Abstract
Patients with indolent lymphoma undertaking recurrent or continuous B cell suppression are at risk of severe COVID‐19. Patients and healthy controls (HC; N = 13) received two doses of BNT162b2: follicular lymphoma (FL; N = 35) who were treatment naïve (TN; N = 11) or received immunochemotherapy (ICT; N = 23) and Waldenström's macroglobulinemia (WM; N = 37) including TN (N = 9), ICT (N = 14), or treated with Bruton's tyrosine kinase inhibitors (BTKi; N = 12). Anti‐spike immunoglobulin G (IgG) was determined by a high‐sensitivity flow‐cytometric assay, in addition to live‐virus neutralization. Antigen‐specific T cells were identified by coexpression of CD69/CD137 and CD25/CD134 on T cells. A subgroup (N = 29) were assessed for third mRNA vaccine response, including omicron neutralization. One month after second BNT162b2, median anti‐spike IgG mean fluorescence intensity (MFI) in FL ICT patients (9977) was 25‐fold lower than TN (245 898) and HC (228 255, p = .0002 for both). Anti‐spike IgG correlated with lymphocyte count (r = .63; p = .002), and time from treatment (r = .56; p = .007), on univariate analysis, but only with lymphocyte count on multivariate analysis (p = .03). In the WM cohort, median anti‐spike IgG MFI in BTKi patients (39 039) was reduced compared to TN (220 645, p = .0008) and HC (p < .0001). Anti‐spike IgG correlated with neutralization of the delta variant (r = .62, p < .0001). Median neutralization titer for WM BTKi (0) was lower than HC (40, p < .0001) for early‐clade and delta. All cohorts had functional T cell responses. Median anti‐spike IgG decreased 4‐fold from second to third dose (p = .004). Only 5 of 29 poor initial responders assessed after third vaccination demonstrated seroconversion and improvement in neutralization activity, including to the omicron variant.
1. INTRODUCTION
In the current SARS‐CoV‐2 pandemic, patients with B cell malignancies are at increased risk of severe COVID‐19 disease and death. Therefore, a comprehensive understanding of the efficacy of vaccination in these patients is a priority. 1 , 2 Follicular lymphoma (FL) is an indolent lymphoma accounting for 20%–30% of all B cell NHLs, 3 characterized by a relapsing and remitting course following treatments over a median survival of approximately 20 years. Waldenström's macroglobulinemia (WM) is a rare indolent lymphoplasmacytic lymphoma productive of monoclonal immunoglobulin M and involving the bone marrow. 4 Management of both FL and WM often involves an initial “watch and wait” period. Patients who require systemic treatment commonly receive combination induction chemotherapy and immunotherapy (with an anti‐CD20 antibody), which reduces both normal and malignant B cells. FL patients often proceed to a “maintenance” period of anti‐CD20 antibody treatment for 2 years, although this practice has reduced due to risk‐mitigation in the COVID‐19 era. 5 Patients with WM are increasingly on continuous therapy with Bruton's tyrosine kinase inhibitors (BTKi) and occasionally B‐cell lymphoma 2 (BCL‐2) inhibitors.
Patients with hematological malignancies are reported to have a reduced humoral response to vaccination. 6 , 7 It is postulated that this is due to both the underlying disease process effect on the immune system, in addition to the influence of immunosuppressive treatments. A prospective cohort study of 885 patients in Lithuania with a hematological malignancy showed a reduced anti‐spike immunoglobulin G (IgG) response to COVID‐19 vaccination, particularly in those with lymphoproliferative disease who had received BTKi therapy and anti‐CD20 therapy. 8 Similar results were seen in an Israeli study of COVID‐19 vaccination in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. 9 The potentially protective role of the T cell response to vaccination is recognized but has not yet been prospectively and longitudinally measured in patients with indolent lymphoma. 10
The available COVID‐19 vaccines in Australia at commencement of this study were the Pfizer‐BioNTech (BNT162b2) mRNA vaccine and the Astra Zeneca (ChAdOx1) nonreplicating viral vector vaccine. Response to these vaccines can be assessed by measuring specific antibody and T cell responses. 11 , 12
Until the emergence of the omicron variant in December 2021, the incidence of COVID‐19 infection in Australia had been relatively low compared to other regions, due to a rigorous viral suppression public health strategy. The confirmed positive cases as a percentage of population was 0.12% in Australia at enrolment to this study in May 2021, compared to 9.84% in the USA at the same time. We were in a unique position to assess the efficacy of COVID‐19 vaccination in a population that had lower rates of exposure to endemic SARS‐CoV‐2 and therefore less potential for confounding of immune responses due to natural infection.
In this study, we comprehensively profiled both the humoral, live viral neutralization and cellular response to COVID‐19 vaccination in patients with FL and WM and determined the impact of differing lymphoma treatment regimens. In addition, we present data from a subset of poor vaccine responders who were encouraged to expedite access to an approved third dose of mRNA (preferably mRNA‐1273) vaccination in October 2021. We chart the rate of ongoing seroconversion and neutralizing activity to early‐clade SARS‐CoV‐2, as well as the delta variant. We also present data of neutralization activity for the omicron variant to this third dose subset of patients.
2. METHODS
2.1. Participants
Patients with FL, WM and healthy age‐matched controls were enrolled in May–June 2021 to a prospective study of immune response after two doses of BNT162b2 administered 21 days apart. The study was approved by the Sydney Local Health District Human Research Ethics Committee (HREC Approval Number: X21‐0120) and all subjects provided written informed consent prior to participation. Recruitment was targeted to obtain comparable proportions of healthy controls (HC), treatment naïve (TN) and treated patients with at least 10 participants in each cohort. All participants had serum specimens collected for antibody measurement and approximately half of participants in each cohort had peripheral blood mononuclear cell (PBMC) specimens collected for T cell analysis (based on logistical limitations). Specimens were collected immediately prior to the first dose (T1), at Day 21 immediately prior to the second dose (T2), and Day 49 (±7d) (T3). A subset of participants had serum collected for antibody assessment prior to and after a third dose of mRNA vaccine (either BNT162b2 or mRNA‐1273).
2.2. High‐sensitivity anti‐spike IgG assay
IgG titers to the SARS‐CoV‐2 spike protein were measured by our previously published high‐sensitivity live‐cell assay. 13 Briefly, HEK293 cells were transfected to express early‐clade SARS‐CoV‐2 spike antigens. Diluted serum (1:80) was added to live spike‐expressing cells. Briefly, in relation to spike expression construct synthesis, codon‐optimized, wild‐type SARS‐CoV‐2 strain Wuhan spike open reading frame with 18 amino acids deleted from their cytoplasmic tail, was cloned within the MCS of a lentiviral expression vector, pLVX‐IRES‐ZsGreen, using EcoRI and XbaI restriction sites, resulting in pSpike‐IRES‐ZsGreen vector. All synthetic gene fragments were ordered through IDT. Cells were then incubated with Alexa Fluor 647‐conjugated anti‐human IgG (H + L) (Thermo Fisher Scientific). Cell events were acquired on LSRII flow cytometer (BD Biosciences), and median fluorescence intensity (MFI), a proxy of antibody titers 14 was analyzed. The threshold for a positive result was determined if the delta MFI (ΔMFI = MFI transfected cells − MFI untransfected cells) was above the positive threshold (mean ΔMFI + 4SD of 24 prepandemic age‐matched controls) in at least two of three quality‐controlled experiments. Low level of spike IgG were defined as <30 000 MFI and were associated to no live virus neutralization. The cut‐off for live virus neutralization was 1:20 and was ≤30 000 MFI. This has been estimated to provide 50% protection against detectable SARS‐CoV‐2 infection. 15 The assay was validated using the WHO20/136 Standard 16 at a starting dilution 1:80 (Figure S1 and Table S1) with a cut‐off for seropositivity of approximately 0.028 BAU/ml. The sensitivity was superior to several commercial assays at 98% (95% confidence interval: 92%–99%). 13 Data were analyzed using FlowJo 10.4.1 (TreeStar), Excel (Microsoft), and GraphPad Prism (GraphPad Software).
2.3. Live virus neutralization assay
In addition to measuring the concentration of anti‐spike IgG we assessed the functionality of these antibodies to prevent cellular infection with live virus, initially using both an early‐clade (A2.2) and delta strain as previously published. 13 Briefly, sera were serially diluted and mixed in duplicate with an equal volume of virus in solution at a median of 1.5 × 103 tissue culture infectious dose per ml. After 1 h of virus‐serum coincubation at 37°C, 40 μl were added to equal volume of freshly trypsinized VeroE6 cells and a HekAT (HAT‐24) cell line selected for SARS‐CoV‐2 permissiveness. The data for VeroE6 is typically the same as the HekAT line, although the former is less sensitive to low responses. The validation of HAT‐24 has been previously described. 17 After 20 h for HAT‐24 and 72 h for VeroE6, cells were stained with NucBlue (Invitrogen) and each well was imaged in its entirety with InCell Analyzer (Cytiva). Nuclei counts decrease with higher viral replication in a dose dependent manner. Samples were compared between convalescent sera, mock controls (defined as 100% neutralization) and infected controls (defined as 0% neutralization). The cut‐off for determining the neutralization endpoint titer of diluted serum samples was set to ≥50% neutralization and the cut‐off of positivity for neutralization on live virus was 1:20.
In December 2021, live omicron viral neutralization was assessed in pre‐ and post‐third dose vaccination sera, adapting the above method. In 29 participants, third dose mRNA vaccines were given to 11 FL cohort patients, 10 patients had mRNA‐1273 and 1 had BNT162b2. In the 18 WM cohort patients, 16 had mRNA‐1273 and 2 had BNT162b2.
2.4. Lymphocyte profiling and antigen‐specific CD4 + and CD8 + T cell responses
Cryopreserved PBMCs were thawed using RPMI (+l‐glut) medium (Thermo Fisher Scientific) supplemented with penicillin/streptomycin (Sigma‐Aldrich), and subsequently stained with antibodies binding to extracellular markers. Ex vivo phenotyping of PBMCs were performed as described previously. 18 The extracellular panel included Live/Dead dye Near InfraRed, CD3 (UCHT1), CD8 (HIL‐72021), CD19 (HIB19) (Biolegend); CD4 (OKT4) (BD Biosciences). Functional T cell assays and FACS staining of 48 hr activated PBMCs were performed as described previously, 18 but with the addition of CD137 (4B4‐1) (Biolegend) to the cultures at 24 h. Final concentration of 10 μg/ml of SARS‐CoV‐2 spike peptide pool (33 15‐mer peptides previously described 19 , 20 ) (Genscript) were added to appropriate wells. 1 μg/ml of 2020 season Influvac Tetra containing virus fragments from four influenza strains; A/Victoria/2570/2019 (H1N1)pdm09‐like strain, A/Hong Kong/2671/2019 (H3N2)‐like strain, B/Washington/02/2019‐like (B/ Victoria lineage) virus, B/Phuket/3073/2013‐like (B/Yamagata lineage) virus (Influenza vaccine‐Mylan Health) were used as a control antigen and SEB (5 μg/ml) was used as a positive control (Thermo Fisher Scientific). The flow cytometry panel included CD3 (UCHT1), CD4 (RPA‐T4), CD8 (RPA‐T8), CD69 (FN50; all Biolegend), CD25 (2A3), CD134 (L106)—BD Biosciences. Samples were acquired on a Cytek Aurora (Biolegend) using the Spectroflo software. Prior to each run, all samples were fixed in 0.5% paraformaldehyde. Data analysis was performed using FlowJo version 10.7.1 (TreeStar).
2.5. Statistics
Differences between groups were determined by the nonparametric Mann–Whitney U test and differences for nonparametric paired data was assessed using the Wilcoxon test. Correlations were determined using simple linear regression. All were calculated using Prism software (GraphPad). Multivariate analyses were performed using R. Bonferroni corrections were used to correct for multiple comparisons.
3. RESULTS
3.1. Participants
Eighty‐five participants received their first dose of BNT162b2 from May to June 2021. These included 35 patients with FL, 37 patients with WM, and 13 HC. One FL patient died from an unrelated cerebrovascular accident before the second vaccine dose. All remaining participants received both vaccines and sampling according to schedule with excellent vaccination time‐point and blood collection adherence at T2: median 21 days (range 20–22 days) and T3: median 49 days (range 47–51 days), following baseline vaccination and baseline bloods at T1. Patient characteristics of all evaluable patients are shown in Table 1. In the FL cohort, 11 were TN, 23 had immunochemotherapy (ICT) with 2 of these continuing on BTKi. In the WM cohort, 9 were TN, 14 had ICT alone, 12 were on BTKi (having prior treatment with ICT), 2 were on venetoclax (having prior treatment with ICT and BTKi). There was no significant difference in gender or age compared to the HC: 8/13 (61.5%) were female, with a median age of 72 (interquartile range [IQR]: 57–74) years. No participant had detectable anti‐spike IgG at T1, confirming no prior SARS‐CoV‐2 exposure. This included one FL patient and seven WM patients who were receiving immunoglobulin replacement therapy. As of January 1, 2022, no participant had contracted COVID‐19.
TABLE 1.
Characteristics of patients
| FL n = 34 | WM n = 37 | HC n = 13 | |
|---|---|---|---|
| Age in years, median (IQR) | 65 (54–71) | 71 (63–74) | 72 (57–74) |
| Gender, male n (%) | 16 (47) | 19 (51.3) | 5 (38.5) |
| Treatment status | |||
| Treatment naïve, n (%) | 11 (32.3) | 9 (24.3) | |
| Anti‐CD20 monoclonal + other, n (%) | 23 (65.7) | 14 (37.8) | |
| Rituximab chemotherapy ± maint., n (%) | 19 (55.9) | 11 (29.7) | |
| Obinutuzumab chemotherapy ± maint., n (%) | 4 (11.8) | – | |
| Completed treatment with anti‐CD20 <6 months, n (%) | 10 (29.4) | 0 (0) | |
| Median time from therapy, months (IQR) | 15.5 (7.5–30.5) | 22 (12–39) | |
| Bruton tyrosine kinase inhibitors, n (%) | 2 (6) | 12 (32.4) | |
| Median time on BTKi, months (IQR) | 56.5 (20–93) | 64.5 (46–75) | |
| Other—Venetoclax, n (%) | – | 2 (5) | |
| Median previous lines, n (IQR) | 1 (1–2) | 2 (1–4) | |
| Median baseline IgG, g/L (IQR) | – | 8.11 (3.93–18.5) | |
| IVIg supplementation, n (%) | 1 (3) | 7 (18.9) | |
| Lymphocyte count, n ×109/L (IQR) | 1.4 (0.78–1.73) | 1.4 (0.93–1.7) |
Abbreviations: BTKi, Bruton's tyrosine kinase inhibitor; FL, follicular lymphoma; HC healthy control; IgG, immunoglobulin G; IQR, interquartile range; WM, Waldenström's macroglobulinemia.
3.2. Anti‐spike IgG levels in patients with FL
Postvaccination anti‐spike IgG response in HC and FL treatment cohorts to T2 are shown in Figure S2. At T3 (Figure 1A), 1 month after two vaccine doses, the median MFI of HCs was 228 255 (IQR: 162 383–249 019), the same as the FL TN cohort: 245 898 (IQR: 180876–251 062; p = .32); and 23‐fold higher than ICT: 9977 (IQR: 0–131 350; p = .002). The median MFI of the TN cohort was also higher than the ICT cohort (p = .001) (see above). Anti‐spike IgG in FL ICT‐treated patients correlated on univariate analysis with lymphocyte count (r = .63; p = .002; Figure S3A) and with time from treatment (r = .56; p = .007; Figure S3B). On multivariate analysis, only lymphocyte count correlated as an independent predictor of anti‐spike IgG MFI (p = .03), with time from treatment being insignificant (p = .16). For patients with an anti‐spike IgG response, the median time from treatment was 26 months (IQR: 13.5–47.5 months). For patients with no anti‐spike IgG response the median time from treatment was 6 months (IQR: 4–14 months).
FIGURE 1.
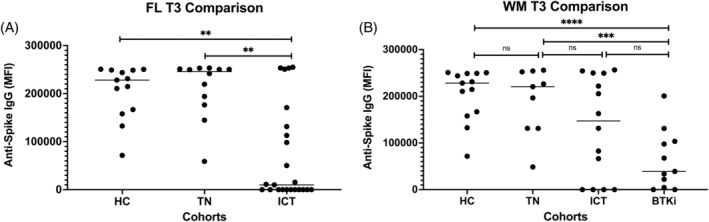
Comparison of anti‐spike IgG response between healthy controls, FL and WM treatment cohorts at T3. In FL (A), there is no difference in response comparing HC with TN, but there is a significantly reduced response in the ICT cohort compared to HC and TN. In WM (B), there is no difference in response comparing HC with TN or ICT, but there is a significantly reduced response BTKi with both HC and TN. A p < .03 was considered statistically significant in A and <.01 in B after Bonferroni correction. BTKi, BTKi‐treated cohort; HC, healthy controls; ICT, immunochemotherapy‐treated cohort; IgG, immunoglobulin G; MFI, delta mean fluorescence intensity; TN, treatment naïve cohort. **p < .01; ***p < .001; ****p < .0001
3.3. Anti‐spike IgG levels in patients with WM
Postvaccination anti‐spike IgG response in HCs and WM cohorts to T2 are shown in Figure S4. At T3 (Figure 1B), the median MFI of HCs was 228 255 (IQR: 162 383–249 019), which was not significantly different from TN: 220 645 (IQR: 131 374–253 237), nor ICT: 147 197 (IQR: 0–249 655); but 6‐fold higher than in those on BTKi therapy: 39 093 (IQR: 4486–103 484; p < .0001). The difference in median MFI between TN‐ and BTKi‐treated patients was also highly significant (p = .0008; values above). There was no significant difference between the ICT and BTKi cohorts (p = .07, values above). No significant relationship was seen on univariate analysis in WM patients in the ICT cohort and their lymphocyte count (r = .51; p = .16) or time from treatment (r = .13; p = .7). Median time from ICT for this cohort was 22 months (IQR: 12–39 months). None of these patients were exposed to BTKi or venetoclax.
3.4. Live virus neutralization after two dose vaccination
Median neutralizing activity of HC to the delta variant: 40 (IQR: 20–80) was equivalent to FL TN: 40 (IQR: 20–80; p = .47) and higher than the median for FL ICT patients: 0 (IQR: 0–70) (p = .02; Figure 2A). There was good correlation between anti‐spike IgG levels and live virus neutralization titers for the delta variant (r = .62, p < .0001; Figure 2C). Median neutralization for HC was comparable to the WM TN 40 (IQR: 10–40; p = .08) and for ICT: 20 (IQR: 0–120; p = .12) but was significantly higher than BTKi: 0 (IQR: 0–0; p < .0001). A full comparison of neutralizing activity for both early‐clade (A2.2) and delta for both VeroE6 and HEK cell lines at T3 are shown in Figure S5 and Table S2. There was comparable neutralization for both cell lines within cohorts, with a nonsignificant trend toward reduced neutralization for the delta variant (Table S2). There was an absence of neutralization in patients with low level anti‐spike IgG. Specifically, the live virus neutralizing activity of the delta variant in the HEK cell line at T3 is shown in Figure 2.
FIGURE 2.
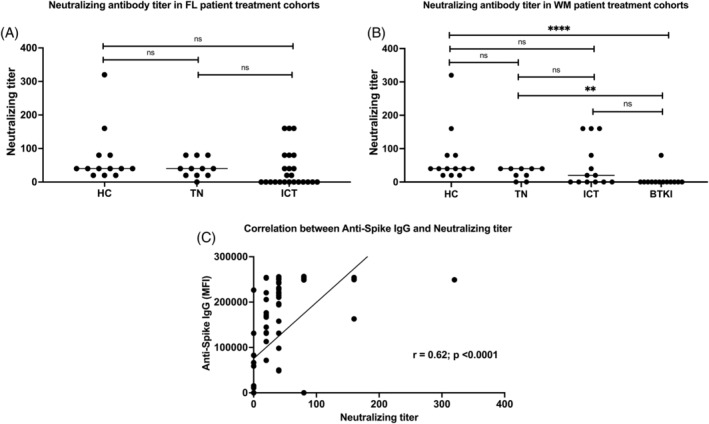
Live SARS‐CoV‐2 neutralization of delta variant in HEK cell line at T3. There is a slight reduction in neutralization of delta variant in FL patients treated with ICT (A) compared to HC. There is significantly lower neutralization of delta variant in WM patients treated with BTKi (B) compared to both HC and TN. Neutralization activity correlates well with anti‐spike IgG (C). A p < 0.03 was considered statistically significant in A and <.01 in B after Bonferroni correction. BTKi, BTKi‐treated cohort; HC, healthy controls; ICT, immunochemotherapy‐treated cohort; IgG, immunoglobulin G; MFI, delta mean fluorescence intensity; TN, treatment naïve cohort. **p < 0.01; ***p < 0.001; ****p < .0001.
3.5. Lymphocyte profiling and antigen‐specific CD4 + and CD8 + T cells
Relative proportions of CD19+ B cells and CD4+ and CD8+ T cells across groups are shown in Table S3. In the FL group, the TN cohort had a reduced proportion of CD8+ T cells compared to HC (at 5.01% vs. 22.7%; p = .004). There was no difference in the proportion of SARS‐CoV‐2 spike specific CD4+ CD25+ CD134+ (OX40) and CD8+ CD69+ CD137+ T cells between groups, as compared to HC (see Figure S6 for an example of flow cytometry plots demonstrating SARS‐CoV‐2 spike specific CD4+ and CD8+ reactive cell events).
The median percentage of B cells of live lymphocytes in WM patients treated with BTKi was 0.11% (IQR: 0.055–1.31), lower than HC 3.08% (IQR: 2.37–5.90; p = .0003). There were no other significant differences between HC and other lymphoma cohorts. WM patients on BTKi also had a reduced proportion of CD4+ T cells with median 46.9% (IQR: 45.8–57.7) compared to HC, median 69.9% (IQR: 56.4–81.6; p = .006), with no significant difference between other lymphoma cohorts. WM patients on BTKi had an elevated proportion of CD8+ T cells with median 45.4% (IQR: 31.9–47.5) compared to HC median of 22.7% (IQR: 10.9–36; p < .01). WM patients treated with ICT also had reduced CD8+ T cells as compared to HC (median 6.79% compared to 22.7%; p = .01).
Data on reactivity to influenza antigens is presented for comparison. There was no difference in the proportion of antigen‐specific CD4+ CD25+ CD134+ (OX40) and CD8+ CD69+ CD137+ T cells between groups, as compared to HC (see Table S4).
3.6. Attrition of anti‐spike IgG occurred between second and third vaccine doses
In FL patients who had seroconverted 28 days post the second BNT162b2 dose and in whom there was pre‐third dose anti‐spike IgG measured (n = 9), the anti‐spike IgG had dropped 4‐fold from a median MFI 82 663 (IQR: 11 061–131 245) to a median MFI 19 942 (IQR: 5802–19 567; p = .004; Figure 3A). The median time between second and third doses was 4 months.
FIGURE 3.
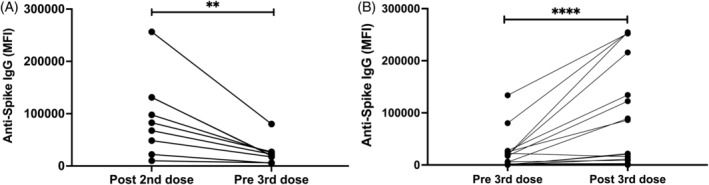
Changes in anti‐spike IgG. (A) There is a significant reduction in anti‐spike IgG following the second dose of BNT162b2 after a median of 4 months, prior to a third mRNA vaccine dose (n = 9). (B) Four weeks following a third mRNA vaccine dose, there was a significant increase in anti‐spike IgG in those who had previously responded and seroconversion in some patients who had previously not responded (n = 29) including FL ICT, WM TN, WM ICT, WM BTKi. BTKi, BTKi‐treated cohort; ICT, immunochemotherapy‐treated cohort; IgG, immunoglobulin G; MFI, delta mean fluorescence intensity; TN, treatment naïve cohort. **p < 0.01; ***p < 0.001; ****p < 0.0001.
3.7. Third primary mRNA vaccine dose resulted in seroconversion in some poor initial responders
Four HC and 29 lymphoma participants had sera collected before and after a third dose mRNA vaccination. The lymphoma cohort comprised 9 FL ICT, 1 WM TN, 6 WM ICT, 10 WM BTKi, 1 WM venetoclax. In composite data of all FL and WM patients (n = 29; Figure S7) there was a 7.5‐fold increase in anti‐spike IgG: median MFI pre‐third dose of 774 (IQR: 246–18 302) compared to median MFI 28 days post‐third dose of 5789 (IQR: 620–87 720; p < .0001; Figure 3B).
In the FL cohort, 2 of 11 (18.2%) had anti‐spike IgG detected prior to, and 4 of 11 (36.4%) had anti‐spike IgG detected 28 days post‐third dose vaccination. In the WM patients, 8 of 18 (44.4%) had detectable anti‐spike IgG before and 11 of 18 (61.1%) had anti‐Spike IgG detected 28 days post‐third dose vaccination.
At Day 28 (Figure S7) following the third vaccine dose, all four HC had neutralization activity to early‐clade, delta and omicron variants, though with a 4–8‐fold reduction in activity against omicron compared to early‐clade and the delta variants.
In the FL cohort, of the eight patients already seropositive, only two had neutralizing activity detected before the third vaccination dose. All eight patients had neutralizing activity detected 28 days following the third dose. The two with neutralizing activity prior to third dose had 2–4‐fold increases in activity titer post‐third dose and both developed omicron neutralization which had not been present prior. All eight patients neutralized early‐clade and the delta variant, and five of eight had activity against omicron.
In the WM cohort, of the two who were already seropositive, one had no detectable neutralizing activity before or after third dose to early‐clade, delta or omicron variants. The other had neutralizing activity which increased 16‐fold for early‐clade and delta variants and 8‐fold for the omicron variant. Of the two additional seroconverters, one had low titer (1:20) neutralizing activity only to the early‐clade variant.
Nine of eighteen WM patients assessed were on BTKi therapy. Two of this cohort seroconverted with the third dose and had low level neutralizing activity. An additional patient who was seropositive before and after the third vaccination dose, had neutralization activity absent before which appeared at a low level following the third dose. All three WM patients on BTKi that seroconverted had withheld their BTKi therapy, of their own volition, immediately prior to the third dose and for 2–4 weeks afterwards.
3.8. WM patients treated with venetoclax had poor humoral response to vaccination
The anti‐spike IgG response and neutralization titer were poor in two WM patients treated with venetoclax, one of whom also had a third vaccine dose. Given the small number of two in this cohort, the data has not been included in the above comparisons, but is shown for completeness in Table S5.
4. DISCUSSION
As the COVID‐19 pandemic continues to evolve, it is imperative to understand which subgroups of the vaccinated population remain at higher risk of contracting the virus and of having severe COVID‐19 disease. Patients with indolent lymphomas comprise a sizable proportion of people with hematological malignancies, and are vulnerable to severe disease. Here we prospectively studied both humoral and cellular immune responses post‐BNT162b2 vaccination in people with FL and WM on a variety of treatment regimens. The longitudinal data generated from this prospective study may help inform future risk assessments, management strategies and the timing of ICT and BTKi therapy with first to third primary and now fourth‐ and subsequent‐dose booster vaccines emerging.
Our results are reassuring in that TN patients with both lymphoma subtypes had similar humoral responses as healthy age‐matched controls, highlighting that in this setting it is the therapies, rather than the disease process, that are detrimental to seroconversion. These results indicate the merit of completing at least two doses of vaccination during the period of initial observation after diagnosis and pretreatment.
The reduced humoral response in 47.8% of patients who have received ICT for FL correlated on univariate analysis with both absolute lymphocyte count and time from treatment, with lymphocyte count remaining significant as a predictive for poor outcome after multivariate analysis in this cohort. This is consistent with other published data in the literature. 21 , 22 Comparable findings were not seen in the WM ICT group with only 28.6% failing to seroconvert, but a limitation was that this cohort does not include patients recently (<12 months) treated with ICT. Therefore, this finding must be interpreted with caution. Of particular concern, were WM patients treated with continuous BTKi therapy, who had approximately 6‐fold lower anti‐spike IgG than the TN cohort following the second vaccine dose and a virtual absence of neutralization activity, consistent with previous reports. 9 , 23
Anti‐spike IgG levels positively correlated to live virus neutralization activity across all groups. Some subjects were observed to have a low level measurable anti‐spike IgG response without neutralization activity, suggesting caution in the interpretation of low‐titer anti‐spike IgG alone in assessing a patient's response to vaccination. Nonetheless, our limited third dose data suggest this initial low‐titer antibody may be enhanced with subsequent vaccination, and the passage of time after ICT. There remains a need to identify a clinically relevant optimal anti‐spike IgG level that consistently predicts neutralization activity. This is challenging given the continuum of serology assays being used, nonstandardization and the high demand for serology standards that have finite supply. However, this will continue to be reassessed with the emergence of new variants of concern where resolution of variant specific spike antibody levels will need to be assessed alongside variant equivalent neutralization assays.
To date, a subgroup of 29 patients, skewed in representation of poor initial responders, were assessed following a third primary dose of an mRNA vaccine (mostly mRNA‐1273), and 5 of 29 (17%) of these patients demonstrated seroconversion. All those who had previously responded had an increase in measurable anti‐spike IgG and neutralization activity. There was a trend for higher neutralization titers for early‐clade and the delta variant compared to lower or absent omicron neutralization, consistent with other emerging data. 17 , 24
Our data, together with previously published reports, 25 suggest that many people with indolent lymphomas will fail to adequately seroconvert following three doses of an mRNA COVID‐19 vaccine, and that viral neutralization may remain compromised. Encouragingly, despite poor humoral response in the ICT‐ and BTKi‐treated populations, our patients had measurable antigen‐specific CD4+ and CD8+ T cells responses of a magnitude comparable to HC. How these functional T cell responses relate to protection from SARS‐CoV‐2 infection requires further characterization. Having a greater number of CD8+ T cells at the time of SARS‐CoV‐2 infection has been found to positivity influence recovery from COVID‐19 in immunosuppressed patients, including those treated with anti‐CD20 monoclonal antibodies. 26 How this relates to vaccine‐induced CD4+ and CD8+ T cells and the potential benefit they confer following infection is still being assessed, but raises the possibility that even in patients who fail to seroconvert there may be benefit from vaccination.
In the next phase of the pandemic, patients with poor immune responses need prioritization for targeted education, shielding, and streamlined medical review in the event of SARS‐CoV‐2 infection. In addition to our planned prospective assessment of a closely monitored treatment interruption to BTKi therapy, our extended cohort of high‐risk patients would be ideal candidates for further study of the long‐acting antibodies tixagevimab/cilgavimab, (AZD7442). 27 This is pertinent given only 196 (3.8%) of the participants in the Phase 3 PRoVENT study were on concurrent immunosuppressive therapy. Additionally, it will be important to assess the tolerance, longevity and neutralization capacity of tixagevimab/cilgavimab in such populations. Both in vitro 17 and in vivo 28 observations of tixagevimab and cilgavimab showed limited activity against the omicron BA1 lineage. Fortunately, activity for tixagevimab/cilgavimab is retained in vivo for omicron BA2 and this latter omicron lineage is supplanting BA1 globally. If repeated vaccination cannot stimulate a native humoral vaccine response in such patients, further developments in either passive antibody therapy and/or broader antivirals may be key to ensuring increased safety of this vulnerable population as the world pivots to living with, rather than eliminating, SARS‐CoV‐2. Future therapeutics will always need to be assessed against both each emerging variant of concern and also their ability to not promote the rapid emergence of resistant variants. 29
In summary, TN FL and WM patients have comparable COVID‐19 vaccine‐induced humoral immunity to HCs. Likewise, most patients distant from ICT have a good humoral response to mRNA vaccination. Patients on BTKi have reduced humoral immunity with markedly low anti‐spike IgG and virtual absence of neutralizing activity to the delta and omicron variants. A small subgroup of patients who received a third dose of mRNA vaccine showed additional seroconversion, with increased IgG titer and neutralization activity in those who had previously responded, though there was a trend toward lower neutralization of the omicron variant. Reassuringly, all patients demonstrate functional CD4+ and CD8+ reactive T cells following two doses of BNT162b2. Despite the impaired humoral response after ICT and while on chronic B cell suppression, this SARS CoV‐2‐specific T cell response may indicate the value of vaccination for all.
Further work needs to be done to more comprehensively investigate the response to third and subsequent vaccinations in larger cohorts and to monitor immunocompromised populations for the emergence of vaccine‐evasive, highly transmissible mutants of SARS‐CoV‐2.
AUTHOR CONTRIBUTIONS
Brendan Beaton, Sarah C. Sasson, Anthony D. Kelleher, and Judith Trotman designed the research. Brendan Beaton, Sarah C. Sasson, Katherine Rankin, Juliette Raedemaeker, Judith Trotman, and Priyanka Hastak wrote the primary manuscript. Brendan Beaton, Katherine Rankin, Juliette Raedemaeker, Alexander Wong, Andrew Warden, Judith Trotman, and Orly Lavee were involved with recruitment of patients and arranging blood collection and processing as well as study logistics. Vera Klemm, C. Mee Ling Munier, Alexandra Carey Hoppe, Fiona Tea, Aleha Pillay, Priyanka Hastak, Alberto O. Stella, Anupriya Aggarwal, Chansavath Phetsouphanh, Stuart Turville, Anthony D. Kelleher, and Fabienne Brilot designed and performed experiments and acquired data, with initial analysis. Brendan Beaton, Sarah C. Sasson, Priyanka Hastak, Katherine Rankin, Juliette Raedemaeker, and Judith Trotman created figures and correlated data with patient characteristics. Brendan Beaton, Sarah C. Sasson, Stuart Turville, Fabienne Brilot, Anthony D. Kelleher, and Judith Trotman supervised the coordination of different clinical and laboratory aspects of the study. Ian D. Caterson assisted with coordinating vaccine availability. All authors reviewed and approved the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The authors acknowledge Mr Serg Duchini and Ms Erica Smeaton, Lymphoma Australia; Mr Peter Smallwood, WMozzies for recruitment support; Diane Criminale and the blood collection service at Concord Hospital, NSW Health Pathology, for sample collection; and Kate Merlin and the clinical trials processing team, St Vincent's Centre for Applied Medical Research Biorepository for sample processing and cryopreservation. Vennila Mathivanan, the Kirby Institute, wrote the supplementary method of spike synthesis. Deborah Cromer and Miles P. Davenport, the Kirby Institute, performed multivariate analysis for a subset of patients. The authors would also like to acknowledge and thank our funders Concord Hospital Hematology Clinical Research Unit, Foundation for a Bloody Great Cause, International Waldenström's Macroglobulinemia Foundation, Lymphoma Australia. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Beaton B, Sasson SC, Rankin K, et al. Patients with treated indolent lymphomas immunized with BNT162b2 have reduced anti‐spike neutralizing IgG to SARS‐CoV‐2 variants, but preserved antigen‐specific T cell responses. Am J Hematol. 2022;1‐9. doi: 10.1002/ajh.26619
Funding information Concord Haematology Clinical Research Unit; Foundation for a Bloody Great Cause; International Waldenstrom's Macroglobulinemia Foundation; Lymphoma Australia
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136(25):2881‐2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Doesum J, Chinea A, Pagliaro M, et al. Clinical characteristics and outcome of SARS‐CoV‐2‐infected patients with hematological diseases: a retrospective case study in four hospitals in Italy, Spain and the Netherlands. Leukemia. 2020;34:2536‐2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casulo C, Barr P. How I treat early‐relapsing follicular lymphoma. Blood. 2019;133(14):1540‐1547. [DOI] [PubMed] [Google Scholar]
- 4. Dimopoulos M, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström's macroglobulinemia. N Engl J Med. 2018;378:2399‐2410. [DOI] [PubMed] [Google Scholar]
- 5. Besson C. It is time to adapt anti‐CD20 administration schedule to allow efficient anti‐SARS‐CoV‐2 vaccination in patients with lymphoid malignancies. Haematologica. 2022;107(3):572‐573. doi: 10.3324/haematol.2021.279457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017;102(10):e397‐e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazza JJ, Yale SH, Arrowood JR, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3(4):214‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maneikis K, Šablauskas K, Ringeleviciute U, et al. Immunogenicity of the BNT162b2 COVID‐19 mRNA vaccine and early clinical outcomes in patients with hematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583‐e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165‐3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen‐specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS‐CoV‐2 mRNA vaccination. Immunity. 2021;54(9):2133‐2142.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrington P, de Lavallade H, Doores KJ, et al. Single dose of BNT162b2 mRNA vaccine against SARS‐CoV‐2 induces high frequency of neutralising antibody and polyfunctional T‐cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;35:3573‐3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marasco V, Carniti C, Guidetti A, et al. T‐cell immune response after mRNA SARS‐CoV‐2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2022;196:548‐558. doi: 10.1111/bjh.17877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tea F, Stella AO, Aggarwal A, et al. SARS‐CoV‐2 neutralizing antibodies: longevity, breadth, and evasion by emerging viral variants. PLoS Med. 2021;18(7):e1003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tea F, Lopez JA, Ramanathan S, et al. Characterization of the human myelin oligodendrocytes glycoprotein antibody response in demyelination. Acta Neuropathol Commun. 2019;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27:1205‐1211. [DOI] [PubMed] [Google Scholar]
- 16. Mattiuzzo G, Bentley E M, Hassall M, et al. Establishment of the WHO international standard and reference panel for anti‐SARS‐CoV‐2 antibody. WHO Expert Committee on Biological Standardization. 2020.
- 17. Aggarwal A, Stella A, Walker G, et al. Platform for isolation and characterization of SARS‐CoV‐2 variants enables rapid characerization of Omicron in Australia. Nat Microbiol. 2022;7(6):896‐908. doi:10/1038/s41564-022-01135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mateus J, Grifoni A, Tarke A, et al. Selective and cross‐reactive SARS‐CoV‐2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pent Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells incuded by SARS‐CoV‐2 in UKconvalescent individuals following COVID‐19. Nat Immunol. 2020;21:1336‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaunders JJ, Munier ML, Seddiki N, et al. High levels of human antigen‐specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J Immunol. 2009;183(4):2827‐2836. [DOI] [PubMed] [Google Scholar]
- 21. Jurgens EM, Ketas TJ, Zhao Z, et al. Serologic response to mRNA COVID‐19 vaccination in lymphoma patients. Am J Hematol. 2021;96(11):E410‐E413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with B‐cell non‐Hodgkin lymphoma. Blood Adv. 2021;5(16):3053‐3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid‐19 vaccination in patients with chronic lymphocytic leukemia. Blood Cancer J. 2021;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS‐CoV‐2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11:337‐343. doi: 10.1080/22221751.2021.2022440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herishanu Y, Rahav G, Levi S, et al. Efficacy of a third BNT162b2 mRNA COVID‐19 vaccine dose in patients with CLL who failed standard two‐dose vaccination. Blood. 2022;139:678‐685. doi: 10.1182/blood.2021014085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in COVID‐19 patients with hematologic cancer. Nat Med. 2021;27(7):1280‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loo Y, McTamney PM, Arends RH, et al. AZD7442 demonstrates prophylactic and therapeutic efficacy in non‐human primates and extended half life in humans. 2021. doi: 10.1101/2021.08.30.21262666 [DOI]
- 28. Benotmane I, Velay A, Thaunat O, et al. Pre‐exposure prophylaxis with Evusheld elicits limited neutralizing activity against the omicron variant in kidney transplant patients. 2022. doi: 10.1101/2022.03.21.22272669 [DOI] [PMC free article] [PubMed]
- 29. Bruel T, Hadjadj J, Maes P, et al. Serum neutralization of SARS‐CoV‐2 omicron sublineages BA.1 and BA.2 in patients receiving monoclongal antibodies. Nat Med. Published online March 23, 2022. doi:10/1016/j.kint.2022.05.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


