Abstract
The COVID‐19 pandemic has influenced organ transplantation decision making. Opinions regarding the utilization of coronavirus disease‐2019 (COVID‐19) donors are mixed. We hypothesize that COVID‐19 infection of deceased solid organ transplant donors does not affect recipient survival. All deceased solid organ transplant donors with COVID‐19 testing results from March 15, 2020 to September 30, 2021 were identified in the OPTN database. Donors were matched to recipients and stratified by the COVID‐19 test result. Outcomes were assessed between groups. COVID‐19 test results were available for 17 694 donors; 150 were positive. A total of 269 organs were transplanted from these donors, including 187 kidneys, 57 livers, 18 hearts, 5 kidney‐pancreases, and 2 lungs. The median time from COVID‐19 testing to organ recovery was 4 days for positive and 3 days for negative donors. Of these, there were 8 graft failures (3.0%) and 5 deaths (1.9%). Survival of patients receiving grafts from COVID‐19‐positive donors is equivalent to those receiving grafts from COVID‐19‐negative donors (30‐day patient survival = 99.2% COVID‐19 positive; 98.6% COVID‐19 negative). Solid organ transplantation using deceased donors with positive COVID‐19 results does not negatively affect early patient survival, though little information regarding donor COVID‐19 organ involvement is known. While transplantation is feasible, more information regarding COVID‐19‐positive donor selection is needed.
Keywords: clinical research/practice, donors and donation: deceased, infection and infectious agents—viral: SARS‐CoV‐2/COVID‐19, infectious disease, patient survival, solid organ transplantation, United Network for Organ Sharing (UNOS)
Abbreviations
- DCD
donation after cardiac death
- IQR
interquartile range
- NAT
nucleic acid test
- OPO
Organ Procurement Organization
- OPTN
Organ Procurement and Transplant Network
- UNOS
United Network for Organ Sharing
- U.S.
United States
1. INTRODUCTION
The COVID‐19 pandemic has influenced decision making related to solid organ transplantation in the United States since early 2020. The first case of coronavirus disease‐2019 (COVID‐19) infection in the United States (U.S.) occurred on January 21, 2020 in the state of Washington. This was followed by the declaration of a state of national emergency in the U.S. on March 13, 2020 (Figure 1). Over the following days, guidance was provided to transplant centers and organ procurement organizations (OPO) regarding the cessation of transplant activities in the U.S. 1 , 2 , 3 , 4 , 5 These initial recommendations led to a temporary decrease in overall waitlist and transplant activities, which returned to baseline within 2–3 months of the start of the pandemic. 5 , 6 , 7 Since this time, the use of organs from deceased donors with active or a history of COVID‐19 infection has been in debate. Several reports recommend against the utilization of COVID‐19‐infected organ donors at the time of their publication owing to several reasons. 8 , 9 , 10 These factors include the risk of blood or allograft tissue transmission, damage to donor organs, lack of effective therapies, exposure of healthcare workers and recovery teams, disease transmission and propagation, and hospital resource utilization.
FIGURE 1.
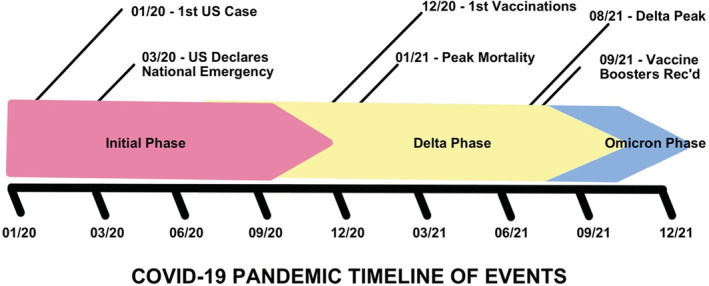
Coronavirus disease‐2019 (COVID‐19) pandemic timeline of events. Rec'd, Recommended
Despite these early recommendations, reports of organ transplantation using donors known to be actively infected with or recovered from COVID‐19 infection began to emerge. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Initial reports came from donors who had previously been infected with COVID‐19 but had subsequently recovered. Later cases included those with lower‐risk donors and recipients who either had a personal history of COVID‐19 infection in the past and/or had antibodies to COVID‐19 present. Most of these studies report good early outcomes after transplantation, except for one lung transplant where the recipient and transplanting surgeon developed COVID‐19 after transplantation. 14 The COVID‐19 strain was confirmed to be the same for all three individuals. The recipient died on post‐transplant day 61. Subsequently, the Organ Procurement and Transplant Network (OPTN) required all OPOs to perform lower respiratory tract testing for COVID‐19 on all potential deceased lung transplant donors. 29 This has been the only reported fatality of a transplant recipient due to transmission of COVID‐19 from a donor.
With this information, several authors have begun recommending the acceptance of low‐risk donors with positive COVID‐19 testing for recipients who would benefit from transplantation and have high expected waitlist mortality. 28 , 32 Risk factors identifying low‐risk donors include a longer time since first positive COVID‐19 test or symptoms, mild severity of disease, reassuring imaging findings, a cycle threshold >35, no recent (<14 days) exposures to individuals with COVID‐19, and no history of acute respiratory distress syndrome or thrombosis. Additionally, the OPTN has provided updated recommendations regarding the acceptance of organs from deceased donors with positive testing or a history of COVID‐19. 29 They state that the risk of transmission of the virus from donors with a history of COVID‐19 greater than 21 days prior to organ recovery is low but recommend caution in accepting these organs. Donors with mild symptoms and 10–21 days since the start of illness or positive test have an unknown safety risk and acceptance of organs should depend on the medical urgency of the candidate, along with several other factors. No recommendations are given for donors diagnosed with active COVID‐19 infection or within 10 days of organ recovery.
Despite these reports and recommendations, there have been few large series of COVID‐19‐positive donors with a very short duration of follow‐up in all cases. 16 , 30 , 31 The OPTN database now contains COVID‐19 test results for all potential donors, since the start of the pandemic, allowing for a larger scale evaluation of outcomes in this population. We hypothesize that the recent COVID‐19 infection of deceased solid organ transplant donors does not negatively affect patient or graft survival.
2. MATERIALS AND METHODS
2.1. Study design
We conducted a retrospective cohort study of the OPTN database, based on data as of September 30, 2021 (received December 27, 2021). The OPTN database includes demographic and clinical data on all waitlisted candidates and donors in the U.S. as submitted by the transplant centers. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN contractor, the United Network for Organ Sharing (UNOS). The study was exempt from IRB approval, as the OPTN database contains no patient identifiers. This study is compliant with the ISHLT's Ethic's statement.
2.2. Patient population
All potential transplant donors with a recovery date between March 15, 2020 and September 30, 2021 (18.5 months) were identified in the deceased donor dataset. Only donors with at least one organ recovered are included in this dataset. Only donors with COVID‐19 nucleic acid test (NAT) results were included in the analysis. Only one COVID‐19 NAT test result was included in the dataset. The timing of this test result, whether it was the first, most recent, or at donor evaluation, is not provided in the dataset. COVID‐19 antigen test results were also available in the dataset but were present for only 2% of donors. These donors all had NAT testing results present; therefore, antigen test results were not included in this analysis. All solid organ transplant recipients with a transplant date between March 15, 2020 and September 30, 2021 were identified in their respective datasets (thoracic, kidney‐pancreas, liver, intestine). The transplant recipient datasets were merged and only variables contained in all datasets were included. The deceased donor dataset and combined recipient datasets were merged, based on donor ID. Donors and recipients without a donor‐recipient pair match were excluded from the analysis. Donor‐recipient pairs were stratified by COVID‐19 NAT result for further analysis. Donor‐recipient pairs with positive COVID‐19 NAT results were further stratified by organ transplanted. Analyses were made on a per graft, not patient, basis, given that some patients received dual organ transplants. For example, patients receiving a combined heart‐kidney transplant were included as both heart and kidney transplants, separately.
The primary study cohort included all donor‐recipient pairs with positive donor COVID‐19 NAT results. The comparison cohort included all donor‐recipient pairs with negative donor COVID NAT results.
2.3. Statistical analysis
Baseline characteristics were compared between groups using summary statistics (median and interquartile range [IQR], number, and percentage). The chi‐square test was used for categorical variables and the Kruskal‐Wallis test was used for continuous variables. The primary study endpoint was patient death across all organs. Secondary study endpoints included patient death stratified by organ transplanted and graft failure across all organs and stratified by organ transplanted. Short‐term patient and graft survival differences between the groups were assessed via the Kaplan–Meier method and compared using the log‐rank test. Univariate relationships between patient and graft survival and several donor‐recipient characteristics were assessed using the Cox proportional hazards model. Variables with a univariate p‐value less than .10 with few (<10%) missing data values were considered for inclusion in multivariate analyses. Type of organ transplanted and COVID‐19 NAT results were included in all models. The Cox proportional hazards model was used to assess independently significant risk factors for patient survival. All graft failures and deaths in the study cohort were reviewed independently. A p‐value of less than .05 was considered significant in this study. R: A Language and Environment for Statistical Computing 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria, 2020) was used to perform statistical calculations.
3. RESULTS
3.1. COVID‐19 testing of donors over time
COVID‐19 testing via NAT was available for 17 694 deceased donors and has steadily increased over time since March 15, 2020 (Figure 2).
FIGURE 2.
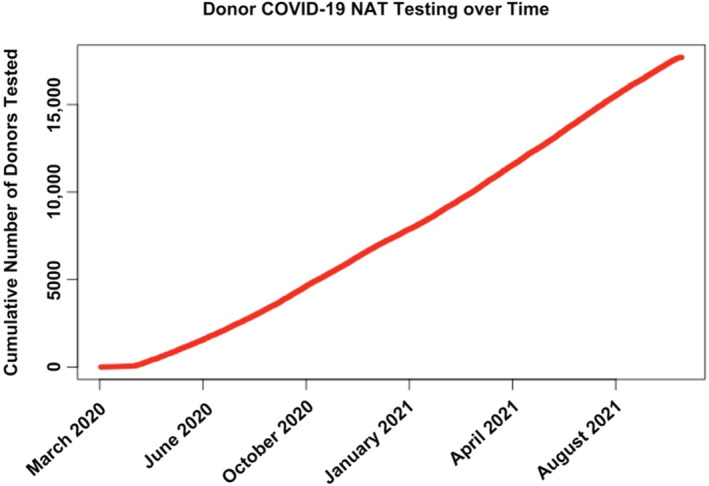
Cumulative COVID‐19 NAT testing of potential transplant donors in the U.S. between March 15, 2020 and September 30, 2021. NAT, nucleic acid test
3.2. Cohort stratification
Sixty‐one‐thousand nine‐hundred‐eighteen transplant recipients across all solid organs between March 15, 2020 and September 30, 2021 were identified (Figure 3). Of these, 44 819 recipients had a matched deceased donor with COVID‐19 NAT results. Additionally, 20 302 potential transplant donors were identified over the same period in the deceased donor dataset (Figure 4). Of these, 17,694 donors (87%) had COVID‐19 NAT results available. There were 150 donors (<1%) with positive COVID‐19 NAT results, 17 167 donors with negative COVID‐19 NAT results, and 377 donors with indeterminate or pending COVID‐19 NAT results.
FIGURE 3.
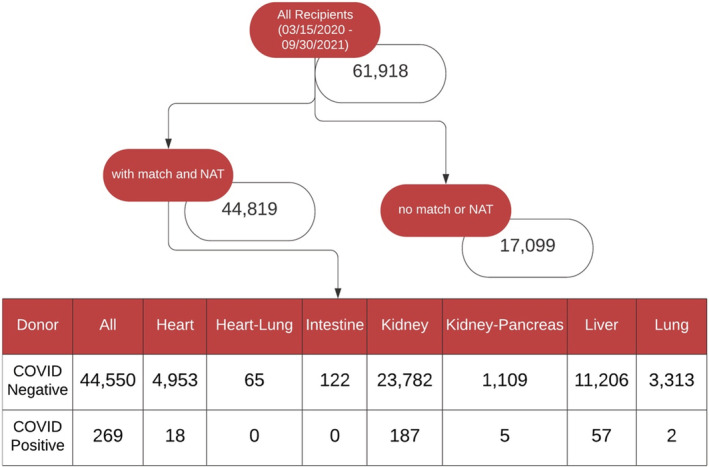
Stratification of all transplant recipients in the U.S. between March 15, 2020 and September 30, 2021. NAT, nucleic acid test
FIGURE 4.
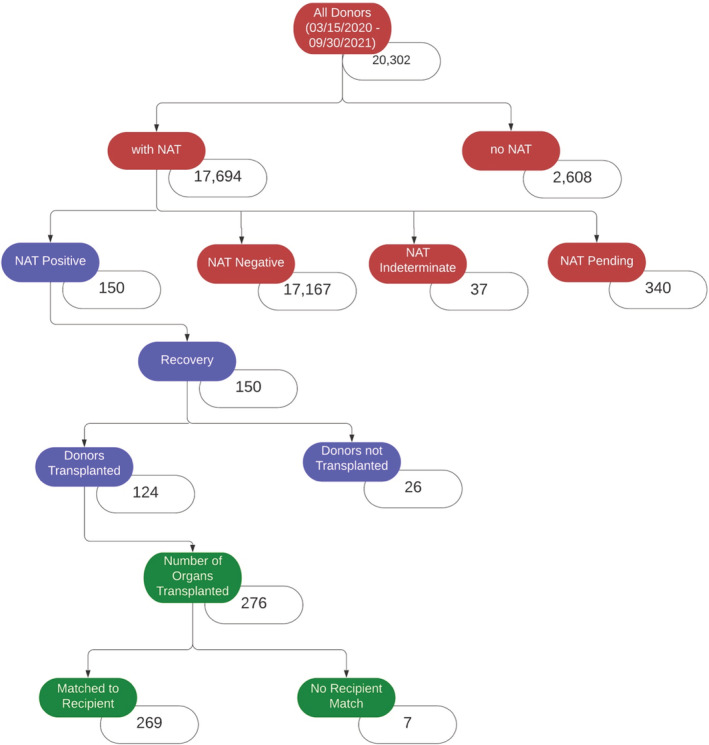
Stratification of all potential transplant donors and organs transplanted from COVID‐19 NAT‐positive donors in the U.S. between March 15, 2020 and September 30, 2021. NAT, nucleic acid test
All 150 potential deceased donors with positive COVID‐19 NAT results underwent recovery of at least one solid organ. Of these, 124 donors had at least one organ transplanted. Two‐hundred‐seventy‐six organs were transplanted from these 124 donors (2.2 organs transplanted per donor). Of these 276 transplanted organs, 269 organs were matched to recipients in the appropriate recipient datasets and comprised the study cohort. Of the 269 matched organs with COVID‐19 NAT‐positive donors, 187 kidneys, 57 livers, 18 hearts, 5 kidney‐pancreases, and 2 lungs were transplanted.
There were four COVID‐19 NAT‐positive‐pediatric deceased donors (0, 6, 14, and 16 years old) from which nine organs (7 kidneys, 2 livers) were transplanted, all into adult recipients. There were three pediatric recipients (8, 10, and 17 years old) who received organs (2 kidneys, 1 heart) from COVID‐19 NAT‐positive donors, all from adult deceased donors.
From the 17 167 donors with negative COVID‐19 NAT results and recipient matches, 44,550 total organs were transplanted and comprised the comparison cohort. Of the 44,550 matched organs with COVID‐19 NAT‐negative donors, 23,782 kidneys, 11,206 livers, 4953 hearts, 1109 kidney‐pancreases, 3313 lungs, 122 intestines, and 65 heart‐lungs were transplanted.
3.3. Timing of transplants using COVID‐19 NAT‐positive donors over time
The first organ transplanted with a COVID‐19 NAT positive deceased donor by organ was: liver (July 22, 2020), heart (August 5, 2020), kidney (August 5, 2020), kidney‐pancreas (April 21, 2021), and lung (June 15, 2021). Transplantation of deceased donors with a positive COVID‐19 NAT has been increasing over time (Figure 5). There have been no reported heart‐lung or intestinal transplants with COVID‐19 NAT‐positive donors as of September 30, 2021. The median time from COVID‐19 NAT testing to organ recovery was 4 days (IQR 2–23) for COVID‐19 NAT‐positive donors and 3 days (IQR 2–5) for COVID‐19 NAT‐negative donors.
FIGURE 5.
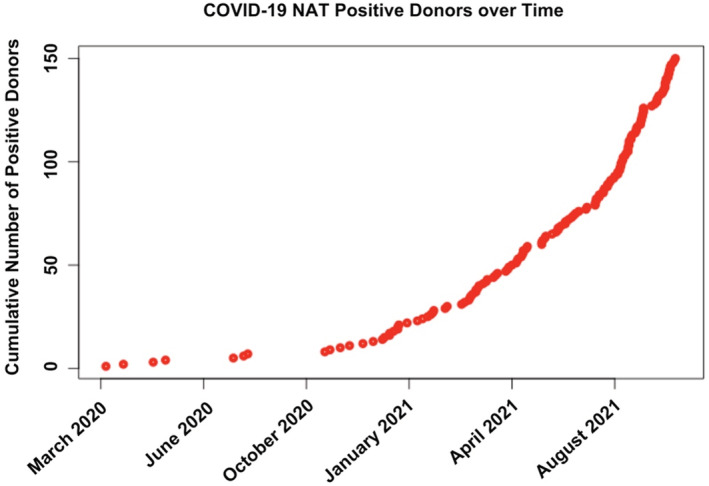
Cumulative number of donors with positive COVID‐19 NAT testing in the U.S. between March 15, 2020 and September 30, 2021. NAT, nucleic acid test
3.4. COVID‐19 NAT‐positive donor demographic and clinical covariates
The median age of COVID‐19 NAT‐positive deceased donors was 38 years (IQR 28–50) and was not significantly different than the age of other donors (p‐value = .309). There were more transplants with male COVID‐19 NAT‐positive donors (72.1% vs. 64%, p = .007). There were relatively more transplants with COVID‐19 NAT‐positive Hispanic donors (22.3% vs. 15.4%) and fewer from white donors (56.1% vs. 64.7%) (p‐value = .007), compared to transplants with COVID‐19 NAT‐negative donors. COVID‐19 NAT‐positive donors were larger (weight 87.5 kg vs. 80 kg, p‐value < .001) (height 175 cm vs. 172.7 cm, p‐value <.001) (BMI 29.4 vs. 26.87, p‐value <.001) than their COVID‐19 NAT‐negative counterparts. There were fewer transplants with COVID‐19 NAT‐positive donors with anoxic cause of death (33.8% vs. 46.2%, p‐value < .001) and they had a lower incidence of downtime (23% vs. 45.4%, p‐value < .001) and cardiopulmonary resuscitation (33.5% vs. 55.6%, p‐value < .001). There were no differences in Kidney Donor Profile Index (KDPI) (40% vs. 40%, p‐value .130) or left ventricular ejection fraction (LVEF) (60% vs. 60%, p‐value .264) between COVID‐19 NAT‐positive and ‐negative donors. Differences in other donor characteristics are described in Table 1. Additionally, demographic and clinical covariate differences between organs for donor‐recipient pairs of COVID‐19 NAT‐positive deceased donors are listed in Table S1.
TABLE 1.
Demographic and clinical covariates of all transplants with COVID‐19 NAT results between March 15, 2020 and September 30, 2021, stratified by COVID‐19 NAT status
| Descriptive statistics | COVID‐19 NAT positive | COVID‐19 NAT negative | |
|---|---|---|---|
| Categorical variable | # (%) | # (%) | p‐value* |
| n | 269 (0.60%) | 44 550 (99.40%) | |
| Organ | |||
| Heart | 18 (6.70%) | 4953 (11.10%) | <.001 |
| Kidney | 187 (69.50%) | 23 782 (53.40%) | |
| Kidney‐pancreas | 5 (1.90%) | 1109 (2.50%) | |
| Liver | 57 (21.20%) | 11 206 (25.20%) | |
| Lung | 2 (0.70%) | 3313 (7.40%) | |
| COVID‐19 NAT location (onor) | |||
| Blood | 0 (0.00%) | 67 (0.20%) | .001 |
| Lower respiratory | 25 (9.30%) | 8152 (18.30%) | |
| Upper respiratory | 241 (89.60%) | 36 060 (80.90%) | |
| Other** | 3 (1.10%) | 271 (0.60%) | |
| DCD | |||
| Yes | 96 (35.70%) | 8351 (18.70%) | <.001 |
| Race (Donor) | |||
| White | 151 (56.10%) | 28 811 (64.70%) | .007 |
| Black | 51 (19.00%) | 7101 (15.90%) | |
| Hispanic | 60 (22.30%) | 6841 (15.40%) | |
| Asian | 2 (0.70%) | 1118 (2.50%) | |
| American Indian/Alaskan | 3 (1.10%) | 305 (0.70%) | |
| Hawaiian/Pacific Islander | 0 (0.00%) | 114 (0.30%) | |
| Multiracial | 2 (0.70%) | 260 (0.60%) | |
| Gender (Donor) | |||
| Male | 194 (72.10%) | 28 503 (64.00%) | .007 |
| Cause of death (Donor) | |||
| Anoxia | 91 (33.80%) | 20 568 (46.20%) | <.001 |
| Cerebrovascular/Stroke | 56 (20.80%) | 9109 (20.40%) | |
| Head trauma | 82 (30.50%) | 13 654 (30.60%) | |
| CNS tumor | 0 (0.00%) | 137 (0.30%) | |
| Other | 20 (7.40%) | 1078 (2.40%) | |
| COVID‐19 infection | 20 (7.40%) | 4 (0.00%) | |
| Downtime (Donor) | |||
| Yes | 62 (23.00%) | 20 096 (45.40%) | <.001 |
| CPR (Donor) | |||
| Yes | 90 (33.50%) | 24 356 (55.60%) | <.001 |
| Age group (Recipient) | |||
| Pediatric | 3 (1.10%) | 2038 (4.60%) | .010 |
| Race (Recipient) | |||
| White | 153 (56.90%) | 22 593 (50.70%) | .504 |
| Black | 59 (21.90%) | 10 616 (23.80%) | |
| Hispanic | 40 (14.90%) | 7655 (17.20%) | |
| Asian | 14 (5.20%) | 2678 (6.00%) | |
| American Indian/Alaskan | 1 (0.40%) | 373 (0.80%) | |
| Hawaiian/Pacific Islander | 1 (0.40%) | 185 (0.40%) | |
| Multiracial | 1 (0.40%) | 450 (1.00%) | |
| Gender (Recipient) | |||
| Male | 173 (64.30%) | 27 766 (62.30%) | .544 |
| Graft status | |||
| Failed | 8 (3.00%) | 2652 (6.00%) | .053 |
| Recipient status | |||
| Died | 5 (1.90%) | 2058 (4.60%) | .045 |
| Continuous variable | Median (IQR) | Median (IQR) | p‐value*** |
|---|---|---|---|
| COVID‐19 NAT to recovery (days) | 4 (2–23) | 3 (2–5) | <.001 |
| Age (years) (Donor) | 38 (28–50) | 37 (26–49) | .309 |
| Age (years) (Recipient) | 54 (43–63) | 55 (43–64) | .433 |
| Graft time since transplant (days) | 83 (31–164) | 220 (106–359) | <.001 |
| Recipient time since transplant (days) | 84 (32–164) | 224 (111–361) | <.001 |
| KDPI (%) | 40 (16–57) | 40 (18–64) | .130 |
| LV ejection fraction (%) | 60 (55–65) | 60 (55–65) | .264 |
Abbreviations: CNS, central nervous system; CPR, cardiopulmonary resuscitation; DCD, donation after cardiac death; KDPI, Kidney Donor Profile Index; LV, left ventricle; NAT, nucleic acid test.
Chi‐square test.
Other = location not indicated.
Kruskal‐Wallis test.
3.5. Demographic and clinical covariates of recipients with COVID‐19 NAT‐positive donors
The median age at transplantation for recipients receiving organs from COVID‐19 NAT‐positive deceased donors was 54 years (IQR 43–63) and was not significantly different than the age of other recipients (p‐value = .433). There were fewer pediatric transplants (1.1% vs. 4.6%, p‐value = .010) and more donations after cardiac death (DCD) (35.7% vs. 18.7%, p‐value < .001) with COVID‐19 NAT‐positive donors. Recipients receiving organs from COVID‐19 NAT‐positive donors weighed more (83.2 kg vs. 80.4 kg, p‐value = .041) than those with COVID‐19 NAT‐negative donors. Differences in other recipient characteristics are described in Table 1 and Table S1.
3.6. Patient and graft survival after transplantation of COVID‐19 NAT‐positive donors
Actuarial survival of patients receiving grafts from deceased donors with positive COVID‐19 NAT is no different than those receiving grafts from donors with negative COVID‐19 NAT (Figure 6, log‐rank test p‐value = .34). The 30‐day post‐transplant patient survival was similar between groups (99.2% survival with COVID‐19 NAT‐positive donor: 98.6% survival with COVID‐19 NAT‐negative donor). Additionally, when stratified by organ transplanted, no differences in actuarial patient survival were found (Figure 7; Figure S1, all p‐values > .32). Actuarial patient survival estimates over the first‐year post‐transplant for all organs are provided in Table 2.
FIGURE 6.
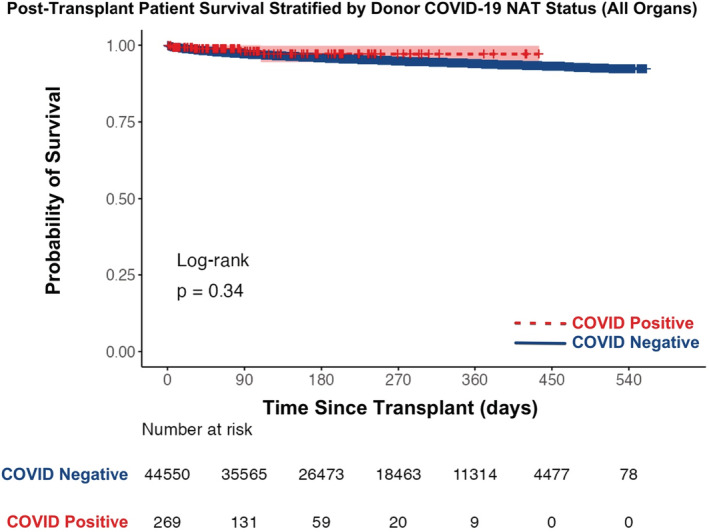
Actuarial post‐transplant patient survival stratified by donor COVID‐19 NAT result between March 15, 2020 and September 30, 2021 (all organs). NAT, nucleic acid test
FIGURE 7.
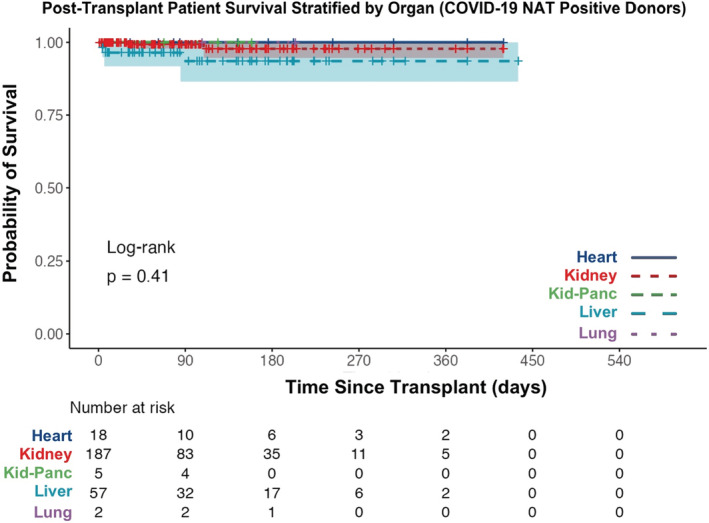
Actuarial post‐transplant patient survival stratified by organ transplanted between March 15, 2020 and September 30, 2021 (COVID‐19 NAT‐positive donors only). NAT, nucleic acid test
TABLE 2.
Actuarial patient survival estimates stratified by organ and donor COVID status
| COVID positive | n | 30‐day | 90‐day | 180‐day | 270‐day | 365‐day |
|---|---|---|---|---|---|---|
| Heart | 18 | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
| Kidney | 187 | 100.0% | 99.3% | 97.8% | 97.8% | 97.8% |
| Kidney‐pancreas | 5 | 100.0% | 100.0% | NA | NA | NA |
| Liver | 57 | 96.5% | 93.5% | 93.5% | 93.5% | 93.5% |
| Lung | 2 | 100.0% | 100.0% | 100.0% | NA | NA |
| All | 269 | 99.2% | 98.0% | 97.1% | 97.1% | 97.1% |
| COVID negative | n | 30‐day | 90‐day | 180‐day | 270‐day | 365‐day |
|---|---|---|---|---|---|---|
| Heart | 4953 | 97.2% | 95.0% | 93.2% | 92.5% | 91.3% |
| Kidney | 23 782 | 99.3% | 98.2% | 97.1% | 96.0% | 95.3% |
| Kidney‐pancreas | 1109 | 99.0% | 98.1% | 97.6% | 96.9% | 96.3% |
| Liver | 11 206 | 97.9% | 96.6% | 95.4% | 94.5% | 93.8% |
| Lung | 3313 | 97.6% | 95.3% | 93.1% | 91.0% | 89.1% |
| All | 44 550 | 98.6% | 97.2% | 95.9% | 94.9% | 94.0% |
Actuarial graft survival from deceased donors with positive COVID‐19 NAT is no different than grafts from donors with negative COVID‐19 NAT (Figure 8, log‐rank test p‐value = .43). The 30‐day post‐transplant graft survival was similar between groups (98.5% survival with COVID‐19 NAT‐positive donor: 97.7% survival with COVID‐19 NAT‐negative donor). Additionally, when stratified by organ transplanted, no differences in actuarial graft survival were found (Figure 9 and Figure S2, all p‐values > .30). Actuarial graft survival estimates over the first‐year post‐transplant for all organs are provided in Table 3.
FIGURE 8.
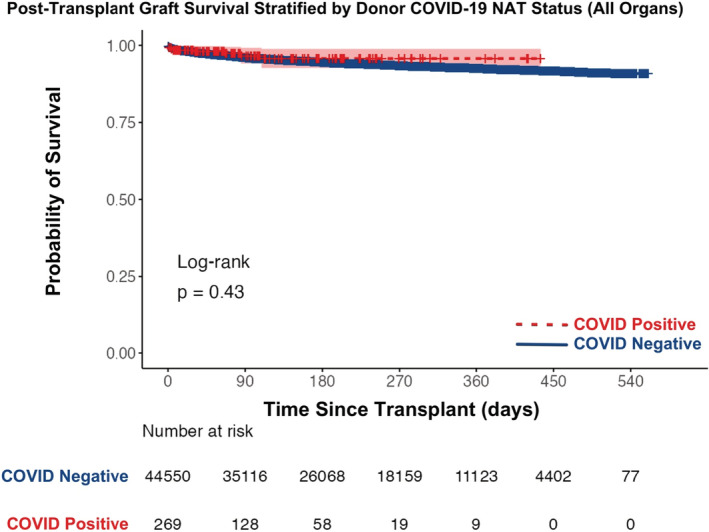
Actuarial post‐transplant graft survival stratified by donor COVID‐19 NAT result between March 15, 2020 and September 30, 2021 (all organs). NAT, nucleic acid test
FIGURE 9.
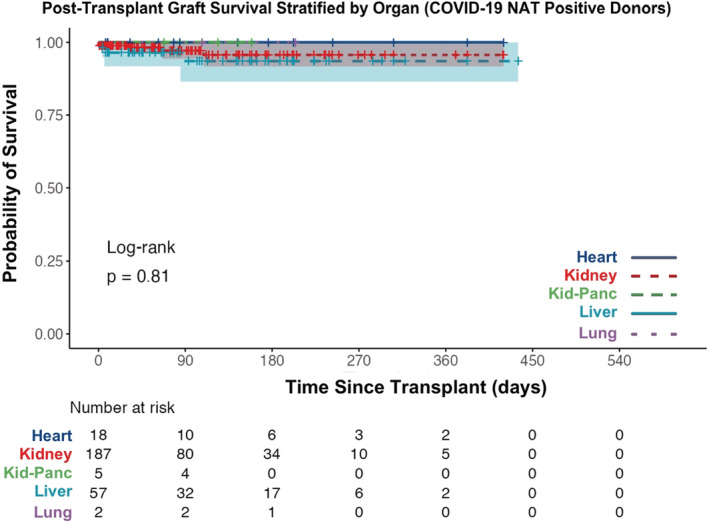
Actuarial post‐transplant graft survival stratified by organ transplanted between March 15, 2020 and September 30, 2021 (COVID‐19 NAT‐positive donors only). NAT, nucleic acid test
TABLE 3.
Actuarial graft survival estimates stratified by organ and donor COVID status
| COVID positive | n | 30‐day | 90‐day | 180‐day | 270‐day | 365‐day |
|---|---|---|---|---|---|---|
| Heart | 18 | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
| Kidney | 187 | 98.9% | 97.2% | 95.7% | 95.7% | 95.7% |
| Kidney‐pancreas | 5 | 100.0% | 100.0% | NA | NA | NA |
| Liver | 57 | 96.5% | 93.5% | 93.5% | 93.5% | 93.5% |
| Lung | 2 | 100.0% | 100.0% | 100.0% | NA | NA |
| All | 269 | 98.5% | 96.6% | 95.7% | 95.7% | 95.7% |
| COVID negative | n | 30‐day | 90‐day | 180‐day | 270‐day | 365‐day |
|---|---|---|---|---|---|---|
| Heart | 4953 | 96.8% | 94.7% | 92.9% | 92.1% | 90.9% |
| Kidney | 23 782 | 98.4% | 96.7% | 95.4% | 94.3% | 93.4% |
| Kidney‐pancreas | 1109 | 98.5% | 97.5% | 97.0% | 96.2% | 95.9% |
| Liver | 11 206 | 96.8% | 95.2% | 93.7% | 92.7% | 92.0% |
| Lung | 3313 | 97.6% | 95.2% | 92.9% | 90.9% | 88.8% |
| All | 44 550 | 97.7% | 96.0% | 94.5% | 93.4% | 92.5% |
In the multivariate regression analysis, a positive COVID‐19 NAT result was not associated with lower patient survival (HR 0.78, 95% CI 0.32–1.87, p‐value = .575). Organ transplanted, race, donor death mechanism, donor race, donor distance, recipient age, and recipient BMI were independently associated with lower patient survival (Table 4).
TABLE 4.
Multivariate Cox proportional hazard model evaluating risk factors for patient survival
| Multivariate Cox proportional hazard model | n = 43 108; LRT = 608.9; p = <.0001 | |
|---|---|---|
| Variable (reference) | HR (95% CI) | p‐value |
| Organ | ||
| Heart (Reference) | xxx | xxx |
| Heart‐lung | 2.63 (1.35–5.11) | .004*** |
| Intestine | 3.19 (1.93–5.27) | <.001*** |
| Kidney | 0.43 (0.37–0.50) | <.001*** |
| Kidney‐pancreas | 0.46 (0.31–0.66) | <.001*** |
| Liver | 0.63 (0.55–0.73) | <.001*** |
| Lung | 1.03 (0.87–1.21) | .757 |
| COVID‐19 NAT result (Donor)‐ Positive | 0.78 (0.32–1.87) | .575 |
| DCD | ||
| Yes | 1.07 (0.94–1.22) | .314 |
| Race (Recipient) | ||
| White (Reference) | xxx | xxx |
| Black | 1.02 (0.90–1.15) | .781 |
| Hispanic | 1.13 (0.99–1.30) | .071 |
| Asian | 1.29 (1.06–1.57) | .011*** |
| American Indian/Alaskan | 1.78 (1.16–2.72) | .008*** |
| Hawaiian/Pacific Islander | 1.24 (0.59–2.63) | .568 |
| Multiracial | 1.16 (0.72–1.84) | .546 |
| Cause of death (Donor) | ||
| Anoxia (Reference) | xxx | xxx |
| Cerebrovascular/Stroke | 1.21 (0.81–1.81) | .343 |
| Head trauma | 0.89 (0.62–1.28) | .539 |
| CNS tumor | 1.43 (0.74–2.75) | .290 |
| Other | 0.70 (0.49–1.00) | .050 |
| COVID‐19 infection | 0.00 (0.00‐Inf) | .988 |
| Death circumstances (Donor) | ||
| Motor vehicle accident (Reference) | xxx | xxx |
| Suicide | 0.84 (0.62–1.13) | .244 |
| Homicide | 0.87 (0.62–1.22) | .415 |
| Child abuse | 1.52 (0.73–3.14) | .264 |
| Accident (Non‐MVA) | 0.92 (0.74–1.14) | .430 |
| Natural causes | 1.01 (0.76–1.33) | .968 |
| None of above | 0.92 (0.71–1.20) | .537 |
| Death mechanism (Donor) | ||
| Intracranial hemorrhage/Stroke (Reference) | xxx | xxx |
| Drowning | 2.00 (1.16–3.44) | .012*** |
| Seizure | 1.03 (0.57–1.84) | .934 |
| Drug intoxication | 1.18 (0.78–1.79) | .444 |
| Asphyxiation | 1.14 (0.71–1.83) | .587 |
| Cardiovascular | 1.10 (0.73–1.66) | .650 |
| Electrical | 1.73 (0.41–7.35) | .458 |
| Gunshot | 1.23 (0.76–1.97) | .405 |
| Stabbing | 0.99 (0.34–2.84) | .979 |
| Blunt Injury | 1.19 (0.78–1.82) | .422 |
| SIDS | 0.00 (0.00‐Inf) | .980 |
| Natural causes | 1.27 (0.84–1.91) | .251 |
| None of above | 1.45 (0.91–2.32) | .119 |
| Inotropes (Donor) | ||
| Yes | 1.05 (0.96–1.16) | .287 |
| Race (Donor) | ||
| White (Reference) | xxx | xxx |
| Black | 1.11 (0.98–1.25) | .090 |
| Hispanic | 1.05 (0.92–1.20) | .479 |
| Asian | 1.34 (1.04–1.74) | .023*** |
| American Indian/Alaskan | 0.82 (0.44–1.54) | .541 |
| Hawaiian/Pacific Islander | 1.74 (0.77–3.90) | .182 |
| Multiracial | 0.84 (0.40–1.78) | .653 |
| Distance to donor (miles) | 1.00 (1.00–1.00) | .022*** |
| Age (years) (Recipient) | 1.02 (1.02–1.03) | <.001*** |
| BMI (kg/m2) (Recipient) | 1.01 (1.01–1.02) | .001*** |
| Time on waitlist (days) | 1.00 (1.00–1.00) | .610 |
| Age (years) (Donor) | 1.00 (0.99–1.00) | .382 |
Note: The primary study outcome variable (COVID‐19 NAT Result (Donor) ‐ Positive) is bolded.
Abbreviations: BMI, body mass index; CI, confidence interval; CNS, central nervous system; DCD, donation after cardiac death; HR, hazard ratio; LRT, likelihood ratio test; MVA, motor vehicle accident; NAT, nucleic acid test; SIDS, sudden infant death syndrome.
Statistically significant results (p < .05).
3.7. Graft failure and death after transplantation of COVID‐19 NAT‐positive donors
Of the 269 transplanted grafts, there were eight graft failures (3.0%) and five deaths (1.9%). There were no pediatric recipient graft losses or deaths. There were three deaths in the liver transplant group, two deaths in the kidney transplant group, and three other graft losses in the kidney transplant group. There were no graft losses or deaths in the heart, kidney‐pancreas, or lung transplant groups, though numbers at risk were small. The median time to death was 31‐days post‐transplant and graft loss was 18.5‐days post‐transplant.
The three deaths after liver transplant were due to: (1) hepatic artery thrombosis at 6 days post‐transplant, (2) generalized sepsis at 85 days post‐transplant, and (3) unknown at 4 days post‐transplant. The two deaths after kidney transplant were due to: (1) respiratory failure at 109 days post‐transplant and (2) unknown at 31 days post‐transplant. The death from respiratory failure occurred in a patient who received a graft from a pediatric donor. The three additional graft losses in the kidney transplant group were due to: (1 & 2) graft thromboses at 90 and 297 days post‐transplant and (3) recurrent disease at 124 days post‐transplant. All organs for recipients with graft loss or death came from different donors. A total of 22 organs (16 kidneys, 4 livers, 2 hearts) were transplanted from these donors and no other graft failures or deaths occurred for other recipients of these organs.
4. DISCUSSION
We present the largest cohort of patients undergoing solid organ transplantation using deceased donors with positive COVID‐19 NAT results thus far presented in the literature. This study not only includes the largest number of recipients but also has the longest follow‐up of any study to date. The median time since transplant for the study cohort was 83 days (IQR 31–164). We provide outcome data for 269 grafts transplanted from 124 deceased donors. Our data shows that overall patient and graft survival for those receiving organs from COVID‐19‐positive deceased donors is equivalent to the survival of those receiving organs from COVID‐19‐negative donors. Additionally, we found that COVID‐19 NAT‐positive donors were more likely to be male, Hispanic, and larger than their COVID‐19 NAT‐negative counterparts, which mimics the general trends seen in the pandemic to date. 33 , 34 , 35
Testing of solid organ transplant donors for COVID‐19 was rapidly initiated at the onset of the pandemic, as can be seen in Figure 2. Eighty‐seven percent of all deceased donors between March 15, 2020 and September 30, 2021 had COVID‐19 testing results available in the database for analysis. Of these, less than 1% (150 donors) were found to have positive COVID‐19 NAT testing prior to donation. Only donors proceeding to the procurement of at least one organ are included in the dataset and it is not known how many other potential COVID‐19 NAT‐positive donors were declined during this time. Figure 5 shows the cumulative incidence of COVID‐19 NAT‐positive deceased donors over time and a trend toward increasing prevalence can be seen over time. This likely represents an increasing comfort of transplant centers to use these donors. Early in the pandemic, little was known about the potential effects of COVID‐19 infection on immunosuppressed individuals. Over time and with the availability of new therapies, comfort grew and utilization increased. Given that the follow‐up time for this study ended on September 30, 2021 at the peak of the delta variant phase of the COVID‐19 pandemic, it should be expected that even greater numbers of donors, many asymptomatic, will likely be found to be positive as follow‐up time extends into the omicron variant phase of the COVID‐19 pandemic and beyond (Figure 1). Additionally, as time passes the number of donors with a history of recovered COVID‐19 infection will continue to increase. Taking these two factors together, it is likely that many future solid organ transplant donors will either have a current or have had a recent past infection with COVID‐19. If the field of transplantation is to continue to flourish in this setting, understanding the risks and outcomes of using these donors will become more urgent over time. This study shows that transplantation in the setting of a positive COVID‐19 NAT result is not only possible but can achieve similar outcomes to those transplanted with organs from COVID‐19 NAT‐negative donors.
At the beginning of the pandemic, little was known about disease transmission and effects on organs for transplant. Additionally, there were no therapeutics known to be effective in preventing or treating disease. Third, variant shifts over time have led to a current variant (omicron) that is readily transmissible through asymptomatic carriers and has lower virulence. 36 After 2 years of the pandemic, we have learned a great deal of information about these unknowns. Information regarding changes to the transmission through hygiene, masking, and social distancing are being sought and potential effects on organs, such as myocarditis, are being studied. 37 Multiple antiviral agents have been studied for use in treating and preventing COVID‐19 and pharmaceuticals and monoclonal antibody use are now more common. Finally, the robust vaccination and booster programs have allowed for nearly all persons in the U.S. to become vaccinated if they choose. This vaccination has been shown to reduce some of the adverse risks of contracting the virus, such as ICU admission and the need for mechanical ventilation, though they may not prevent infection all together. 38 , 39 The combination of changing, less virulent strains of the virus, appropriate pre‐transplant vaccination, pre‐exposure prophylactics, and post‐exposure therapeutics, has likely led and will likely continue to lead to increasing comfort with the idea of accepting donors for transplantation, even in the setting of active COVID‐19 infection.
Our data show that not only is the transplantation of these organs possible and increasing in frequency, but the outcomes of these transplants are at least equal to those of COVID‐19 NAT‐negative donor transplants. Our data is robust for outcomes of kidney (n = 187 + 5 kidney‐pancreas) and liver (n = 57) transplants, but there is a paucity of data for heart (n = 18) and lung (n = 2) transplants and no data for intestinal (n = 0) transplants. Data to support the use of COVID‐19‐positive deceased donors in children is lacking currently as well. The current study included only four pediatric donors and three pediatric recipients.
The impact of COVID‐19 infection on these donors must be considered as well. While these donors may have had a positive COVID‐19 NAT result at the time of organ offer, the timing of COVID‐19 symptoms (if any) in relationship to donation is not known. Only 7.4% of COVID‐19 NAT‐positive donors were noted to have COVID‐19 as a cause of death in the dataset. It is not possible to ascertain which of the other donors may have had significant COVID‐19 disease that led to death. Additionally, though COVID‐19 donors appeared to have similar organ function based on KDPI and LVEF, evidence for COVID‐19 organ involvement is not available in the data reviewed. It is entirely plausible that many COVID‐19 NAT‐positive donors suffered death due to an unrelated cause and were found to have a positive test at donation screening. Additionally, there are potentially donors with false‐positive tests in the COVID‐19 NAT‐positive group, along with false‐negative tests in the COVID‐19 NAT‐negative group.
Whether death (n = 5) or graft loss (n = 8) in the study population was influenced by donor COVID‐19 NAT status is not known as well. In our cohort, no deaths were obviously attributed to COVID‐19, though two were documented as unknown, one related to sepsis, and one related to respiratory failure, which could potentially be due to COVID‐19 infection. Additionally, two kidney graft losses and one liver death were attributed to graft or hepatic artery thrombosis, which raises concerns about hypercoagulability in this group. Furthermore, the single reported fatality in the literature of a lung transplant recipient after transmission of COVID‐19 from the donor was not captured by our method of data analysis in the study. 14 In this case, it is likely that this donor‐recipient pair had missing data, which did not allow for a proper matching to take place. Nonetheless, even if all deaths were attributed to COVID‐19, the overall survival of the cohort is equal to that of the rest of the population. It is not known, however, if any evidence of organ damage due to COVID‐19 infection may be present in these organs and survival is not necessarily the only relevant outcome. There has not been enough follow‐up time for most individuals to assess for long‐term organ damage via this database review. With this, continued post‐transplant evaluation for late sequelae of COVID‐19 on graft function must occur. Additionally, COVID‐19 transmission to the recipient is not specifically reported in the dataset, nor would it be reported through UNOS patient safety as an unexpected event. Thus, its prevalence is unknown, though at some point it may be expected to occur.
Despite these reassuring outcomes, other factors must be considered when assessing the impact of COVID‐19 NAT‐positive deceased donor utilization, which is not readily apparent in the data available in the dataset. Resource utilization and potential risk to OPO, donor hospital, recipient hospital, and transplant team staff must be considered. 8 While transplantation of solid organs is a life‐saving endeavor, intensive care unit and operating room utilization for such procedures must be balanced against the needs of other individuals. Additionally, the risk of exposure to known COVID‐19‐positive donors requires additional consideration regarding the allocation of personal protective equipment and the development of novel protocols for infection prevention. Not only can potential exposure and infection of healthcare staff lead to morbidity or mortality in these individuals, but it also reduces these caregiver's availability for other duties, which have already been overwhelmed at times during the pandemic.
5. LIMITATIONS
There are several limitations to the outcomes of this study, which could not be overcome, given the lack of granular data regarding aspects of the COVID‐19 infection itself. The largest limitation is that the COVID‐19 NAT‐positive donors included in this report account for only a small proportion of all potential COVID‐19 NAT‐positive donors. No information is available on those donors who did not proceed with organ recovery, nor is it known why organs from these donors were accepted. Data regarding patient follow up is provided by the transplanting center and may be missing, incomplete, or not up to date. There is limited data available for organs other than kidney or liver. There is little data available for child donors or recipients. There is no information available on the degree of donor COVID‐19 infection, symptoms, organ involvement, or therapies. There is no information regarding the cycle thresholds of these tests. There is a potential patient selection bias as well with some centers offering COVID‐19‐positive donor transplants to recipients at higher urgency. There is no information available about peri‐transplant therapies employed in these patients to minimize the risk of transmission to recipients, such as remdesivir, monoclonal antibody administration, and vaccination status of the donor or recipient. Additionally, there are changing risks of COVID‐19 infection and morbidities over time and as new variants arise.
6. CONCLUSIONS
Solid organ transplantation using grafts from a cohort of deceased donors with positive COVID‐19 NAT results did not negatively affect early post‐transplant patient or graft survival. Though these results are reassuring, the lack of granular data in the OPTN database regarding many aspects of donor selection, including the extent and timing of COVID‐19 infection in these donors must be considered. To fully understand the risks involved with the utilization of COVID‐19 NAT‐positive donors, expanded data collection must be undertaken to address many of the limitations of this study.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
No funding was received to conduct this study.
Bock MJ, Vaughn GR, Chau P, Berumen JA, Nigro JJ, Ingulli EG. Organ transplantation using COVID‐19‐positive deceased donors. Am J Transplant. 2022;00:1‐14. doi: 10.1111/ajt.17145
Contributor Information
Matthew J. Bock, Email: mbock@ucsd.edu.
Gabrielle R. Vaughn, Email: gvaughn@ucsd.edu
Peter Chau, Email: pchau@ucsd.edu.
Jennifer A. Berumen, Email: jberumen@health.ucsd.edu
John J. Nigro, Email: jnigro@ucsd.edu
Elizabeth G. Ingulli, Email: eingulli@health.ucsd.edu
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the Corresponding author. The data are publicly available through requests made to UNOS/OPTN.
REFERENCES
- 1. American Society of Transplantation . COVID‐19 (Coronavirus): FAQs for Organ Transplantation . Updated March 10, 2020. https://www.myast.org/faqs‐organ‐transplantation. Accessed April 1, 2020.
- 2. Association of Organ Procurement Organizations . COVID‐19 (Coronavirus) Bulletin . Updated on March 10, 2020. https://www.aopo.org. Accessed April 01, 2020.
- 3. American Society of Transplant Surgeons . Organ Retrieval for Transplantation in the COVID‐19 Era . Updated March 27, 2020. https://asts.org/advocacy/covid‐19‐resources/asts‐covid‐19‐strike‐force/asts‐covid‐19‐strike‐force‐organ‐retrieval‐guidance. Accessed April 1, 2020.
- 4. International Society for Heart and Lung Transplantation . Guidance for Cardiothoracic Transplant and Ventricular Assist Device Centers regarding the SARS‐CoV‐2 pandemic . Updated March 21, 2020. https://ishlt.org/ishlt/media/documents/SARS‐CoV‐2_Guidance‐for‐Cardiothoracic‐Transplant‐and‐VAD‐center.pdf. Accessed April 1, 2020.
- 5. Goff RR, Wilk AR, Toll AE, McBride MA, Klassen DK. Navigating the COVID‐19 pandemic: initial impacts and responses of the organ Procurement and transplantation Network in the United States. Am J Transplant. 2021;21(6):2100‐2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cholankeril G, Podboy A, Alshuwaykh OS, et al. Early impact of COVID‐19 on solid organ transplantation in the United States. Transplantation. 2020;104:2221‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashfaq A, Gray GM, Carapellucci J, et al. Impact of Coronavirus‐2019 on pediatric and adult heart transplantation waitlist activity and mortality in the United States: a descriptive approach. Lancet Reg Health Am. 2021;3:100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah MB, Lynch RJ, El‐Haddad H, Doby B, Brockmeier D, Goldberg DS. Utilization of deceased donors during a pandemic: argument against using SARS‐CoV‐2–positive donors. Am J Transplant. 2020;20(7):1795‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michaels MG, La Hoz RM, Danziger‐Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;20(7):1768‐1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Natori Y, Anjan S, Simkins J, et al. Small bowel transplantation from SARS‐CoV‐2 respiratory PCR positive donors: is it safe? Transpl Infect Dis. 2021;23(6):e13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Querrey M, Kurihara C, Manerikar A, et al. Lung donation following SARS‐CoV‐2 infection. Am J Transplant. 2021;21(12):4073‐4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de la Villa S, Valerio M, Salcedo M, et al. Heart and liver transplant recipients from donor with positive SARS‐CoV‐2 RT‐PCR at time of transplantation. Transpl Infect Dis. 2021;23:e13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ceulemans LJ, Van Slambrouck J, De Leyn P, et al. Successful double‐lung transplantation from a donor previously infected with SARS‐CoV‐2. Lancet Respir Med. 2021;9(3):315‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS‐CoV‐2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21(8):2885‐2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong HL, Kim SH, Choi DL, Kwon HH. A case of coronavirus disease 2019–infected liver transplant donor. Am J Transplant. 2020;20(10):2938‐2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kute VB, Godara S, Guleria S, et al. Is it safe to be transplanted from living donors who recovered from COVID‐19? Experience of 31 kidney transplants in a multicenter cohort study from India. Transplantation. 2021;105(4):842‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koval CE, Poggio ED, Lin YC, Kerr H, Eltemamy M, Wee A. Early success transplanting kidneys from donors with new SARS‐CoV‐2 RNA positivity: a report of 10 cases. Am J Transplant. 2021;21:3743‐3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhand A, Gass A, Nishida S, et al. Successful transplantation of organs from a deceased donor with early SARS‐CoV‐2 infection. Am J Transplant. 2021;21:3804‐3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neidlinger NA, Smith JA, D'Alessandro AM, et al. Organ recovery from deceased donors with prior COVID‐19: a case series. Transpl Infect Dis. 2021;23(2):e13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sigler R, Shah M, Schnickel G, et al. Successful heart and kidney transplantation from a deceased donor with PCR positive COVID‐19. Transpl Infect Dis. 2021;23:e13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frattaroli P, Anjan S, Coro A, et al. Is it safe to perform abdominal transplantation from SARS‐CoV‐2 polymerase chain reaction positive donors? Transpl Infect Dis. 2021;23:e13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barros N, Ermel A, Mihaylov P, Lacerda M, Fridell J, Kubal C. Deceased donor liver transplantation from a SARS‐CoV‐2–positive donor to a SARS‐CoV‐2–positive recipient. Liver Transpl. 2021;27(12):1849‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puodziukaite L, Serpytis M, Kundrotaite A, et al. Kidney transplantation from a SARS‐CoV‐2‐positive donor for the recipients with immunity after COVID‐19. Transpl Infect Dis. 2021;23(4):e13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molnar MZ, Hall IE, Raghavan D, et al. Kidney transplantation from SARS‐CoV‐2–positive deceased donor. Am J Transplant. 2021;22:1280‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meshram HS, Kute VB, Patel H, Desai S, Chauhan S, Dave RB. A case report of successful kidney transplantation from a deceased donor with terminal COVID‐19‐related lung damage: ongoing dilemma between discarding and accepting organs in COVID‐19 era! Transpl Infect Dis. 2021;23:e13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romagnoli R, Gruttadauria S, Tisone G, et al. Liver transplantation from active COVID‐19 donors: a lifesaving opportunity worth grasping? Am J Transplant. 2021;21(12):3919‐3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jayasekera CR, Vikram HR, Rifat Z, et al. Solid organ transplantation from SARS‐CoV‐2–infected donors to uninfected recipients: a single‐center experience. Transplant Direct. 2022;8(2):e1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wall AE, McKenna GJ, Onaca N, et al. Utilization of a SARS‐CoV‐2–positive donor for liver transplantation. Proc (Bayl Univ Med Cent). 2022;35(1):62‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Organ Procurement and Transplantation Network . Summary of current evidence and information– donor SARS‐CoV‐2 Testing & Organ Recovery from donors with a history of COVID‐19. Updated January 21, 2022. https://optn.transplant.hrsa.gov/media/kkhnlwah/sars‐cov‐2‐summary‐of‐evidence.pdf. Accessed February 1, 2022.
- 30. Kute VB, Fleetwood VA, Meshram HS, Guenette A, Lentine KL. Use of organs from SARS‐CoV‐2 infected donors: is it safe? A contemporary review. Curr Transplant Rep. 2021;8:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eichenberger EM, Kaul DR, Wolfe CR. The pandemic provides a pathway: what we know and what we need to know about using COVID positive donors. Transpl Infect Dis. 2021;23(5):e13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kates OS, Fisher CE, Rakita RM, Reyes JD, Limaye AP. Use of SARS‐CoV‐2‐infected deceased organ donors: should we always “just say no?”. Am J Transplant. 2020;20(7):1787‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess deaths associated with COVID‐19, by age and race and ethnicity—United States, January 26–October 3, 2020. Morb Mortal Wkly Rep. 2020;69(42):1522‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ho JS, Fernando DI, Chan MY, Sia CH. Obesity in COVID‐19: a systematic review and meta‐analysis. Ann Acad Med Singapore. 2020;49(12):996‐1008. [PubMed] [Google Scholar]
- 35. Nguyen NT, Chinn J, De Ferrante M, Kirby KA, Hohmann SF, Amin A. Male gender is a predictor of higher mortality in hospitalized adults with COVID‐19. PLoS One. 2021;16(7):e0254066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brüssow H. COVID‐19: omicron–the latest, the least virulent, but probably not the last variant of concern of SARS‐CoV‐2. Microbial Biotechnology. 2022;15:1927‐1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Assistance Publique ‐ Hôpitaux de Paris . (2020, June ‐ ). Descriptive and Retrospective Analysis of Acute Myocarditis Associated With Pandemic COVID‐19 in Children. Identifier: NCT04420468. Updated May 24, 2021. https://clinicaltrials.gov/ct2/show/NCT04420468. Accessed June 10, 2022.
- 38. Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of BNT162b2 vaccine against critical Covid‐19 in adolescents. N Engl J Med. 2022;386:713‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID‐19 hospitalization and disease severity. JAMA. 2021;326(20):2043‐2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available on request from the Corresponding author. The data are publicly available through requests made to UNOS/OPTN.


