Abstract
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has been associated with a high risk of adverse outcomes in solid organ transplant (SOT) recipients in the pre-vaccination era. In this retrospective cohort study, we examined the incidence and severity of COVID-19 in kidney and liver transplant recipients in Denmark in the post-vaccination era, from December 27, 2020, to December 27, 2021. We included 1428 SOT recipients with 143 cases of first-positive SARS-CoV-2 PCR test. The cumulative incidence of first-positive SARS-CoV-2 PCR test 1 year after initiation of vaccination was 10.4% (95% CI: 8.8–12.0), and the incidence was higher in kidney than in liver transplant recipients (11.6% [95% CI: 9.4–13.8] vs. 7.4% [95% CI: 5.1–9.8], p = .009). After the first-positive SARS-CoV-2 PCR test, the hospitalization rate was 31.5% (95% CI: 23.9–39.1), and 30-day all-cause mortality was 3.7% (95% CI: 0.5–6.8). Hospitalization was lower in vaccinated than in unvaccinated SOT recipients (26.4% [95% CI: 18.1–34.6] vs. 48.5% [95% CI: 31.4–65.5], p = .011), as was mortality (1.8% [95% CI: 0.0–4.3] vs. 9.1% [95% CI: 0.0–18.9], p = .047). In conclusion, SOT recipients remain at high risk of adverse outcomes after SARS-CoV-2 infections, with a lower risk observed in vaccinated than in unvaccinated SOT recipients.
KEYWORDS: clinical research/practice, infection and infectious agents – viral: SARS-CoV-2/COVID-19, infectious disease, patient survival, solid organ transplantation, vaccine
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; DDV, Danish Vaccination Register; ICU, intensive care unit; IQR, interquartile range; MiBa, Danish Microbiology Database; mRNA, messenger RNA; mTOR, mammalian target of rapamycin; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome corona virus-2; SOT, solid organ transplant
1. INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), was associated with substantial morbidity and mortality in solid organ transplant (SOT) recipients in the pre-vaccination era with reports on hospitalization rates ranging from 31 to 89%,1, 2, 3, 4, 5 intensive care unit (ICU) admission ranging from 11% to 33%,1, 2, 3, 4 , 6 and mortality ranging from 5% to 27%.1, 2, 3, 4 , 6, 7, 8
Development and administration of vaccines is the mainstay of preventive efforts to curb the COVID-19 pandemic, and at-risk individuals have been prioritized for early vaccination with messenger RNA (mRNA) vaccines.9 In a phase III trial, the BNT162b2 mRNA vaccine demonstrated excellent efficacy in protection from symptomatic COVID-19 within the first 2 months following vaccination with a declining efficacy from 2 to 6 months after vaccination.10 However, SOT recipients were excluded from the phase III trials,11 and data on real-life vaccine effectiveness in SOT recipients are lacking. Reports suggest that in the first months after vaccination, SARS-CoV-2 vaccines effectively protect SOT recipients against infections.12, 13, 14 However, humoral immunity wanes over time in both immunocompetent individuals15 , 16 and SOT recipients,17 , 18 and a higher proportion of SOT recipients reach low concentrations of antibodies and neutralizing capacity of antibodies 6 months after vaccination than immunocompetent individuals.18 Furthermore, reports showing that protection against infection wanes over time in immunocompetent individuals10 , 19, 20, 21 have led to administration of a third and, subsequently, a fourth vaccine dose to SOT recipients in many countries.9
After vaccination, SOT recipients remain at higher risk of SARS-CoV-2 infections than immunocompetent individuals.22 A Spanish study found lower mortality in vaccinated SOT recipients than in unvaccinated SOT recipients after mRNA vaccination,14 and an English study reported that vaccination with BNT162b2 did not reduce risk of death after SARS-CoV-2 infection in SOT recipients. However, to date, no large study has investigated SARS-CoV-2-related morbidity and mortality in a homogenously vaccinated cohort of SOT recipients in the post-vaccination era. Furthermore, no large study including mild and asymptomatic infections has investigated morbidity and mortality in SOT recipients after SARS-CoV-2 infection in the post-vaccination era.
We aimed to determine the incidence of first-positive SARS-CoV-2 polymerase chain reaction (PCR) test in SOT recipients in the first year after vaccination was initiated in Denmark. Furthermore, we aimed to investigate treatments, hospitalizations and ICU admissions 30 days after first-positive SARS-CoV-2 PCR test, and 30-day mortality in SOT recipients with first-positive SARS-CoV-2 PCR test who were vaccinated or unvaccinated at the time of infection.
2. METHODS
2.1. Study design
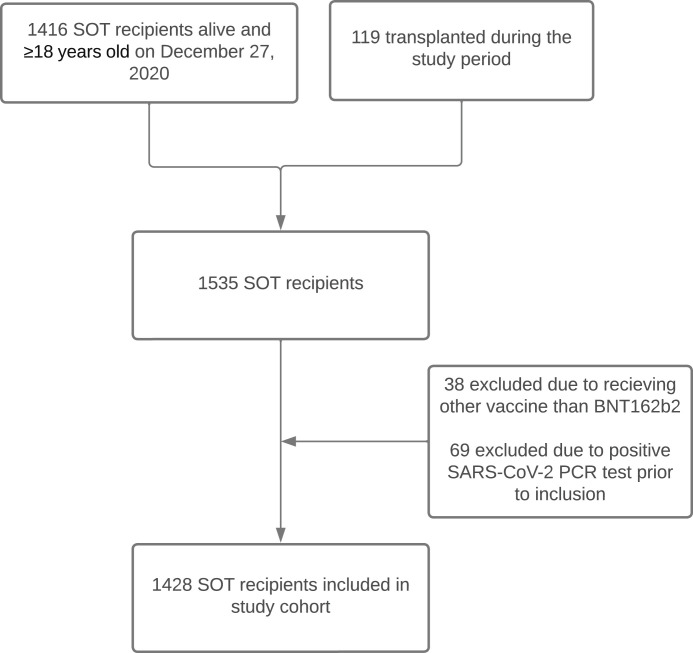
In this retrospective cohort study, we included kidney and liver transplant recipients transplanted at Copenhagen University Hospital, Rigshospitalet, Denmark between January 1, 1990 and December 1, 2021, who were ≥ 18 years old, living in Denmark, alive on December 27, 2020, received either BNT162b2 vaccination or no vaccination and had no history of positive SARS-CoV-2 PCR test prior to inclusion. We excluded SOT recipients who had positive SARS-CoV-2 PCR prior to the inclusion date or received vaccines other than BNT162b2 ( Figure 1). Rigshospitalet is a large tertiary hospital with a knowledge center and highly specialized functions in the field of solid organ transplantation, and it is the only liver transplant center in Denmark. In Denmark, vaccination against SARS-CoV-2 was initiated on December 27, 2020. SOT recipients were prioritized for early vaccination, and the vast majority of SOT recipients received BNT162b2 if vaccinated (>99%).
FIGURE 1.
Consort diagram. A flow diagram showing how many SOT recipients were eligible to be included in the study cohort and the reasons for exclusion in SOT recipients not included.
Data on clinical information, including demographics, comorbidities, transplant type, date of death, and use of immunosuppressive medication were collected from medical records. In patients with a positive SARS-CoV-2 PCR test, data on hospitalizations, COVID-19-related treatments, and admissions to intensive care units (ICU) were collected from medical records. Data on admissions to all healthcare facilities in Denmark were accessible and were used in the study. Data on vaccination status were collected from the Danish Vaccination Register (DDV). Since 2015 it has been mandatory to register all vaccines administered to Danish patients in DDV.23 Data on SARS-CoV-2 PCR tests were collected from the Danish Microbiology Database (MiBa),24 which contains complete data on all microbiological samples in Denmark including samples from both general practices, test centers, and hospitals. Other than SARS-CoV-2 PCR, rapid antigen test has been widely available and free of charge in Denmark. In case of a positive rapid antigen test, patients were recommended to have a PCR test. Furthermore, close contacts and symptomatic patients were recommended to have PCR rather than rapid antigen tests. The complete national coverage of MiBa and DDV23 combined with extensive and easily accessible free of charge PCR testing in Denmark during the study period allowed us to report all vaccinations and positive SARS-CoV-2 PCR tests from all sectors of the universal Danish healthcare system. In Denmark, extensive whole-genome sequencing of positive SARS-CoV-2 PCR tests was performed throughout 2021, with approximately 80% of positive PCR samples being successfully sequenced until the beginning of December 2021, when positive cases increased outnumbering the sequencing capacity after the introduction of the omicron variant.25
The study was conducted following the declaration of Helsinki, and according to Danish legislation, the retrieval of data without collection of informed consent was approved by the institutional review board at the Center for Regional Development (R-20051155).
2.2. Definitions
A participant was defined as vaccinated 14 days after receiving the second dose of BNT162b2 vaccine. A participant was defined as having received a third dose 14 days after receiving the third dose of BNT162b2 vaccine. None of the participants received a fourth dose during the study period. An unvaccinated participant was defined as having received zero doses, having received one dose or being within the first 14 days after the second dose.
The duration of follow-up was defined as the number of days from “December 27, 2020” to “the time of the first-positive SARS-CoV-2 PCR test,” “the time of death,” or “December 27, 2021,” whichever came first. Therefore, the duration of follow-up was shorter than 1 year for some patients.
COVID-19-related hospitalizations were defined as a hospital admission lasting more than 24 h within 30 days of a positive SARS-CoV-2 PCR test, where the patient presented at least one COVID-19-related symptom (cough, fever, myalgia, headache, dyspnea, sore throat, diarrhea, nausea/vomiting, loss of smell and taste, abdominal pain or rhinorrhea).26 In addition, if the patient was already hospitalized at the date of first-positive SARS-CoV-2 PCR test, the admission was defined as a COVID-19-related admission if the patient presented COVID-19-related symptoms as described above or was treated for COVID-19.
Admission to ICU was defined as an admission to ICU during a COVID-19-related hospitalization.
Participants who were transplanted after December 27, 2020 were defined as having delayed entry to the study.
2.3. Study period
We started follow-up on December 27, 2020, when the first patient in Denmark was vaccinated, and ended the follow-up on December 27, 2021. For participants with delayed entry, the start of follow-up was the day of transplantation. In total, 107 participants had delayed entry. To assess incidence rates and cumulative incidences of first-positive SARS-CoV-2 PCR test, we censored the follow-up time on December 27, 2021, at the time of the first-positive SARS-CoV-2 PCR test, or at the time of death, whichever came first. To assess cumulative incidence of hospitalizations, admission to ICU, and mortality after the first-positive SARS-CoV-2 PCR test, all participants with a positive SARS-CoV-2 PCR test were followed for at least 30 days after the date of the first-positive test or to the time of death, whichever came first.
2.4. Statistics
Normality was assessed by QQ-plots/normal probability plots. Continuous data were reported with medians and interquartile range (IQR). Categorical data were reported as frequency counts and percentages, and independency was tested by chi-square test or Fisher’s test, as appropriate. Differences in continuous data were tested using the Mann–Whitney U test.
Incidence rates of first-positive SARS-CoV-2 PCR test in SOT recipients were calculated as the number of first-positive SARS-CoV-2 PCR tests per 1000 person-years at risk. Byar’s approximation to the Poisson distribution was used to calculate 95% confidence intervals (CI). The cumulative incidence of first-positive SARS-CoV-2 PCR test in the follow-up period was calculated using the Aalen–Johansen estimator with death as a competing risk. Gray’s test was used to test for the difference in cumulative incidence of first-positive SARS-CoV-2 PCR test in liver and kidney transplant recipients.
In a sensitivity analysis, we excluded transplant recipients transplanted after “December 27, 2020” to see if exclusions of participants with delayed entry affected the results.
Descriptive statistics were used to describe the characteristics of vaccinated and unvaccinated SOT participants with a positive SARS-CoV-2 PCR test.
Cumulative incidence of hospital admissions or admissions to ICU 30 days after first-positive SARS-CoV-2 PCR test was calculated using the Aalen–Johansen estimator with death as a competing risk. To test for differences in 30-day cumulative incidence of hospitalizations and admissions to ICU in vaccinated and unvaccinated SOT recipients after first-positive SARS-CoV-2 PCR test Gray’s test was used. Mortality 30 days after first-positive SARS-CoV-2 PCR test was calculated using the Kaplan–Meier estimator. The log-rank test was used to test for difference in 30-day mortality after first-positive SARS-CoV-2 PCR test in vaccinated and unvaccinated SOT recipients. To test for difference in 30-day cumulative incidence of hospitalizations in liver and kidney transplant recipients after first-positive SARS-CoV-2 PCR test Gray’s test was used.
A Cox regression model with monoclonal antibodies as time-updated variable was used to determine the risk factors of hospital admission due to COVID-19 in the first 30 days after the first-positive PCR test. Age, gender, type of transplanted organ, number of vaccine doses, treatment with outpatient monoclonal antibodies comorbidities, time from transplantation to infection, and immunosuppressive maintenance therapy were considered possible risk factors. In a multivariable analysis, we included age, transplanted organ, number of vaccine doses before infection, and outpatient monoclonal antibody treatment. The proportional hazard assumptions were tested using score residuals and found to be valid.
Analyses were performed using R version 4.0.3 (R Core Team, 2020, Vienna, Austria) and the packages epiR,27 survival, prodlim,28 fmsb, timereg, and cmprsk.29
3. RESULTS
3.1. Baseline clinical characteristics of SOT recipients
We included 1428 SOT recipients without a previous positive SARS-CoV-2 PCR test (Figure 1), of those 905 were kidney and 523 were liver transplant recipients. The median age at the start of follow-up (December 27, 2020) was 54 years (IQR 45–63), and 851 (59.6%) SOT recipients were male. The median time from transplantation to the start of follow-up was 6.1 years (range 0.0–30.0), with a minor difference between liver and kidney transplant recipients ( Table 1). Furthermore, the distribution of comorbidities and use of immunosuppressive maintenance treatment differed between liver and kidney transplant recipients (Table 1).
TABLE 1.
Baseline characteristics of all liver and kidney transplant recipients
| All SOT recipients, n = 1428 | Liver transplant recipients, n = 523 (36.6%) | Kidney transplant recipients, n = 905 (63.4%) | |
| Age, median (IQR) | 54 (45–63) | 55 (46–63) | 53 (44–63) |
| Sex (male), n (%) | 851 (59.6%) | 294 (56.2%) | 557 (61.5%) |
| Time since transplantation at end of follow-up in years, median (IQR) | 6.1 (2.6–10.4) | 5.1 (1.8–9.7) | 6.6 (3.0–10.7) |
| Comorbidities, n (%) | |||
| Cardiovascular diseasea | 717 (68.5%) | 145 (36.2%) | 572 (88.5%) |
| Chronic pulmonary disease | 115 (11.0%) | 42 (10.5%) | 73 (11.3%) |
| Diabetes mellitus | 253 (24.2%) | 89 (22.2%) | 164 (25.4%) |
| Immunosuppressive treatment, n (%) | |||
| No antimetabolites | 206 (14.4%) | 121 (23.1%) | 85 (9.4%) |
| Mycophenolate | 1095 (76.7%) | 366 (70.0%) | 729 (80.6%) |
| Azathioprine | 127 (8.9%) | 36 (6.9%) | 91 (10.1%) |
| Calcineurin inhibitor (Ciclosporin, Tacrolimus) | 1277 (89.4%) | 475 (90.8%) | 802 (88.6%) |
| mTOR inhibitor (Sirolimus, Everolimus) | 86 (6.0%) | 32 (6.1%) | 54 (6.0%) |
| Corticosteroids | 1082 (75.8%) | 256 (48.9%) | 826 (91.3%) |
| Number of vaccine doses at time of death or December 27, 2021 | |||
| 0 | 74 (5.2%) | 21 (4.0%) | 53 (5.9%) |
| 1 | 5 (0.4%) | 3 (0.6%) | 2 (0.2%) |
| 2 | 127 (8.9%) | 45 (8.6%) | 82 (9.1%) |
| 3 | 1222 (85.6%) | 454 (86.8%) | 768 (84.9%) |
Abbreviations: IQR, interquartile range; mTOR, mammalian target of rapamycin.
Missing data from 381 participants (122 liver transplant recipients and 259 kidney transplant recipients).
3.2. Follow-up
The study comprised of 1316 person-years of follow-up with a median follow-up of 365 days (IQR 365–365). Reasons for follow-up of <365 days were death in 42 SOT recipients (2.9%), SARS-CoV-2 infection during the study period in 143 SOT recipients (10%), and delayed entry in 107 SOT recipients transplanted after December 27, 2020 (7.5%). Seventy-eight of the 107 SOT recipients transplanted after December 27, 2020 were vaccinated before transplantation. Although, only four of those were infected during follow-up. For vaccinated SOT recipients, the median time from the start of follow-up to the time that the SOT recipient was considered vaccinated (i.e., >14 days after the second dose) was 76 days (IQR 46–94, Figure S1). The median (IQR) time from vaccination to a positive PCR was 247 (191–272) days and ranged from 14 to 317 days.
3.3. Incidence rate and cumulative incidence of first-positive SARS-CoV-2 PCR test
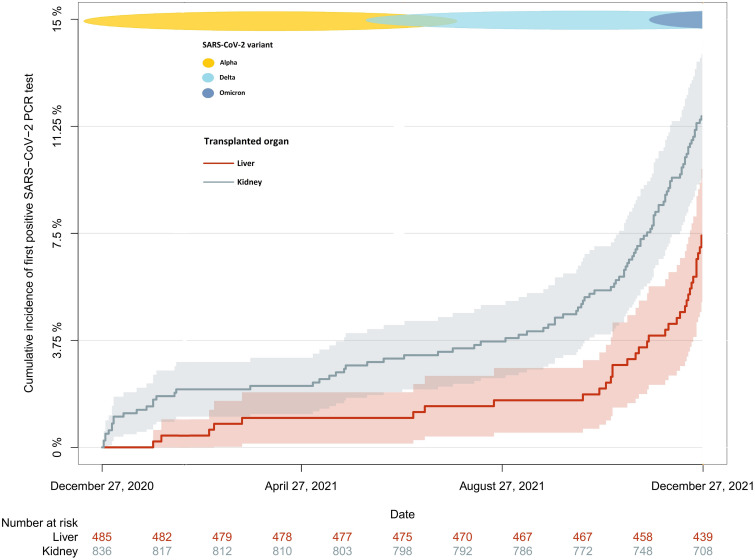
During follow-up, 143 SOT recipients had at least one positive SARS-CoV-2 PCR test. The incidence rate of first-positive SARS-CoV-2 PCR test was 109 per 1000 person-years (95% CI: 92–128), and the median time from the start of follow-up to first-positive SARS-CoV-2 PCR test was 314 days (IQR 179–346). In kidney transplant recipients the incidence rate was 127 per 1000 person-years (95% CI: 105–153), while it was 77 per 1000 person-years (95% CI: 56–105) in liver transplant recipients. The incidence rate ratio was 1.64 (1.13–2.38, p = .008). The cumulative incidence of first-positive SARS-CoV-2 PCR test on December 27, 2021, was 10.4% (95% CI: 8.8–12.0). The cumulative incidence of first-positive SARS-CoV-2 PCR test was higher in the kidney transplant recipients compared to the liver transplant recipients (11.6% [95% CI: 9.4–13.8] vs. 7.4% [95% CI: 5.1–9.8), p = .009, Figure 2). In a sensitivity analysis excluding participants with delayed entry, we found that all estimates and p-values remain robust (Table S1).
FIGURE 2.
Cumulative incidence of first-positive SARS-CoV-2 PCR test in kidney and liver transplant recipients during the study period (December 27, 2020 to December 27, 2021). Cumulative incidence of first-positive SARS-CoV-2 PCR test in kidney (gray) and liver (red) transplant recipients from December 27, 2020 to December 27, 2021 excluding participants with delayed entry. In Denmark, the alpha variant was first reported in November 2020, and from March until June 2021 it was the dominant variant. The delta variant became the dominant variant from July 2021 until December 2021, and the first cases of omicron were detected in late November 2021. [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Clinical characteristics of SOT recipients at the time of the first-positive SARS-CoV-2 PCR test
Of the 143 SOT recipients with a positive SARS-CoV-2 PCR test, 33 were unvaccinated at the time of infection, and 110 were vaccinated ( Table 2). There were no significant differences in clinical characteristics between vaccinated and unvaccinated SOT recipients with positive SARS-CoV-2 PCR test, nor was there a difference in the type of transplanted organ (Table 2). However, the median date of first-positive SARS-CoV-2 PCR test was February 1, 2021, in unvaccinated and November 24, 2021, in vaccinated SOT recipients. Consequently, SARS-CoV-2 variant differed between vaccinated and unvaccinated positive SARS-CoV-2 PCR tests, wild-type being most frequent among those who were unvaccinated at time of infection and delta being most frequent among those who were vaccinated at the time of infection (p < .001, Table 2). Furthermore, in Denmark, preventive use of monoclonal antibodies after SARS-CoV-2 infection in high-risk groups became available in December 2021. Until then, the use of mAbs after SARS-CoV-2 infection was not universal. Therefore, more SOT recipients who were vaccinated at the time of first-positive SARS CoV- 2 PCR test received monoclonal antibody treatment than those who were unvaccinated at the time of first-positive SARS-CoV-2 PCR test (34.5% vs. 6.1%, p = .001, Table 2).
TABLE 2.
Clinical characteristics of SOT recipients at the time of first-positive SARS-CoV-2 PCR test according to vaccination status at time of infection
| All SOT recipients, n = 143 | Unvaccinated, n = 33 (23.1%) | Vaccinated, n = 110 (76.9%) | p-value | |
| Age at time of first-positive SARS-CoV-2 PCR test, median (IQR) | 49 (40–59) | 46 (31–60) | 50 (42–58) | 0.369 |
| Sex (male), n (%) | 91 (63.6%) | 17 (51.5%) | 74 (67.3%) | 0.149 |
| Time from transplantation to first-positive SARS-CoV-2 PCR test in years, median (IQR) | 6.0 (2.5–10.0) | 5.0 (2.0–10.0) | 6.0 (3.0–9.8) | 0.751 |
| Transplant type, n (%) | ||||
| Kidney | 105 (73.4%) | 26 (78.8%) | 79 (71.8%) | 0.569 |
| Liver | 38 (26.6%) | 7 (21.2%) | 31 (28.2%) | |
| Comorbidities, n (%) | ||||
| Cardiovascular disease | 95 (66.4%) | 23 (69.7%) | 72 (65.5%) | 0.808 |
| Chronic pulmonary disease | 17 (11.9%) | 5 (15.2%) | 12 (10.9%) | 0.543 |
| Diabetes mellitus | 32 (22.4%) | 6 (18.2%) | 26 (23.6%) | 0.674 |
| Immunosuppressive treatment at time of first-positive SARS-CoV-2 PCR test, n (%) | ||||
| No antimetabolites | 13 (9.1%) | 2 (6.1%) | 11 (10.0%) | 0.593 |
| Mycophenolate | 121 (84.6%) | 28 (84.8%) | 93 (84.5%) | |
| Azathioprine | 9 (6.3%) | 3 (9.1%) | 6 (5.5%) | |
| Calcineurin inhibitor (Ciclosporin, Tacrolimus) | 135 (94.4%) | 31 (93.9%) | 104 (94.5%) | 1.00 |
| mTOR inhibitor (Sirolimus, Everolimus) | 4 (2.8%) | 2 (6.1%) | 2 (1.8%) | 0.228 |
| Corticosteroids | 122 (85.3%) | 29 (87.9%) | 93 (84.5%) | 0.783 |
| Number of vaccine doses at time of first-positive SARS-CoV-2 PCR test, n (%) | ||||
| 0 | 29 (20.3%) | 29 (87.9%) | 0 (0%) | <0.001 |
| 1 | 4 (2.8%) | 4 (12.1%) | 0 (0%) | |
| 2 | 46 (32.2%) | 0 (0%) | 46 (41.8%) | |
| 3 | 64 (44.8%) | 0 (0%) | 64 (58.2%) | |
| SARS-CoV-2 varianta, n (%) | ||||
| Wild-type | 8 (11.3%) | 8 (61.5%) | 0 (0.0%) | <0.001 |
| Alpha | 7 (9.9%) | 2 (15.4%) | 5 (8.6%) | |
| Delta | 51 (71.8%) | 3 (23.1%) | 48 (82.8%) | |
| Omicron | 5 (7.0%) | 0 (0.0%) | 5 (8.6%) | |
| Outpatient monoclonal antibody treatment with Sotrovimab | 37 (28.0%) | 2 (6.1%) | 35 (31.8%) | 0.003 |
| Outpatient monoclonal antibody treatment with Regn-Cov2 | 3 (2.7%) | 0 (0%) | 3 (2.7%) | 1.00 |
Abbreviations: IQR, interquartile range; mTOR, mammalian target of rapamycin; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory coronavirus 2.
Missing data from 72 infections (20 unvaccinated and 52 vaccinated).
3.5. Outcomes after first-positive SARS-CoV-2 PCR test in SOT recipients
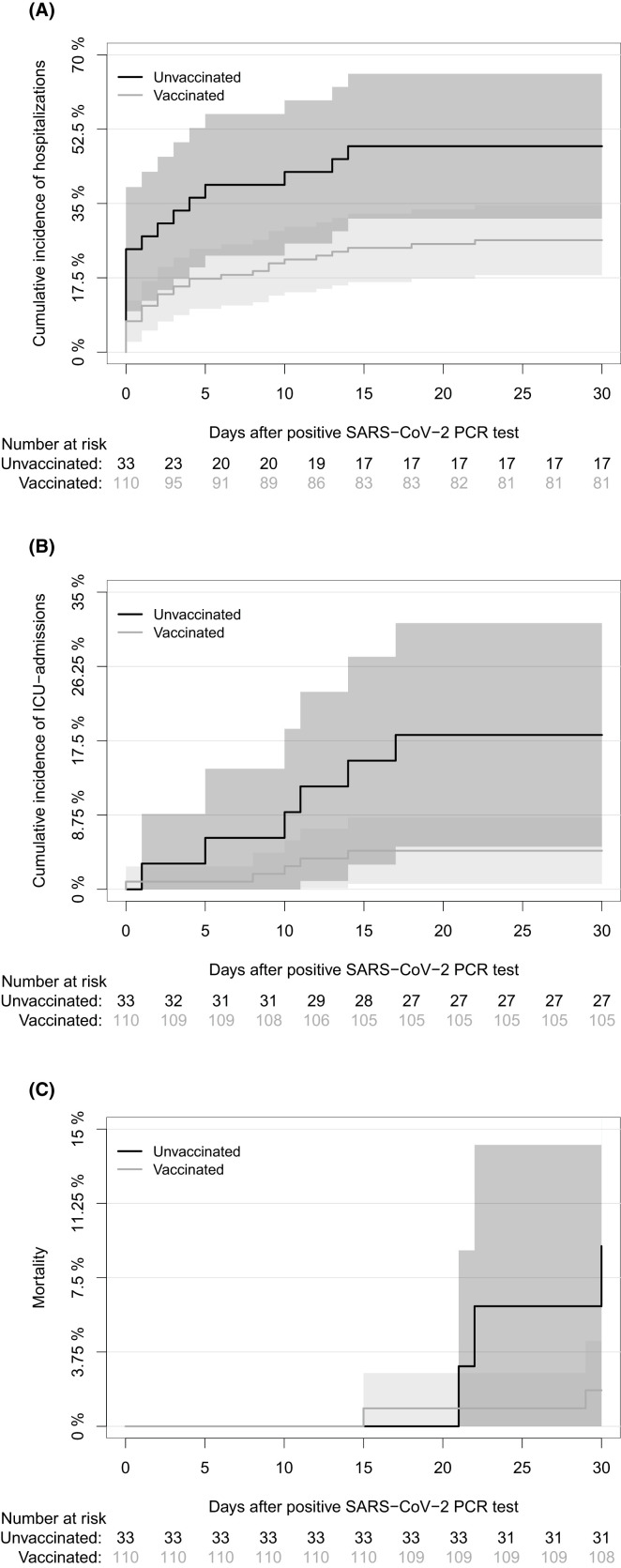
Forty-five SOT recipients were hospitalized due to COVID-19 within 30 days of their first-positive SARS-CoV-2 PCR test. The 30-day cumulative incidence of hospitalizations after first-positive SARS-CoV-2 PCR test was 31.5% (95% CI: 23.9–39.1), and this was lower in vaccinated than unvaccinated SOT recipients (26.4% [95% CI: 18.1–34.6] vs. 48.5% [95% CI: 31.4–65.5], p = .011, Figure 3). Eleven SOT recipients were admitted to ICU during hospitalization due to COVID-19. The 30-day cumulative incidence of ICU admissions was 7.7% (95% CI: 3.3–12.1), and this was lower in vaccinated than unvaccinated SOT recipients (4.5% [95% CI: 0.7–8.4] vs. 18% [95% CI: 5.0–31.3], p = .011, Figure 3). The 30-day all-cause mortality after first-positive SARS-CoV-2 PCR test was 3.5% (95% CI: 0.5–6.5) and there was a lower 30-day all-cause mortality in the vaccinated than in unvaccinated SOT recipients (1.8% [95% CI: 0.0–4.3] vs. 9.1% [95% CI: 0.0–18.9], p = .047, Figure 3).
FIGURE 3.
Cumulative incidence of hospitalizations, ICU admissions, and mortality 30 days after first-positive SARS-CoV-2 PCR test in vaccinated and unvaccinated SOT recipients. (A) Cumulative incidence of hospitalizations in vaccinated and unvaccinated SOT recipients due to COVID-19 in the first 30 days after positive SARS-CoV-2 PCR test with day of first-positive SARS-CoV-2 PCR test as day 0. (B) Cumulative incidence of ICU admissions in vaccinated and unvaccinated SOT recipients due to COVID-19 in the first 30 days after first-positive SARS-CoV-2 PCR test with day of first-positive SARS-CoV-2 PCR test as day 0. (C) All-cause mortality in vaccinated and unvaccinated SOT recipients in the first 30 days after first-positive SARS-CoV-2 PCR test with day of first-positive SARS-CoV-2 PCR test as day 0. The yellow line represents SOT recipients who were vaccinated at time of infection, while black line represents SOT recipients who were unvaccinated at time of infection. [Color figure can be viewed at wileyonlinelibrary.com]
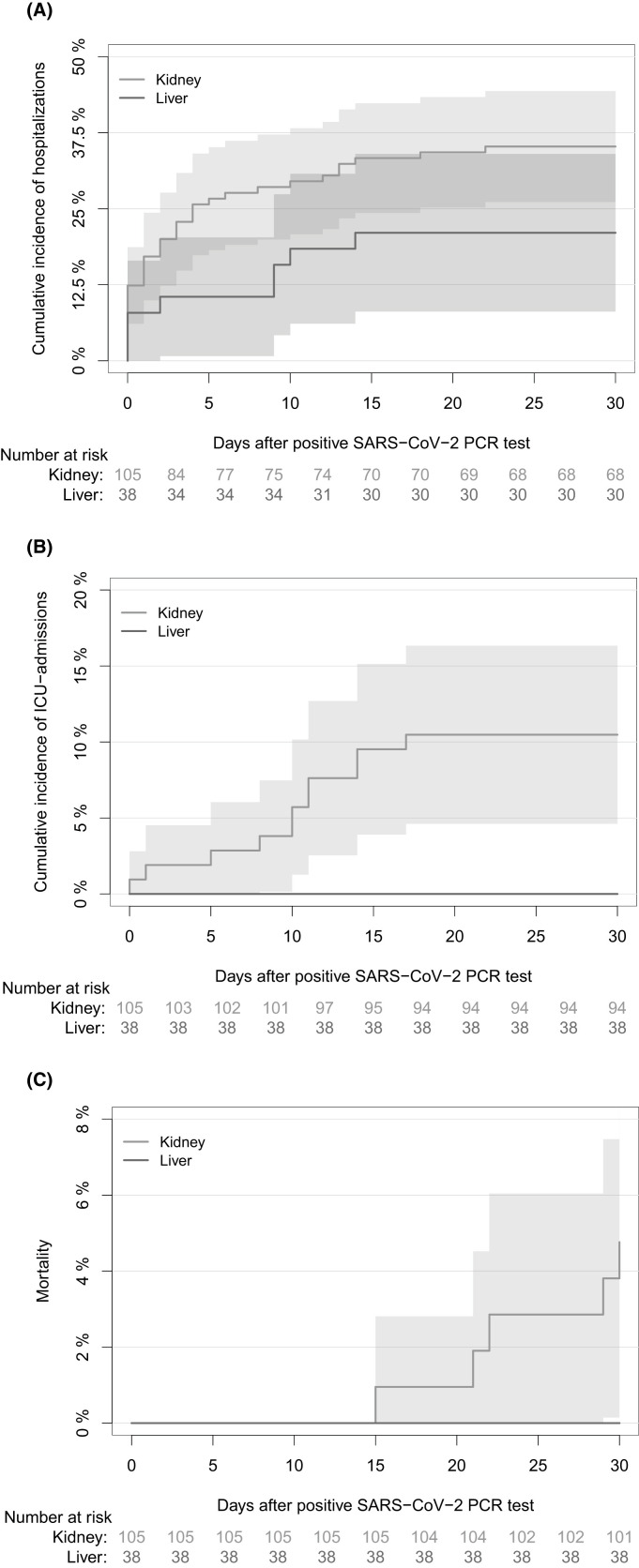
There was no significant difference in 30-day cumulative incidence of hospitalizations after first-positive SARS-CoV-2 PCR test between kidney and liver transplant recipients (35.2% [95% CI: 26.1–44.4] vs. 21.1% [95% CI: 8.1–34.0], p = .101, Figure 4). None of the liver transplant recipients were admitted to ICU or died within 30 days of the first-positive SARS-CoV-2 PCR test. In kidney transplant recipients the 30-day cumulative incidence of ICU admissions after first-positive SARS-CoV-2 PCR test was 10.5% (95% CI: 4.6–16.3, Figure 4), and the 30-day all-cause mortality was 4.8% (95% CI: 0.7–8.2, Figure 4). Furthermore, 30-day cumulative incidence of hospitalizations was lower among participants receiving outpatient mAb treatment than those who did not after first-positive SARS-CoV-2 PCR test (41.7% [95% CI: 32.2–51.3], p < .001, Figure S2).
FIGURE 4.
Cumulative incidence of hospitalizations, ICU admissions, and mortality 30 days after first-positive SARS-CoV-2 PCR test in kidney and liver transplant recipients. (A) Cumulative incidence of hospitalizations in SOT recipients due to COVID-19 in the first 30 days after first-positive SARS-CoV-2 PCR test with the day of first-positive SARS-CoV-2 PCR test as day 0. (B) Cumulative incidence of ICU admissions in liver and kidney transplant recipients in the first 30 days after first-positive SARS-CoV-2 PCR test with the day of first-positive SARS-CoV-2 PCR test as day 0. (C) All-cause mortality in liver and kidney transplant recipients in the first 30 days after first-positive SARS-CoV-2 PCR test with day of first-positive SARS-CoV-2 PCR test as day 0. The red line represents liver transplant recipients and the grey line represents kidney transplant recipients. [Color figure can be viewed at wileyonlinelibrary.com]
3.6. Treatment of SOT recipients hospitalized due to COVID-19
The median duration of hospital stay due to COVID-19 was 6 days (IQR 4–12). Among the 45 SOT recipients who were hospitalized, there was a tendency toward a shorter stay at hospital in those who were vaccinated at the time of infection compared to those who were unvaccinated (median 4 days vs 12 days, p = .058). Among the hospitalized SOT recipients, 42% received oxygen therapy, 42% received Remdesivir treatment, 64.4% received dexamethasone treatment, 4.4% received convalescent plasma, and 51% received monoclonal antibody treatment ( Table 3). In-hospital treatments did not differ between those who were vaccinated and unvaccinated at the time of infection except for monoclonal antibody treatment, which was more frequent in the vaccinated SOT recipients than the unvaccinated (62.5% vs. 18.8%, p = .004, Table 3).
TABLE 3.
Clinical characteristics, treatment, and outcomes in SOT recipients hospitalized due to COVID-19
| All SOT recipients, n = 45 | Unvaccinated, n = 16 (35.6%) | Vaccinated, n = 29 (64.4%) | p-value | |
| Age at time of first-positive SARS-CoV-2 PCR test, median (IQR) | 55 (45–66) | 47 (33–66) | 56 (47–61) | 0.331 |
| Sex (male), n (%) | 32 (71.1%) | 7 (43.8%) | 25 (86.2%) | 0.005 |
| Time from transplantation to first-positive SARS-CoV-2 PCR test in years, median (IQR) | 5.0 (2.0–8.0) | 4.5 (2.0–10.2) | 5.0 (3.0–8.0) | 0.962 |
| Transplant type, n (%) | ||||
| Kidney | 37 (82.2%) | 14 (87.5%) | 23 (79.3%) | 0.692 |
| Liver | 8 (17.8%) | 2 (12.5%) | 6 (20.7%) | |
| Comorbidities, n (%) | ||||
| Cardiovascular disease | 33 (73.3%) | 11 (68.8%) | 22 (75.9%) | 0.728 |
| Chronic pulmonary disease | 6 (13.3%) | 4 (25.0%) | 2 (6.9%) | 0.166 |
| Diabetes mellitus | 13 (28.9%) | 4 (25.0%) | 9 (31.0%) | 0.743 |
| Immunosuppressive treatment at time of first-positive SARS-CoV-2 PCR test, n (%) | ||||
| No antimetabolites | 5 (11.1%) | 1 (6.2%) | 4 (13.8%) | 0.833 |
| Mycophenolate | 38 (84.4%) | 14 (87.5%) | 24 (82.8%) | |
| Azathioprine | 2 (4.4%) | 1 (6.2%) | 1 (3.4%) | |
| Calcineurin inhibitor (Ciclosporin, Tacrolimus) | 41 (91.1%) | 15 (93.8%) | 26 (89.7%) | 1.00 |
| mTOR inhibitor (Sirolimus, Everolimus) | 3 (6.7%) | 1 (6.2%) | 2 (6.9%) | 1.00 |
| Corticosteroids | 40 (88.9%) | 15 (93.8%) | 25 (86.2%) | 0.641 |
| Number of vaccine doses at time of first-positive SARS-CoV-2 PCR test, n (%) | ||||
| 0 | 13 (28.9%) | 13 (81.2%) | 0 (0%) | <0.001 |
| 1 | 3 (6.7%) | 3 (18.8%) | 0 (0%) | |
| 2 | 17 (37.8%) | 0 (0%) | 17 (58.6%) | |
| 3 | 12 (26.7%) | 0 (0%) | 12 (41.4%) | |
| SARS-CoV-2 varianta, n (%) | ||||
| Wild-type | 6 (26.1%) | 6 (75.0%) | 0 (0.0%) | <0.001 |
| Alpha | 2 (8.7%) | 1 (12.5%) | 1 (6.7%) | |
| Delta | 15 (65.2%) | 1 (12.5%) | 14 (93.3%) | |
| Omicron | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| In-hospital treatment, n (%) | ||||
| Oxygen therapy | 19 (42.2%) | 8 (50.0%) | 11 (37.9%) | 0.639 |
| Remdesivir | 19 (42.2%) | 5 (31.2%) | 14 (48.3%) | 0.429 |
| Dexamethasone | 29 (64.4%) | 10 (62.5%) | 19 (65.5%) | 1.00 |
| Monoclonal antibody treatment with Sotrovimab | 16 (35.6%) | 1 (6.3%) | 15 (51.7%) | 0.003 |
| Monoclonal antibody treatment with REGN-COV2 | 7 (15.6%) | 2 (12.5%) | 5 (17.2%) | 1.00 |
| Convalescent plasma | 2 (4.4%) | 2 (12.5%) | 0 (0%) | 0.121 |
| Hospital stay length, median (IQR) | 6 (4–12) | 12 (5–18) | 4 (3–7) | 0.058 |
| Admission to ICU, n (%) | 11 (24.4%) | 6 (37.5%) | 5 (17.2%) | 0.161 |
| Death within 30 days of positive SARS-CoV-2 PCR test, n (%) | 5 (11.1%) | 3 (18.8%) | 2 (6.9%) | 0.330 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; mTOR, mammalian target of rapamycin; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory coronavirus 2.
Missing data from 22 infections (8 unvaccinated and 14 vaccinated).
3.7. Risk factors for hospital admission after infection
In the univariable Cox proportional hazards model, participants who received three doses of vaccine or patients who received outpatient monoclonal antibodies had an HR of 0.11 ([95% CI, 0.01–0.76], p < .026) and 0.31 ([95% CI, 0.15–0.67], p < .003) for hospital admission, respectively. In the multivariable model, containing age, type of transplanted organ, number of vaccine doses, and outpatient monoclonal antibodies, patients who had three doses of vaccine had an HR of 0.42 ([95% CI, 0.19–0.89], p < .025). Moreover, treatment with outpatient monoclonal antibodies had an HR of 0.15 ([95% CI, 0.02–1.05], p = .057) for the hospital admission ( Table 4).
TABLE 4.
Risk factors for hospital admission after infection. Univariable and multivariable Cox regression with monoclonal antibody treatment as a time-updated variable
| Unadjusted model |
Adjusted modela |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age per 10 years | 7.50 | 0.82–68.34 | 0.074 | 6.05 | 0.71–51.28 | 0.099 |
| Male sex | 1.47 | 0.77–2.82 | 0.136 | — | — | — |
| Transplantation type (liver Tx as reference) | 1.85 | 0.87–3.95 | 0.110 | 1.93 | 0.52–0.89 | 0.096 |
| Transplanted within the last year from the start of follow-up or after follow-up | 1.34 | 0.53–3.40 | 0.542 | — | — | — |
| Number of vaccines before the infection (0 and 1 as reference) | ||||||
| 2 | 0.64 | 0.33–1.24 | 0.185 | 0.70 | 0.35–1.38 | 0.299 |
| 3 | 0.312 | 0.15–0.67 | 0.003 | 0.42 | 0.19–0.89 | 0.025 |
| Immunosuppressive treatment | ||||||
| Antimetabolite at time of infection (none as reference) | ||||||
| Mycophenolate | 0.81 | 0.33–1.97 | 0.648 | — | — | — |
| Azathioprine | 0.59 | 0.11–3.30 | 0.550 | — | — | — |
| Corticosteroids at time of infection | 1.49 | 0.60–3.71 | 0.386 | — | — | — |
| CNI or mTOR at time of infection (CNI as reference) | ||||||
| mTOR | 2.82 | 0.74–10.77 | 0.128 | — | — | — |
| None | 1.47 | 0.34–6.31 | 0.607 | — | — | — |
| Outpatient monoclonal antibody treatment | 0.11 | 0.01–0.76 | 0.026 | 0.15 | 0.02–1.05 | 0.057 |
| Comorbidities | ||||||
| Cardiovascular disease | 1.51 | 0.79–2.90 | 0.219 | — | — | — |
| Chronic pulmonary disease | 1.15 | 0.50–2.66 | 0.746 | — | — | — |
| Diabetes mellitus | 1.50 | 0.80–2.84 | 0.209 | — | — | — |
Abbreviations: CI, confidence interval; CNI, calcineurin inhibitor; HR, hazard ratio; mTOR, mamalian target of rapamycin.
The adjusted model is adjusted for age, transplantation type, number of vaccination, and outpatient monoclonal antibody treatment.
4. DISCUSSION
In this large retrospective cohort study with real-life data from liver and kidney transplant recipients in Denmark, the cumulative incidence of first-time positive SARS-CoV-2 PCR test was 10% in the first year after BNT162b2 vaccination was initiated. Thirty days after first-positive SARS-CoV-2 PCR test, the cumulative incidence of hospitalizations was 31.5%. Vaccinated transplant recipients had a lower incidence of hospitalization, ICU admission, and a lower 30-day mortality, and there was a tendency toward shorter hospital stay in vaccinated than in unvaccinated SOT recipients. Risk of hospital admission was 58% lower in participants who received three vaccine doses. Moreover, there was a tendency toward lower risk of hospital admission in participants who received outpatient monoclonal antibodies. The cumulative incidence of first-positive SARS-CoV-2 PCR test was higher in kidney transplant recipients than in liver transplant recipients, but there were no significant differences in outcome between the groups.
Vaccination against SARS-CoV-2 in Denmark was initiated on December 27, 2020 and SOT recipients were prioritized for early vaccination. We started follow-up on December 27, 2020, and 1 year later, the cumulative incidence of first-time SARS-CoV-2 PCR test was 10%. Importantly, we found that most SOT recipients did not need hospital admission and lower rates of ICU admission and mortality than previously reported. Studies from the pre-vaccination era found hospitalization rates ranging from 31% to 89%,1, 2, 3, 4, 5 ICU admission ranging from 11% to 33%,1, 2, 3, 4 , 6 and mortality ranging from 5% to 27%.1, 2, 3, 4 , 6, 7, 8 Several factors may contribute to the lower morbidity and mortality observed. First, extensive and easily accessible SARS-CoV-2 PCR testing combined with Danish national registries provides us with excellent opportunities to detect SOT recipients with mild or even asymptomatic infections. In settings without such opportunities, morbidity and mortality might be overestimated. Importantly, we found incidence of hospitalization, ICU admission, and mortality 30 days after first-positive SARS-CoV-2 PCR test to be lower in vaccinated than in unvaccinated SOT recipients. No previous studies have estimated the impact of SARS-CoV-2 vaccination on hospitalization and ICU admissions in SOT recipients, however, it has been shown that vaccination with mRNA vaccines in the general population reduces the risk of hospitalization, disease progression, and death.30 We found that receiving three vaccine doses decrease the risk of hospital admission by 58%. Although, our study is descriptive, and we cannot conclude on causality or conclude that lower rates of hospitalization or mortality in vaccinated SOT recipients are due to vaccines. In a recent study from England, BNT162b2 vaccination was not found to reduce the risk of death in SOT recipients.31 Furthermore, treatment and management of SOT recipients with COVID-19 has evolved since the start of the pandemic with implementation of evidence-based treatments such as Dexamethasone,32 Remdesivir,33 , 34 and monoclonal antibodies35 , 36 that may contribute to lower morbidity and mortality. We showed that participants who received outpatient monoclonal antibodies had a tendency to lower risk of hospital admission. Finally, the virulence of the dominant variant may also affect morbidity and mortality. In Denmark, the alpha variant was first reported in November 2020, and from mid-March until June 2021, it was the dominant variant.37 The delta variant became the dominant variant from July 2021 until December 2021,38 and the first cases of omicron were detected in November 2021.39 The median date of the first-positive SARS-CoV-2 PCR test was February 1, 2021, for unvaccinated and November 24, 2021, for the vaccinated transplant recipients. SARS-CoV-2 variants were only reported for about half of the positive SARS-CoV-2 PCR tests. Still, the wild-type and delta variants were the most prevalent SARS-CoV-2 variants among unvaccinated and vaccinated SOT recipients, respectively, compatible with the dominant variants in February and November in the general population of Denmark.25 The severity of COVID-19 and risk of hospitalization, ICU admission, and death are not similar among SARS-CoV-2 variants, with the highest risk of adverse outcomes after infection with the delta variant in the general population.40, 41, 42 A recent study reported a lower risk of adverse outcomes after infection with the omicron variant compared to the delta variant in the general population,43 however, it remains unknown if this difference also applies to SOT recipients. The severity of COVID is dependent to multiple factors, among them, omicron replicates less efficiently in the lower respiratory tract which can explain a lower severity of COVID.44
The cumulative incidence of first SARS-CoV-2 PCR test in kidney transplant recipients was higher than in liver transplant recipients. In line with our findings, kidney transplant recipients were more likely to have SARS-CoV-2 infection than liver transplant recipients in a cohort of SOT recipients from the United States from the pre-vaccination era.5 Within the post-vaccination era, a recent study from Spain reported a 2.1 times higher incidence ratio for SARS-CoV-2 infection in kidney transplant recipients than in liver transplant recipients.14 We observed no significant difference between kidney and liver transplant recipients in cumulative incidence of hospitalization. However, in our cohort only kidney transplant recipients were admitted to ICU or died within 30-days after first-positive SARS-CoV-2 PCR test. This is in line with previous studies reporting that COVID-19 in liver transplant recipients was less severe and had fewer adverse outcomes than in kidney transplant recipients.45, 46, 47 The kidney transplant recipients in our cohort had a higher burden of comorbidity than liver transplant recipients, including diabetes and cardiovascular diseases which are known risk factors for ICU admission and death.48 The impact of comorbidities is supported by a study from Sweden, where 30-day all-cause mortality was 17% in liver transplant recipients and 9.3% in kidney transplant recipients, and in this study liver transplant recipients had a higher burden of comorbidities.2 Finally, we previously found kidney transplant recipients to develop less robust antibody responses to BNT162b2 vaccination than liver transplant recipients.18 Robust antibody responses have been shown to be of importance in protection against disease progression,49 inferring that differences in immunological responses between kidney and liver transplant recipients may be of importance.
There was a tendency toward a shorter hospital stay among hospitalized SOT recipients in those who were vaccinated than in those who were unvaccinated at time of first-positive SARS-CoV-2 PCR test. Treatments were the same for vaccinated and unvaccinated, except for outpatient and in-hospital monoclonal antibodies, which more frequently were prescribed to the vaccinated SOT recipients. It has been reported that monoclonal antibodies can prevent disease progression in SOT recipients,35 however, vaccination status and SARS-CoV-2 variant were not reported in that study. The impact of monoclonal antibodies seems to depend on the SARS-Cov-2 variant with novel variants exhibiting antibody evasive properties,50 and the association between monoclonal antibody treatments and length of hospital stay in SOT recipients needs more investigation.
Our study had some limitations. First, treatments and preventive strategies changed during the study period making it difficult to estimate the impact of each measure. Furthermore, the retrospective nature of the study restricts any attempt to conclude on causality between treaments, preventive strategies, and clinical outcomes. Lastly, the median date of first-positive SARS-CoV-2 PCR test and consequently the SARS-CoV-2 variants for unvaccinated and vaccinated SOT recipients were different, which might affect the results. Our study also had strengths; this is the first large real-life study of morbidity and mortality in SOT recipients who were homogenously vaccinated with mRNA vaccine. Furthermore, we had complete nationwide SARS-CoV-2 PCR test data from MiBa. This combined with extensive and easily accessible testing provides the opportunity to report asymptomatic and mild SARS-CoV-2 infections allowing qualified estimations of hospitalization and mortality rates. Finally, although we did not report the variant in all positive SARS-CoV-2 PCR tests, extensive whole-genome sequencing in Denmark allowed us to report the variant in half of the positive SARS-CoV-2 PCR tests.
In conclusion, SOT recipients remain at high risk of SARS-CoV-2 infections and adverse outcomes after SARS-CoV-2 infections even in the vaccination era. Vaccinated SOT recipients seem to be at lower risk of adverse outcomes than unvaccinated SOT recipients after SARS-CoV-2 infection, however, further studies looking into the effectiveness of preventive measures such as monoclonal antibodies and additional vaccinations are warranted. Finally, investigations of morbidity and mortality associated with emerging SARS-CoV-2 variant infections in SOT recipients are needed, as is continued surveillance of SARS-CoV-2 vaccines’ effectivenes in protecting SOT recipients from SARS-CoV-2 associated morbidity and mortality as new variants emerge.
Acknowledgments
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Figure S1
Figure S2
Table S1
REFERENCES
- 1.Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105(6):1365–1371. doi: 10.1097/TP.0000000000003670. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Søfteland JM, Friman G, von Zur-Mühlen B, et al. COVID-19 in solid organ transplant recipients: a national cohort study from Sweden. Am J Transplant. 2021;21(8):2762–2773. doi: 10.1111/AJT.16596. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study. Am J Transplant. 2021;21(7):2509–2521. doi: 10.1111/AJT.16428. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coll E, Fernández-Ruiz M, Padilla M, et al. COVID-19 in solid organ transplant recipients in Spain throughout 2020: catching the wave? Transplantation. 2021;105(10):2146–2155. doi: 10.1097/TP.0000000000003873. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinson AJ, Agarwal G, Dai R, et al. COVID-19 in solid organ transplantation: results of the national COVID cohort collaborative. Transpl Direct. 2021;7(11):e775. doi: 10.1097/TXD.0000000000001234. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira MR, Arcasoy S, Farr MA, et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23(4):e13637. doi: 10.1111/TID.13637. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumortier J, Duvoux C, Roux O, et al. Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45(4):101639. doi: 10.1016/J.CLINRE.2021.101639. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295–1303. doi: 10.1111/AJT.16424. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Interim Clinical Considerations for Use of COVID-19 Vaccines. CDC. Accessed February 20, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 10.Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMOA2110345. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malinis M, Cohen E, Azar MM. Effectiveness of SARS-CoV-2 vaccination in fully vaccinated solid organ transplant recipients. Am J Transpl. 2021;21(8):2916–2918. doi: 10.1111/AJT.16713. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslam S, Adler E, Mekeel K, Little SJ. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021;23(5):e13705. doi: 10.1111/TID.13705. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bestard O, Jouve T, Castells L, et al. Reconciling short-term clinical and immunological outcomes of SARS-CoV-2 vaccination in solid organ transplant recipients. Am J Transpl. 2022;22(2):673–675. doi: 10.1111/AJT.16855. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMOA2114583. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Alós L, Armenteros JJA, Madsen JR, et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat Commun. 2022;13(1):1614. doi: 10.1038/S41467-022-29225-4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alejo JL, Mitchell J, Chiang TPY, et al. Six-month antibody kinetics and durability in SARS-CoV-2 mRNA vaccinated solid organ transplant recipients. Transplantation. 2022;106(1):E109–E110. doi: 10.1097/TP.0000000000003975. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamm SR, Møller DL, Pérez-Alós L, et al. Decline in antibody concentration 6 months after two doses of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients and healthy controls. Front Immunol. 2022;13:564. doi: 10.3389/FIMMU.2022.832501. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMOA2114114. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMOA2114228. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182(2):153–162. doi: 10.1001/JAMAINTERNMED.2021.7024. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grove Krause T, Jakobsen S, Haarh M, Mølbak K. The Danish vaccination register. Euro Surveill. 2012;17(17):2. doi: 10.2807/ESE.17.17.20155-EN. doi: [DOI] [PubMed] [Google Scholar]
- 24.Voldstedlund M, Haarh M, Mølbak K. The Danish microbiology database (MiBa) 2010 to 2013. Euro Surveill. 2014;19(1):20667. doi: 10.2807/1560-7917.ES2014.19.1.20667. doi: [DOI] [PubMed] [Google Scholar]
- 25.Danish Covid-19 Genome Consortium. Accessed March 2, 2022. https://covid19genomics.dk/statistics
- 26.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance — United States, January 22–may 30, 2020. Morb Mortal Wkly Rep. 2020;69(24):759–765. doi: 10.15585/MMWR.MM6924E2. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark Steven- A, Sergeant E, Nunes T, et al. EpiR: tools for the analysis of epidemiological data. R Package 2.0.19. Published online 2021.
- 28.Gerds TA. Prodlim: Product-Limit Estimation for Censored Event History Analysis. R Package Version 2019.11.13. Published 2019. Accessed March 2, 2022. https://cran.r-project.org/web/packages/prodlim/index.html
- 29.Gray B. cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2–10. Published 2020. Accessed March 2, 2022. https://cran.r-project.org/web/packages/cmprsk/index.html
- 30.Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callaghan CJ, Mumford L, Curtis RMK, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106(3):436–446. doi: 10.1097/TP.0000000000004059. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horby P, Lim W, Emberson J, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMOA2021436. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMOA2007764. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMOA2015301. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahearn AJ, Thin Maw T, Mehta R, et al. A programmatic response, including Bamlanivimab or Casirivimab-imdevimab administration, reduces hospitalization and death in COVID-19 positive abdominal transplant recipients. Transplantation. 2022;106(2):e153–e157. doi: 10.1097/TP.0000000000003953. doi: [DOI] [PubMed] [Google Scholar]
- 36.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody Sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMOA2107934. doi: [DOI] [PubMed] [Google Scholar]
- 37.Lyngse FP, Mølbak K, Skov RL, et al. Increased transmissibility of SARS-CoV-2 lineage B.1.1.7 by age and viral load. Nat Commun. 2021;12(1):7251. doi: 10.1038/s41467-021-27202-x. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyngse FP, Mølbak K, Denwood M, et al. Effect of Vaccination on Household Transmission of SARS-CoV-2 Delta VOC. medRxiv. Published Online January 2022:2022.01.06.22268841. doi: 10.1101/2022.01.06.22268841 [DOI]
- 39.Espenhain L, Funk T, Overvad M, et al. Epidemiological characterisation of the first 785 SARS-CoV-2 Omicron variant cases in Denmark, December 2021. Euro Surveill Bull Eur sur les Mal Transm = Eur Commun Dis Bull. 2021;26(50) doi: 10.2807/1560-7917.ES.2021.26.50.2101146. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bager P, Wohlfahrt J, Fonager J, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage B.1.1.7 in Denmark: an observational cohort study. Lancet Infect Dis. 2021;21(11):1507–1517. doi: 10.1016/S1473-3099(21)00290-5. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bager P, Wohlfahrt J, Rasmussen M, Albertsen M, Krause TG. Hospitalisation associated with SARS-CoV-2 delta variant in Denmark. Lancet Infect Dis. 2021;21(10):1351. doi: 10.1016/S1473-3099(21)00580-6. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cevik M, Mishra S. SARS-CoV-2 variants and considerations of inferring causality on disease severity. Lancet Infect Dis. 2021;21(11):1472–1474. doi: 10.1016/S1473-3099(21)00338-8. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui KPY, Ho JCW, Cheung M, et al. SARS-CoV-2 omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–720. doi: 10.1038/s41586-022-04479-6. doi: [DOI] [PubMed] [Google Scholar]
- 45.Ślusarczyk A, Tracz A, Gronkiewicz M, Jureczko L, Bączkowska T. Outcomes of COVID-19 in hospitalized kidney and liver transplant recipients: a single-center experience. Polish Arch Intern Med. 2021;131(11):16070. doi: 10.20452/pamw.16070. doi: [DOI] [PubMed] [Google Scholar]
- 46.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021;35(1):100588. doi: 10.1016/j.trre.2020.100588. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinson AJ, Dai R, Agarwal G, et al. Sex and organ-specific risk of major adverse renal or cardiac events in solid organ transplant recipients with COVID-19. Am J Transplant. 2021;22:1–15. doi: 10.1111/AJT.16865. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2021;72(9):e206–e214. doi: 10.1093/cid/ciaa1012. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 2021;12(1):1–12. doi: 10.1038/s41467-021-22958-8. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 omicron sublineages. Nat. 2022 doi: 10.1038/s41586-022-04594-4. Published online March 3, 2022:1–1. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.