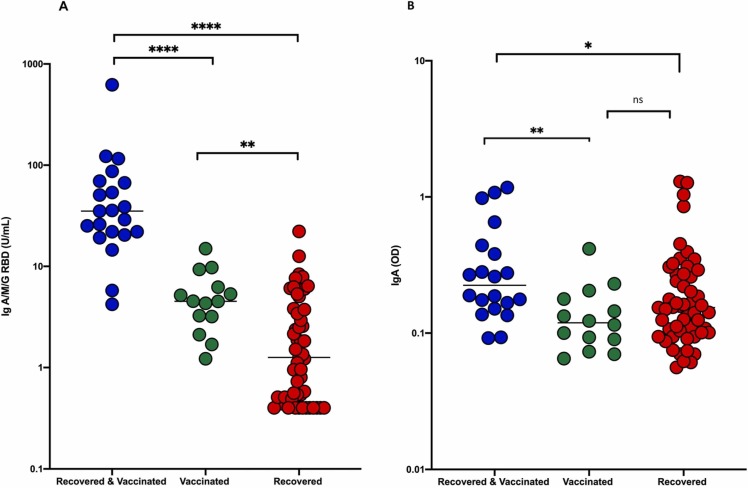
Transfer of anti-S1-RBD-SARS-CoV-2 antibody into breast milk was particularly profound in women who had recovered from COVID-19 and were vaccinated during lactation (RV). The median titer was significantly higher compared to the R group, as well as in the V group (p-value =< 0.0001, Fig. 2 A). Furthermore, the V group had significantly higher titers of anti-S1-RBD-SARS-CoV-2 Ig antibodies in breast milk, compared to R (p-value=< 0.01, Fig. 2 A). The qualitative anti-SARS-CoV-2 S1 IgA assay showed the same tendency (Fig. 2 B), although less pronounced and in this case the difference between the groups V and R was not statistically significant.
Fig. 2.
Comparison between median antibodiy levels in breast milk according to the three different groups RV (blue), V (green) and R (red) assessed by anti-S1-RBD-SARS-CoV-2 assay (A) and anti-SARS-CoV-2 IgA ELISA (B)*p < 0.05, * *p < 0.01, * **p < 0.001, and * ** *p < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Long term response
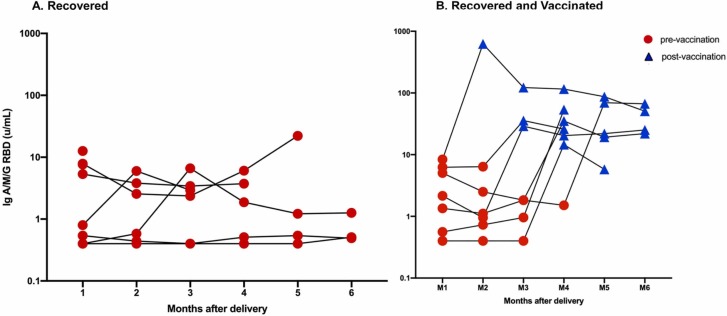
To investigate the SARS-CoV-2 antibody kinetics in breast milk samples were analyzed up to 6 months after delivery ( Fig. 3). Although a moderate anti-RBD IgA/M/G decline over the months of follow-up could be detected, we observed that a single dose of vaccine could induce a significant increase in SARS-CoV-2 specific antibodies titers to the breast milk.
Fig. 3.
6 months-follow-up post delivery kinetic humoral response of antibodies anti-S1-RBD-SARS-CoV-2 in breast milk according to the R (A) and the RV (B) groups.
3.4. Virus neutralization assay
The neutralization test could be performed in 8 breast milk samples from 6 participants: 5 samples from the RV group (2 before and 3 after vaccination), 1 sample from the R group and 2 samples from the V group. Interestingly all breast milk samples with positive anti-RBD Ig were found to neutralize both an early pandemic SARS-CoV-2 isolate (Pfefferle et al., 2020) and the new Omicron variant (B.1.1.529) in vitro. However, the neutralization titers were not directly correlated with the concentration of anti-S1-RBD-SARS-CoV-2 Ig antibodies and in general lower antibody titers were found against Omicron variant (supplementary Table 2).
4. Discussion
In the present study, we identified a robust and persistent SARS-CoV-2 antibody transfer to breast milk in women with previous COVID-19 infection and/or vaccination. Women who recovered from SARS-CoV-2 infection in pregnancy and were subsequently vaccinated during the lactation period had the highest antibody levels in breast milk compared to those only infected or vaccinated. This is consistent with previous studies in human milk (Juncker et al., 2021) as well as in serum (Gils et al., 2021, Manisty et al., 2021) where just one dose of the BNT162b2 vaccine elicited significantly higher antibody levels in previously infected participants. We showed a positive correlation between SARS-CoV-2 antibody levels in serum and in human milk, in line with previous studies (Demers-Mathieu et al., 2021, Gonçalves et al., 2021, Whited and Cervantes, 2022). Furthermore, in our study antibodies persisted in breast milk up to 300 days. Although we could not distinguish directly between the different subgroups, the results of the three SARS-CoV-2 assays (total Ig, IgA and IgG specific) indicate that IgA must be the predominant immunoglobulins with anti SARS-CoV-2 activity in breast milk, considering the complete absence of IgG Trimeric antibodies in breast milk. Studies, focusing in IgA titers in breastmilk comfirmed our data (Fox et al., 2022, Juncker et al., 2021).
Neutralizing effects of antibodies against SARS-CoV-2 were shown in several studies in recovered (Favara et al., 2020, Pace et al., 2021) and vaccinated (Fox et al., 2022, Narayanaswamy et al., 2022, Perez et al., 2022, Yeo et al., 2021, Young et al., 2021) lactating women. In our study the neutralization titer of antibodies doubled directly after one booster dose of messenger RNA Covid-19 vaccine in a recovered women. Neutralizing capacities detected in the neutralization assay only moderately correlated with anti-S1-RBD-SARS-CoV-2 Ig levels especially in samples with low levels of spike antibodies indicating that either crossreactive antibodies against circulating corona viruses (Wec et al., 2020) or antibodies targeting the M protein of the cell membrane might be functionally relevant. In addition to an early pandemic SARS-CoV-2 isolate, we evaluated the neutralizing capacity of breast milk antibodies against current dominant SARS-CoV-2 Omicron variant. Although still detectable, a fourfold decrease of the neutralizing titers was found compared to the early pandemic isolate. These findings are in accordance with other studies on serum antibodies that showed Omicron evaded neutralization by antibodies from recovered patients or individuals who were vaccinated with two doses mRNA vaccine with 12- to 44-fold higher efficiency than the Delta variant (Hoffmann et al., 2022, Muik et al., 2022). With three booster mRNA or heterologous vaccinations only moderate Omicron evasion was observed, although none of the included women in our study had a comparable profile (Hoffmann et al., 2022).
There are some limitations in this study. First, the sample size for each group of women was small. Secondly, due to the small amount of breast milk leftovers the neutralization test had to be limited to 8 samples. Nevertheless, a coherent human milk antibody kinetic was found within the groups. Furthermore, six months follow-up was not available for all participants. However, to our knowledge, our study shows one of the longest follow up of antibodies in breast milk of COVID-19 recovered and/or vaccinated women.
In conclusion, we confirmed the presence of antibodies in breast milk with neutralization effectivity against two SARS-CoV-2 variants including the emerging Omicron variant. The highest titers were observed in reconvalescent women vaccinated in the lactation period. Our data support a beneficial effect of vaccination of COVID-19-recovered breast-feeding women to enhance maternal immune response and humoral passive protection in newborns. The key aspect for future studies will be to correlate maternal antibody levels with infant immune protection.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the German Research Foundation (KFO296: AR232/25–2, STA 1549/2–1, DI2103/2–2, KH4617/1–2, BR1730/7–1, Germany) to P.A., A.D and K.H. and the Authority for Science, Research and Equality, Hanseatic City of Hamburg (State Research Funding, LFF-FV73, Germany) to P.A. and A.D.
Author contributions
F.O., M.L., P.C.A. and A.T. developed the study and designed most of the experimental set-ups. A.T., G.H. and L.R. enrolled the subjects and collected samples. K.H., A.D. and A.T. oversaw clinical management of patients. N.F. and L.R. coordinated the sample collection, quality control and assessments. F.O., D.N., S.P., M.Ä. and M.L. performed most of the analyses, processed the experimental data and designed most of the figures. R.P., N.P. and P.E. performed the neutralization assays. The original draft of the manuscript was written by F.O. and A.T. and edited by M.L. and P.C.A., further writing, review and editing were done by all authors. P.C.A., M.L. and A.T. supervised the project.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jri.2022.103685.
Appendix A. Supplementary material
Supplementary material
.
References
- Albrecht M., Arck P.C. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front Immunol. 2020;11:555. doi: 10.3389/fimmu.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirthalingam G., Andrews N., Campbell H., Ribeiro S., Kara E., Donegan K., Fry N.K., Miller E., Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384:1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- Anti- SARS-CoV -2 TrimericS IgG assay. Liaison DiaSorin, n.d.
- Anti-SARS-CoV-2 IgA . Euroimmun, n.d.
- Blencowe H., Lawn J., Vandelaer J., Roper M., Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J. Epidemiol. 2010;39:i102–i109. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child mortality and COVID -19 - UNICEF DATA [WWW Document], n.d. URL https://data.unicef.org/topic/child-survival/covid-19/ (accessed 2.8.22).
- Chow E.J., Englund J.A. SARS-CoV-2 infections in children. Infect. Dis. Clin. 2022:0. doi: 10.1016/j.idc.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A.-R.Y., McMahan K., Yu J., Tostanoski L.H., Aguayo R., Ansel J., Chandrashekar A., Patel S., Apraku Bondzie E., Sellers D., Barrett J., Sanborn O., Wan H., Chang A., Anioke T., Nkolola J., Bradshaw C., Jacob-Dolan C., Feldman J., Gebre M., Borducchi E.N., Liu J., Schmidt A.G., Suscovich T., Linde C., Alter G., Hacker M.R., Barouch D.H. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers-Mathieu V., DaPra C., Medo E. Comparison of severe acute respiratory syndrome coronavirus 2-specific antibodies’ binding capacity between human milk and serum from coronavirus disease 2019-recovered women. Breast Med. 2021;16:393–401. doi: 10.1089/bfm.2020.0381. [DOI] [PubMed] [Google Scholar]
- Elecsys anti-SARS-CoV-2 . Roche, n.d.
- Favara D.M., Ceron-Gutierrez M.L., Carnell G.W., Heeney J.L., Corrie P., Doffinger R. Detection of breastmilk antibodies targeting SARS-CoV-2 nucleocapsid, spike and receptor-binding-domain antigens. Emerg. Microbes Infect. 2020;9:2728–2731. doi: 10.1080/22221751.2020.1858699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery D.D., Gouma S., Dhudasia M.B., Mukhopadhyay S., Pfeifer M.R., Woodford E.C., Triebwasser J.E., Gerber J.S., Morris J.S., Weirick M.E., McAllister C.M., Bolton M.J., Arevalo C.P., Anderson E.M., Goodwin E.C., Hensley S.E., Puopolo K.M. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pedia. 2021;175:594–600. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Marino J., Amanat F., Oguntuyo K.Y., Hahn-Holbrook J., Lee B., Zolla-Pazner S., Powell R.L. The IgA in milk induced by SARS-CoV-2 infection is comprised of mainly secretory antibody that is neutralizing and highly durable over time. PLoS ONE. 2022;17 doi: 10.1371/journal.pone.0249723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fow A., Marino J., Amanat F., Krammer F., Hahn-Holbrook J., Zolla-Pazner S., Powell R.L. Robust and specific secretory IgA against SARS-CoV-2 detected in human milk. iScience. 2020;23(11) doi: 10.1016/j.isci.2020.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gils M.J., van, Willigen H.D., van, Wynberg E., Han A.X., Straten K., van der, Verveen A., Lebbink R., Dijkstra M., Burger J.A., Oomen M., Tejjani K., Bouhuijs J.H., Appelman B., Lavell A.H.A., Poniman M., Caniels T.G., Bontjer I., Vught L.A., van, Vlaar A.P.J., Sikkens J.J., Bomers M.K., Sanders R.W., Kootstra N.A., Russell C., Prins M., Bree G.J., de, Jong M.D., de, Group, Rec S. Single-dose SARS-CoV-2 vaccine in a prospective cohort of COVID-19 patients. medRxiv 2021. 05. 2021;25:21257797. doi: 10.1101/2021.05.25.21257797. [DOI] [Google Scholar]
- Gonçalves J., Juliano A.M., Charepe N., Alenquer M., Athayde D., Ferreira F., Archer M., Amorim M.J., Serrano F., Soares H. Secretory IgA and T cells targeting SARS-CoV-2 spike protein are transferred to the breastmilk upon mRNA vaccination. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasa N.B., Olson S.M., Staat M.A., Newhams M.M., Price A.M., Boom J.A., Sahni L.C., Cameron M.A., Pannaraj P.S., Bline K.E., Bhumbra S.S., Bradford T.T., Chiotos K., Coates B.M., Cullimore M.L., Cvijanovich N.Z., Flori H.R., Gertz S.J., Heidemann S.M., Hobbs C.V., Hume J.R., Irby K., Kamidani S., Kong M., Levy E.R., Mack E.H., Maddux A.B., Michelson K.N., Nofziger R.A., Schuster J.E., Schwartz S.P., Smallcomb L., Tarquinio K.M., Walker T.C., Zinter M.S., Gilboa S.M., Polen K.N., Campbell A.P., Randolph A.G., Patel M.M. Overcoming COVID-19 investigators, overcoming COVID-19 network, 2022. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 States, July 2021-January. MMWR Morb. Mortal. Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Krüger N., Schulz S., Cossmann A., Rocha C., Kempf A., Nehlmeier I., Graichen L., Moldenhauer A.-S., Winkler M.S., Lier M., Dopfer-Jablonka A., Jäck H.-M., Behrens G.M.N., Pöhlmann S. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185(447–456) doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley W.L., Theil P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–474. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncker H.G., Mulleners S.J., van Gils M.J., Bijl T.P.L., de Groot C.J.M., Pajkrt D., Korosi A., van Goudoever J.B., van Keulen B.J. Comparison of SARS-CoV-2-Specific Antibodies in Human Milk after mRNA-Based COVID-19 Vaccination and Infection. Vaccines. 2021;9:1475. doi: 10.3390/vaccines9121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann T.R., Marchant A., Way S.S. Vaccination strategies to enhance immunity in neonates. Science. 2020;368:612–615. doi: 10.1126/science.aaz9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A., Lui B.G., Wallisch A.K., Bacher M., Mühl J., Reinholz J., Ozhelvaci O., Beckmann N., Güimil Garcia R.C., Poran A., Shpyro S., Finlayson A., Cai H., Yang Q., Swanson K.A., Türeci Ö., Şahin U. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375:678–680. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswamy V., Pentecost B.T., Schoen C.N., Alfandari D., Schneider S.S., Baker R., Arcaro K.F. Neutralizing antibodies and cytokines in breast milk after coronavirus disease 2019 (COVID-19) mRNA vaccination. Obstet. Gynecol. 2022;139:181–191. doi: 10.1097/AOG.0000000000004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace R.M., Williams J.E., Järvinen K.M., Belfort M.B., Pace C.D.W., Lackey K.A., Gogel A.C., Nguyen-Contant P., Kanagaiah P., Fitzgerald T., Ferri R., Young B., Rosen-Carole C., Diaz N., Meehan C.L., Caffé B., Sangster M.Y., Topham D., McGuire M.A., Seppo A., McGuire M.K. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio. 2021;12:e03192–20. doi: 10.1128/mBio.03192-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S.E., Luna Centeno L.D., Cheng W.A., Marentes Ruiz C.J., Lee Y., Congrave-Wilson Z., Powell R.L., Stellwagen L., Pannaraj P.S. Human Milk SARS-CoV-2 antibodies up to 6 months after vaccination. Pediatrics. 2022;149 doi: 10.1542/peds.2021-054260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl S.H., Uzan-Yulzari A., Klainer H., Asiskovich L., Youngster M., Rinott E., Youngster I. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S., Huang J., Nörz D., Indenbirken D., Lütgehetmann M., Oestereich L., Günther T., Grundhoff A., Aepfelbacher M., Fischer N. Complete genome sequence of a SARS-CoV-2 strain isolated in Northern Germany. Microbiol Resour. Announc. 2020:9. doi: 10.1128/MRA.00520-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero Ramírez D.S., Lara Pérez M.M., Carretero Pérez M., Suárez Hernández M.I., Martín Pulido S., Pera Villacampa L., Fernández Vilar A.M., Rivero Falero M., González Carretero P., Reyes Millán B., Roper S., García Bello M.Á. SARS-CoV-2 antibodies in breast milk after vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052286. [DOI] [PubMed] [Google Scholar]
- Ryan L., Plötz F.B., van den Hoogen A., Latour J.M., Degtyareva M., Keuning M., Klingenberg C., Reiss I.K.M., Giannoni E., Roehr C., Gale C., Molloy E.J. Neonates and COVID-19: state of the art. Pedia Res. 2022;91:432–439. doi: 10.1038/s41390-021-01875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Rai H., Gautam D.N.S., Prajapati P.K., Sharma R. Emerging evidence on omicron (B.1.1.529) SARS-CoV-2 variant. J. Med Virol. 2022 doi: 10.1002/jmv.27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Prahl M., Gaw S.L., Narasimhan S., Rai D., Huang A., Flores C., Lin C.Y., Jigmeddagva U., Wu A.H.B., Warrier L., Levan J., Nguyen C.B.T., Callaway P., Farrington L., Acevedo G.R., Gonzalez V.J., Vaaben A., Nguyen P., Atmosfera E., Marleau C., Anderson C., Misra S., Stemmle M., Cortes M., McAuley J., Metz N., Patel R., Nudelman M., Abraham S., Byrne J., Jegatheesan P. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. medRxiv 2021. 05. 2021;01:21255871. doi: 10.1101/2021.05.01.21255871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A. Omicron emerges. N. Sci. 2021;252:7. doi: 10.1016/S0262-4079(21)02140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wec A.Z., Wrapp D., Herbert A.S., Maurer D.P., Haslwanter D., Sakharkar M., Jangra R.K., Dieterle M.E., Lilov A., Huang D., Tse L.V., Johnson N.V., Hsieh C.-L., Wang N., Nett J.H., Champney E., Burnina I., Brown M., Lin S., Sinclair M., Johnson C., Pudi S., Bortz R., Wirchnianski A.S., Laudermilch E., Florez C., Fels J.M., O’Brien C.M., Graham B.S., Nemazee D., Burton D.R., Baric R.S., Voss J.E., Chandran K., Dye J.M., McLellan J.S., Walker L.M. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020;369:731–736. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited N., Cervantes J. Antibodies against SARS-CoV-2 in human breast milk after vaccination: a systematic review and meta-analysis. Breastfeed. Med. 2022;17:475–483. doi: 10.1089/bfm.2021.0353. [DOI] [PubMed] [Google Scholar]
- Yeo K.T., Chia W.N., Tan C.W., Ong C., Yeo J.G., Zhang J., Poh S.L., Lim A.J.M., Sim K.H.Z., Sutamam N., Chua C.J.H., Albani S., Wang L.-F., Chua M.C. Neutralizing activity and SARS-CoV-2 vaccine mRNA persistence in serum and breastmilk after BNT162b2 vaccination in lactating women. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.783975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Seppo A.E., Diaz N., Rosen-Carole C., Nowak-Wegrzyn A., Cruz Vasquez J.M., Ferri-Huerta R., Nguyen-Contant P., Fitzgerald T., Sangster M.Y., Topham D.J., Järvinen K.M. Association of human milk antibody induction, persistence, and neutralizing capacity with SARS-CoV-2 infection vs mRNA vaccination. JAMA Pedia. 2021 doi: 10.1001/jamapediatrics.2021.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material