Dear Editor,
Vaccination is an effective means to contain the spread of COVID‐19 infection. While data on the safety of COVID‐19 vaccines remain limited in patients with leprosy, there have been a plethora of articles reporting the adverse effect and flare of pre‐existing dermatological diseases such as psoriasis, atopic dermatitis and lichen planus after COVID‐19 vaccination. 1 , 2 , 3
We surveyed 35 patients with leprosy visiting our leprosy clinic from July 2021 to December 2021; all patients received the Oxford–AstraZeneca ChAdOx1 nCoV‐19 vaccine. Twenty‐one of 35 patients (60%) had received both vaccine doses. Two of them developed Erythema nodosum leprosum (ENL), and one developed Type 1 lepra reaction shortly after receiving the first dose of the vaccine.
Case 1: A female in her forties who was a treated case of borderline lepromatous (BL) leprosy presented with ENL 1 week after the first dose of ChAdOx1 nCoV‐19 vaccine. She was investigated, and her reverse transcription‐polymerase chain reaction (RT‐PCR) was negative for COVID‐19. Her complete blood count showed marked leucocytosis and neutrophilia and rest of her routine investigations were within normal limits. She was started on oral prednisolone 30 mg, and ENL subsided within 2 weeks of therapy. Prednisolone was tapered and stopped after 12 weeks, and she did not have any recurrence. After 16 weeks of the first dose, she received her second dose of the vaccine without any exacerbation.
Case 2: A 45‐year male who received a first dose of ChAdOx1 nCoV‐19 vaccine developed fever, joint pain and painful erythematous nodules after 8 days of vaccination. He had completed the multidrug therapy (MDT) for lepromatous leprosy (LL) 8 months back and was not receiving any medication after that. No previous history of leprosy reaction was present nor any triggering factors were found for ENL. The patient was started on prednisolone 40 mg, and symptoms improved after a week of therapy. He was continued on tapering doses of prednisolone over a period of 12 weeks and did not have any recurrence after stopping prednisolone. The second dose of vaccine received after 4 months was uneventful.
Case 3: A 35‐year‐old female developed a hypoaesthetic, well‐defined erythematous, oedematous annular plaque approximately 25 × 7 cm over her upper back with few satellite lesions 14 days after receiving the first dose of ChAdOx1 nCoV‐19 vaccine (Fig. 1a). A similar plaque was present over the left arm with an approximate size of 10 × 6 cm. Slit skin smear examination from the ear lobes and upper back was negative. Histopathological examination revealed features suggestive of borderline tuberculoid (BT) leprosy with Type 1 reaction (Fig. 1b). Type 1 reaction following vaccination led to unmasking of the disease in this case and she was started on WHO multidrug multibacillary regimen (MDT MBR) and oral prednisolone.
Figure 1.
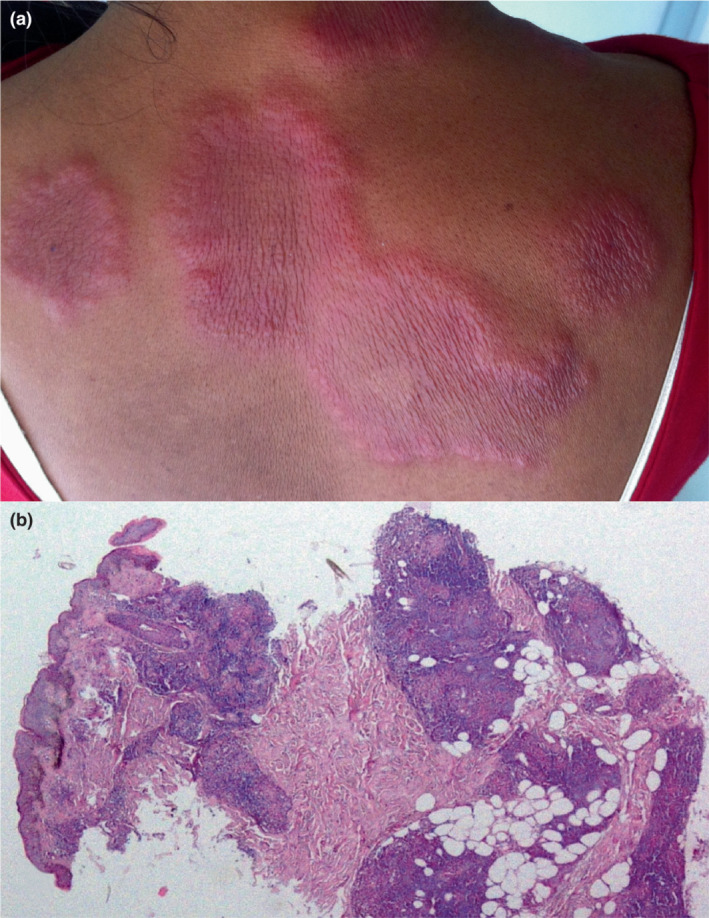
(a) Erythematous, oedematous annular plaques in patient with Type 1 reaction. (b) Skin biopsy showing epithelioid cell granulomas composed of histiocytes and lymphocytes and mild perivascular lymphomononuclear infiltrate. (H&E, 4×).
Vaccines are known to cause lepra reactions or worsening of pre‐existing reactions. 4 , 5 Robello et al. 6 reported two cases of ENL after receiving the first dose of ChAdOx1‐S/nCoV‐19 vaccine. A patient with borderline lepromatous (BL) leprosy developed acute foot drop with Type 2 Lepra after 5 days of receiving the COVID‐19 vaccine. 7 Aponso et al. 8 reported a case of Type 1 lepra reaction unveiling multibacillary leprosy after receiving the Pfizer‐BioNTech COVID‐19 vaccine.
COVID‐19 vaccines cause inflammatory cascade via neutrophilia and increased Th1 T cell responses (due to increased tumour necrosis factor (TNF)‐α and interferon (IFN)‐γ production by CD4+ T cells) leading to raised cell‐mediated immunity towards leprosy bacilli, thereby possibly causing both types of lepra reactions. 9 Higher immune alteration and SARS‐CoV‐2 spike‐specific effector T‐cell response are noted with the first dose of the adenovirus‐vectored ChAdOx1 nCoV‐19 vaccine beginning by day 7 and maximizing by day 14, which possibly explain the reactions triggered by first dose in our cases. 10
We have not seen any significant effect of COVID‐19 vaccinations on the incidence or severity of reactions and neuritis in our leprosy patients. It is to be noted that none of our patients who were on treatment with MDT developed any adverse effects after vaccination. Two cases that developed ENL had completed the treatment in the past, while one case was unveiled with a Type 1 reaction after receiving the vaccine. The vaccine for COVID‐19 appears to be safe for use in leprosy patients, and all patients should be encouraged to get vaccinated against COVID‐19.
Funding source
Nil.
Conflicts of interest
Nil.
Acknowledgement
The patients in this manuscript have given written informed consent to publication of their case details.
Data availability statement
Data available on request from the authors.
References
- 1. Sotiriou E, Tsentemeidou A, Bakirtzi K, Lallas A, Ioannides D, Vakirlis E. Psoriasis exacerbation after COVID‐19 vaccination: a report of 14 cases from a single Centre. J Eur Acad Dermatol Venereol 2021; 35: e857–e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Potestio L, Napolitano M, Bennardo L, Fabbrocini G, Patruno C. Atopic dermatitis exacerbation after Covid‐19 vaccination in Dupilumab‐treated patients. J Eur Acad Dermatol Venereol 2022; 36: e409–e411. [DOI] [PubMed] [Google Scholar]
- 3. Herzum A, Burlando M, Molle MF, Micalizzi C, Cozzani E, Parodi A. Lichen planus flare following COVID‐19 vaccination: a case report. Clin Case Rep 2021; 9: e05092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol 2017; 8: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandre MK, Poenaru SM, Boggild AK. Erythema nodosum Leprosum triggered by antecedent influenza vaccine and respiratory tract infection: a case report. J Cutan Med Surg 2019; 23: 114–116. [DOI] [PubMed] [Google Scholar]
- 6. Rebello PFB, Pennini SN. Erythema nodosum leprosum and active leprosy after ChAdOx1‐S/nCoV‐19 recombinant vaccine. A report of two cases. Lep Review 2021; 92: 421–426. [Google Scholar]
- 7. Panda AK, Begum F, Panda M, Jena AK. Trigger of type 2 lepra reaction with acute foot drop following Covid‐19 vaccination. J Eur Acad Dermatol Venereol 2022; 36: e334–e335. [DOI] [PubMed] [Google Scholar]
- 8. Aponso S, Hoou LC, Wei YY, Salahuddin SA, Yit PJ. Multibacillary leprosy unmasked by COVID‐19 vaccination. JAAD Case Reports 2022; 19: 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahin U, Muik A, Derhovanessian E et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586: 594–599. [DOI] [PubMed] [Google Scholar]
- 10. Folegatti PM, Ewer KJ, Aley PK et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet 2020; 396: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


