To the Editor,
COVID‐19 vaccine reactions may lead to unnecessary avoidance of future doses. Evidence‐based information about vaccine adverse effects decreases vaccine hesitancy. 1 Urticaria can occur in 2% of individuals with each mRNA COVID‐19 vaccine dose. 2 This study describes urticaria and angioedema (U/AE) events occurring after COVID‐19 vaccinations.
Vaccine‐related reactions were abstracted from the COVID‐19 Vaccine Allergy Case Registry (https://allergyresearch.massgeneral.org/), a US‐based registry open for voluntary reporting of vaccine allergy cases. U/AE events attributed to COVID‐19 vaccines entered by clinicians between February 13, 2021, to February 8, 2022, were reviewed. Data were summarized using descriptive statistics and univariable tests were used to compare variables. Data were analyzed in SAS (v9.4). The registry was approved by the Mass General Brigham Human Research Committee with a waiver of informed consent.
There were 481 reactions from 46 US states entered by clinicians (64% Moderna, 27% Pfizer‐BioNTech, and 9% Other/unknown); 60 (12%) cases were U/AE events (mean age: 48 years; range: 9–85 years; SD ± 19.0 years; Table 1, Figure 1). The majority of cases were female (n = 50, 83%). Race was White (n = 47, 78%), Asian (n = 5, 8%), Black (n = 3, 5%), Other (n = 2, 4%), and Native Hawaiian/Pacific Islander (n = 1, 2%); two (3%) had Hispanic/Latino ethnicity. Seven (12%) patients reported a history of chronic U/AE, and 24 (40%) had atopic comorbidities (allergic rhinitis, asthma, and/or atopic dermatitis).
TABLE 1.
Demographics, clinical presentation, and resolution of urticaria and/or angioedema (U/AE) events related to the COVID‐19 vaccines. Data shown as n (%) unless specified
| All (n = 60) | Moderna (n = 29) | Pfizer‐ BioNTech (n = 31) | |
|---|---|---|---|
| Mean Age (Range ± SD)* | 48y (9–85 y ± 19.0) | 55 y (24–85 y ± 18.0) | 42 y (9–72 y ± 17.6) |
| Female | 50 (83) | 25 (86) | 25 (81) |
| Race | |||
| White | 47 (78) | 23 (79) | 24 (77) |
| Asian | 5 (8) | 2 (7) | 3 (10) |
| Black | 3 (5) | 0 (0) | 3 (10) |
| Native Hawaiian/Pacific | 1 (2) | 1 (3) | 0 |
| Islander | |||
| Other/Missing | 4 (7) | 3 (10) | 1 (3) |
| Ethnicity | |||
| Hispanic/Latino | 2 (3) | 1 (3) | 1 (3) |
| Past Medical History | |||
| Atopic comorbidities a | 24 (40) | 13 (45) | 11 (35) |
| History of U/AE | 7 (12) | 4 (14) | 3 (10) |
| Vaccine Dose | |||
| Dose 1 | 46 (77) | 20 (69) | 26 (84) |
| Dose 2 | 14 (23) | 9 (31) | 5 (16) |
| Latency* | |||
| Immediate (<4 h) | 19 (32) | 6 (21) | 13 (42) |
| Non‐immediate (4–24 h) | 13 (22) | 3 (10) | 10 (32) |
| Delayed (>24 h) | 28 (47) | 20 (69) | 8 (26) |
| Resolution Time of Reaction b | |||
| <6 h | 11 (19) | 3 (10) | 8 (26) |
| 6–24 h | 9 (15) | 4 (13) | 5 (16) |
| 1–2 d | 4 (7) | 1 (3) | 3 (10) |
| 2–4 d | 10 (17) | 3 (10) | 7 (23) |
| 5–7 d | 5 (8) | 3 (10) | 2 (6) |
| >1 week* | 11 (18) | 9 (31) | 2 (6) |
| Treatment(s) c | |||
| H1‐Antihistamines | 42 (70) | 21 (72) | 21 (68) |
| H2‐Antihistamines | 8 (13) | 5 (17) | 3 (10) |
| Corticosteroids (oral) | 16 (27) | 7 (24) | 9 (29) |
| Corticosteroids (topical) | 5 (8) | 5 (17) | 0 (0) |
| Corticosteroids (intravenous) | 2 (3) | 1 (3) | 1 (3) |
| Epinephrine (intramuscular) d | 3 (5) | 0 (0) | 3 (10) |
| No treatment | 13 (24) | 7 (24) | 6 (19) |
| Treatment Location e ,* | |||
| Home | 18 (30) | 11 (38) | 7 (23) |
| Hospital Admission d | 2 (3) | 0 (0) | 2 (6) |
| Emergency Department | 11 (18) | 3 (10) | 8 (26) |
| Urgent Care | 5 (8) | 4 (14) | 1 (3) |
| Ambulatory Clinic | 9 (15) | 5 (17) | 4 (13) |
| Retail/Vaccine Clinic | 3 (5) | 0 (0) | 3 (10) |
Note: *Univariable statistical testing between Moderna and Pfizer‐BioNTech significant, p < .05.
Abbreviations: U/AE, urticaria and/or angioedema.
Includes asthma, atopic dermatitis, or allergic rhinitis.
10 cases had unknown resolution time.
19 cases had multiple treatments and 6 cases had missing treatment.
There were four cases who had either epinephrine administered or hospitalization: Case 1) A white non‐Hispanic female patient receiving her first Pfizer‐BioNTech vaccine developed urticaria as well as throat closing sensation, dyspnea, and nausea >24 h after vaccination. She presented to the emergency department, received intramuscular epinephrine, antihistamines, and corticosteroids. She required hospitalizations and symptom resolution required 5–7 d. A baseline tryptase was 4.4 ng/mL, and acute tryptase was 5.3 ng/mL. Case 2) A white, Hispanic female patient receiving her first Pfizer‐BioNTech vaccine developed urticaria, angioedema, and headache starting 4–24 h after vaccination. She was treated in the emergency department with intramuscular epinephrine, antihistamines, and corticosteroids with resolution within 1 h. Case 3) A Black, non‐Hispanic male was in the vaccine clinic being observed after his first Pfizer‐BioNTech vaccine when he developed lip swelling, urticaria, and persistent throat clearing. He was treated with intramuscular epinephrine with resolution within 1 h. Case 4) A White, non‐Hispanic female patient receiving her first Pfizer‐BioNTech vaccine developed urticaria with hoarseness and difficulty breathing (without stridor or wheeze) from 4 ‐24 h after vaccination. She was treated in the emergency department and required hospitalization.
Highest acuity location shown. One case had missing treatment location.
FIGURE 1.
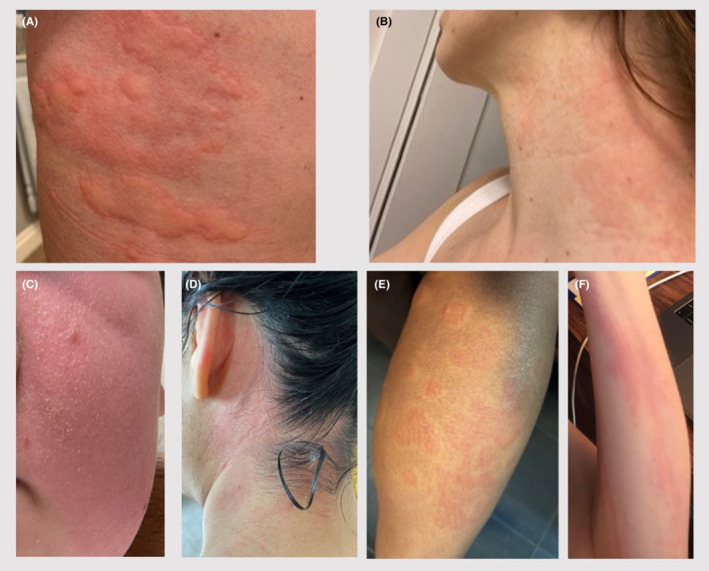
Cases of Urticaria and/or Angioedema after mRNA COVID‐19 Vaccination. Photographs of urticaria and angioedema events. (A) Generalized urticaria after Pfizer‐BioNTech COVID‐19 vaccine dose 1. (B) Erythema and generalized urticaria after Pfizer‐BioNTech vaccine dose 1. (C) Scattered urticaria and facial angioedema after Pfizer‐BioNTech vaccine dose 2. (D) Generalized urticaria after Moderna vaccine dose 2. (E) Generalized urticaria after Moderna vaccine dose 1. (F) Generalized urticaria and dermatographism after Moderna vaccine dose 2
All U/AE events occurred after an mRNA COVID‐19 vaccine (n = 29 [48%] Moderna; n = 31 [52%] Pfizer‐BioNTech), and most with the first dose (n = 46, 77%). The onset time for U/AE was variable, but just 19 (32%) cases were immediate (<4 h; 15 cases [25%] ≤1 h) following vaccination. U/AE onset was also between 4 and 24 h (n = 13, 22%) and delayed (>24 h) following vaccination (n = 28, 47%). Onset of U/AE was more often immediate with Pfizer‐BioNTech compared to Moderna (41% vs 21%; p = .08), and U/AE was more often delayed with Moderna compared to Pfizer‐BioNTech (69% vs 26%; p < .001). Resolution times were diverse, but Moderna U/AE more commonly lasted >1 week than Pfizer‐BioNTech U/AE (31% vs 6%; p = 0.014).
Treatments included antihistamines and corticosteroids. Three (5%) cases, all after Pfizer‐BioNTech, were treated with intramuscular epinephrine. Treatment was not required for 13 (24%) cases. U/AE events were treated at home (n = 18, 30%), the emergency department (n = 11, 18%), and ambulatory care clinics (n = 9, 15%); two (3%) cases required hospitalization, both after Pfizer‐BioNTech vaccine. There were no intensive care unit admissions or deaths. Of 27 (45%) patients skin tested to vaccine and/or excipients, all tests were negative.
Of 481 patients with COVID‐19 vaccine reactions in this registry, 12% experienced U/AE events following an mRNA COVID‐19 vaccine. Although >30 million doses of Janssen COVID‐19 vaccine have been administered in the United States, most US vaccinations (>95%) have been with mRNA vaccines, and no U/AE events were reported after Janssen vaccine. 3 Most U/AE events occurred following the first COVID‐19 vaccine dose (77%) with events occurring comparably after Pfizer‐BioNTech and Moderna vaccines. Prior studies suggest that some cutaneous reactions may be more commonly observed with the Moderna compared to Pfizer‐BioNTech. 4 , 5 In this study, U/AE events after Pfizer‐BioNTech appeared to be associated with an earlier onset, higher severity, and quicker resolution than U/AE events secondary to Moderna.
Similar to other studies, U/AE events following COVID‐19 vaccines were predominantly observed in females (83%) and in individuals of White race (78%). 2 , 3 , 4 , 5 , 6 While a biologic underpinning may explain U/AE events in females, racial differences will require additional study, as racial differences in COVID‐19 vaccination uptake, healthcare access/use, and/or skin reaction recognition/reporting may contribute to these observed differences. U/AE after vaccination may not be the result of an immunoglobulin (Ig) E‐mediated allergy or other hypersensitivity reaction to the vaccine or its excipients. Alternative explanations include non‐IgE‐mediated mechanisms and vaccine reactogenicity, the physical manifestation of a host's immune inflammatory response to a vaccine.
Current US‐based guidance suggests patients with reaction onset within 4 h or any severe symptoms be referred to an allergist/immunologist. 7 Given emergent data on reaction recurrence being rare though potentially severe, 2 , 8 , 9 and our findings that U/AE events were largely delayed in onset and seemingly benign in course, and with negative allergy assessments, U/AE events following mRNA COVID‐19 vaccination may not absolutely contraindicate future doses.
CONFLICT OF INTEREST
KB reports grants from NIH/NIAID (K01 AI125631, R01 AI150295), AHRQ (R01HS025375), MGH Executive Committee on Research, MGH Department of Medicine (DOM) Transformative Scholar Award, and MGH DOM COVID‐19 Junior Investigator Support Initiative; personal fees from Weekley, Schulte, Valdes, Murman, Tonelli, Vasios, Kelly, Stroll, P.A and Piedmont Liability Trust; and royalties from UpToDate, during the conduct of the study, outside the submitted work. SA reports grants from NIH/NIAID (R01 AI135197 and UM2 AI130836) and DBV Technologies, outside the submitted work. AP serves on the advisory board for CSL Behring, Pharming, Takeda, and Blueprint Medicines, outside the submitted work. EF serves as the principal investigator for the American Academy of Dermatology COVID‐19 Dermatology Registry and receives grants from the International League of Dermatologic Societies (ILDS), and is an author and receives royalties from UpToDate. AB receives research grants from Takeda and Kalvista and serves on the advisory board for Takeda, Biocryst, Kavista, and Pharvaris outside the submitted work. The remaining authors have nothing to disclose.
ACKNOWLEDGEMENTS
The authors thank Linette Milkovich RN and Elizabeth Hartigan RN.
REFERENCES
- 1. Rief W. Fear of adverse effects and COVID‐19 vaccine hesitancy: recommendations of the treatment expectation expert group. JAMA Health Forum. 2021;2(4):e210804. doi: 10.1001/jamahealthforum.2021.0804 [DOI] [PubMed] [Google Scholar]
- 2. Robinson LB, Fu X, Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID‐19 vaccines. JAMA Dermatol. 2021;157(8):1000‐1002. doi: 10.1001/jamadermatol.2021.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. COVID Data Tracker . Centers for Disease Control and Prevention. https://covid.cdc.gov/covid‐data‐tracker/#datatracker‐home. Accessed June 3, 2022.
- 4. Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med. 2021;384(13):1273‐1277. doi: 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. doi: 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Catala A, Munoz‐Santos C, Galvan‐Casas C, et al. Cutaneous reactions after SARS‐CoV‐2 vaccination: a cross‐sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142‐152. doi: 10.1111/bjd.20639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Interim Clinical Considerations for Use of COVID‐19 Vaccines Currently Approved or Authorized in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/covid‐19/clinical‐considerations/covid‐19‐vaccines‐us.html. Accessed February 19, 2022.
- 8. Chu DK, Abrams EM, Golden DBK, et al. Risk of second allergic reaction to SARS‐CoV‐2 vaccines: a systematic review and meta‐analysis. JAMA Intern Med. 2022;182(4):376‐385. doi: 10.1001/jamainternmed.2021.8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pitlick MM, Joshi AY, Gonzalez‐Estrada A, Chiarella SE. Delayed systemic urticarial reactions following mRNA COVID‐19 vaccination. Allergy Asthma Proc. 2022;43(10):40‐43. doi: 10.2500/aap.2022.43.210101 [DOI] [PMC free article] [PubMed] [Google Scholar]


